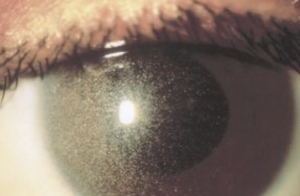
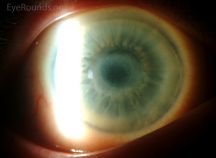
Metabolic Keratopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
A number of systemic metabolic disorders of genetic origin affect the anterior portion of the eye. Many of the corneal manifestations of systemic disease are alterations in corneal clarity and function caused by abnormal storage of metabolic substances, such as proteins, carbohydrates, and lipids.
Metabolic keratopathies (MK) are usually autosomal recessive and a single enzyme defect or absence often accounts for the clinical manifestations. Various gene loci have been determined along with its associated biochemical defect in patients with MK.
Disease Entity
Disease
MK can be confused with corneal dystrophies as both are bilateral and are often associated with systemic involvement. The following points can be used to distinguish between the two:
- MK affects the peripheral as well as the central cornea.
- The corneal changes in metabolic disorders may involve more than one layer of the cornea.
- There is typically progression over time in patients with MK.
Etiology
The clinical manifestations are dependent upon the enzymatic defects and accumulation of specific toxic metabolic substances. Patients can present with a wide range of symptoms (including ocular, cardiac, neurological deficits) and even death at an early age.
General Pathology
Enzymatic defects along the normal path of metabolism cause accumulation of toxic byproducts which circulate in the body. Depending upon the level of toxicity, the result may be disabling or even life-threatening.
Metabolic Disorders
Diabetes Mellitus
Epidemiology
Diabetes mellitus (DM) affects 451 million adults between the ages of 20 to 99 years of age worldwide. Diabetic keratopathy affects 50-70% of patients with DM.
Pathogenesis
- Insulin deficiency and/or resistance
- Enzymatic abnormalities
- Increase in polyol metabolism
- Increase in glycation of protein components
- Increase in advanced glycation end products (AGE)
- Structural & functional abnormalities
- Basement membrane thickening
- Decreased corneal nerve bundles
- Loss of nerve-derived growth factors (IGF-1, substance P)
Treatment
- Topical lubricants
- Patching
- Bandage contact lens
- Topical growth factors (IGF-1 and Substance P and Epidermal Growth Factor)
- Oral and topical aldose reductase inhibitor
- Cenegermin
Disorders of lysosomal storage
Lysosomes are organelles that digest protein, nucleic acids, lipids, and carbohydrates.
A. Mucopolysaccharidoses (MPS)
MPS occurs with a prevalence of 1:10,000 births. Screening is performed by measuring urine glycosaminoglycans (GAGs). Diagnosis is based on blood assays.
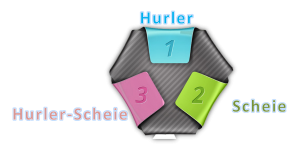
1. MPS I
- Defective enzyme: α-iduronidase
- Accumulation: Dermatan & heparan sulfate (glycosaminoglycans, GAGs)
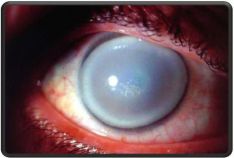
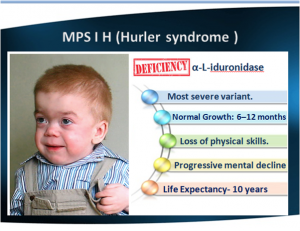
- Hurler's is the most severe form. Hurler is characterized by severe mental impairment.
- Scheie is the mildest form.
- All 3 forms can present with severe corneal clouding that mostly involves the stroma (sparing the endothelium and Descemet's membrane.
2. MPS II (Hunter's syndrome)
- Defective enzyme: iduronate-2-sulfatase
- Accumulation: Dermatan & heparan sulfate (GAGs)
- No to minimal corneal changes.
3. MPS III (Sanfilippo)
- Defective enzyme: Deficiencies in 4 different enzymes have been identified, but are clinically indistinguishable
- Accumulation: GAGs
- Presents between age 2-6yrs
- Rapid mental deterioration
- No corneal changes, though mild to severe retinopathy can be detected
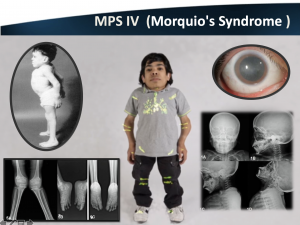
4. MPS IV (Morquio)
- Defective enzyme: N-acetylgalactosamine-6-sulfatase
- Accumulation: Keratan sulfate, dermatan sulfate (GAGs)
- Characterized by severe skeletal changes & spinal cord compression
- Mild corneal opacification
- PKP is of limited use due to concomitant retinopathy
5. MPS VI (Maroteaux-Lamy)
- Defective enzyme: N-acetylgalactosamine-4-sulfatase
- Accumulation: Dermatan sulfate
- Normal intelligence, otherwise very similar to Hurler’s syndrome in regard to systemic manifestations
- Corneal clouding that may benefit from a PKP
6. MPS VII (Sly syndrome)
- Defective enzyme: ß-glucuronidase
- Accumulation: Heparan, dermatan, and chondroitin sulfate
- Corneal opacification
7. MPX IX (Natowicz)
- Defective enzyme: hyaluronidase
- Accumulation: Hyaluronic acid
- No ocular symptoms
B. Lipidoses
1. Generalized gangliosidosis (GM) 1
- Defective enzyme: ß-galactosidases A, B, and C
- Accumulation: GM 1 ganglioside
- Autosomal recessive
- Early life psychomotor retardation, skeletal deformities, and organomegaly
- Death by 2 years of age
- Mild and diffuse corneal clouding
- Other ocular manifestations include nystagmus, cherry-red spot of the macula, retinal hemorrhages, and optic atrophy
2. GM 2 gangliosidosis (Tay-Sachs)[1]
- Defective enzyme: Beta Hexosaminidase-A
- Accumulation: GM2 gangliosides
- Diagnosis: no or low level of Hex A enzyme activity and normal or elevated levels of Beta Hexosaminidase B, targeted gene testing
- Autosomal Recessive
- Gene: mutation in HEXA gene located at 15q23
- There are three main types of Tay-Sachs disease: infantile, juvenile, and adult-onset types.
- Infantile: presents within 3-6 months but may present as early as one week or through prenatal non hydrops fetalis
- Early presentation: Motor weakness, irritability, sensory hypersensitivity, exaggerated startle, hypotonic, developmental delays
- Later symptoms: spasticity and seizures, macrocephaly, decerebrate posturing and vegetative state by age 2 years
- Prolonged QT and nonspecific T waves changes have been reported
- Juvenile: presents between ages two to ten
- Early presentation: incoordination, muscle weakness, ataxia, spasticity
- Later Symptoms: vegetative state with decerebrate posturing 5-10 years after diagnosis and death a few years later
- Adult: adolescence or early adulthood
- Less aggressive form that presents with various neurological and psychiatric symptoms
- Ocular manifestations:
- Infantile
- Cherry-Red spots due to GM2 ganglioside accumulation in the retinal ganglion cells, concentrating in the macula margins
- Narrowing of retinal vessels
- Nystagmus
- Optic atrophy
- Complete vision loss
- Juvenile
- Cherry-Red Spot
- Optic atrophy and retinitis pigmentosa later in life
- Infantile
3. GM 2 gangliosidosis (Sandhoff)[2][3]
- Defective enzyme: Beta Hexosaminidase A and B
- Accumulation: GM2 gangliosides
- Autosomal Recessive
- Clinically indistinguishable from Tay-Sachs Disease
- Ocular manifestations:
- Total vision loss
- Cherry-Red Spot in the Macula
4. GM 2 gangliosidosis (GM2 activator deficiency)[4]
- Defective enzyme: GM2 Activator
- Accumulation: GM2 gangliosides
- Diagnosis: proband with findings of GM2 gangliosidosis, normal beta hexosaminidase enzyme, molecular genetic testing
- Autosomal Recessive
- Gene: GM2A
- Symptoms include weakness and loss of motor skills starting at 4 to 12 months, hypotonia, exaggerated startle response, hyperreflexia, seizures
- Ocular manifestations:
- Cherry-Red Spot in the Macula
5. Fabry Disease
- Defective enzyme: α–galactosidase
- Accumulation: α-galactosidase A (sphingolipid)
- Diagnosis: WBC enzyme assay, though gene sequencing is the most reliable
- X-linked recessive
- Renal failure, peripheral neuropathy, and cardiovascular disease
- Corneal changes (corneal verticillata) are the most consistent and earlier ocular abnormality
- Confocal microscopy can show intracellular inclusions
- Aneurysmal dilations and tortuosity of the conjunctival and retinal vessels
- Posterior subcapsular cataract
- Treatment
- Renal transplantation
- Enzyme replacement therapy (available in the US since 2003), can decrease the appearance of corneal verticillate
6. Multiple sulfatase deficiency
- Defective enzyme: Cα-formylglycine-generating enzyme
- Accumulation: Sulfatides
- Diagnosis: WBC & plasma enzyme assay
- Autosomal recessive
- Presents in infancy, death usually by age 10
- Subtle corneal opacities
- Other ocular findings include macular changes, cataract, and optic atrophy
- Clinical features
- Neurological deficiencies
- Ichthyosis
- Coarse facies
7. Farber lipogranulomatosis[5][6]
- Defective enzyme: Ceramidase or Acid Ceramidase
- Autosomal Recessive
- Gene: ASAH1
- Classic symptoms: Painful swollen joints, subcutaneous nodules (lipogranulomas), hoarse voice due to laryngeal nodules
- Other symptoms: developmental delay, visceromegaly, respiratory involvement, neurological symptoms
- Potential ocular manifestations:
- Cherry-Red Spot on the Macula
- Corneal opacity
- Visual fixation instability
- Nystagmus
8. Gaucher disease[7][8]
- Defective enzyme: Glucocerebrosidase
- Accumulation: Glucocerebroside lipids
- Diagnosis: less than 15% mean normal activity of glucocerebrosidase in peripheral blood leukocytes
- Autosomal Recessive
- Gene: GBA1
- There are five types of Gaucher disease including type 1, type 2, type 3, perinatal lethal and cardiovascular.
- Common symptoms include hepatosplenomegaly, severe joint pain, parkinsonism, yellow skin pigmentation
- Ocular Manifestations:
- Ocular motor apraxia in Type III
- Strabismus
- Corneal opacity
- Cherry-Red Spot on the Macula
9. Niemann-Pick A & B[9]
- Defective enzyme: Acid Sphingomyelinase (ASM)
- Accumulation: sphingomyelin and its precursor lipids accumulate in lysosomes, particularly in macrophages
- Autosomal Recessive
- Gene: Type A and B are caused by missense mutations in sphingomyelin phosphodiesterase 1 (SMPD1)
- Diagnosis: blood sample to evaluate the activity of ASM in leukocytes, gene testing
- Type A presents in the first few months of life, typically before three months
- Neurological symptoms appear by one year of age
- Hepatosplenomegaly, thrombocytopenia, interstitial lung disease, slowed bone growth, neurological manifestations such as ataxia, developmental delay, peripheral neuropathy
- Ocular manifestations:
- Bright cherry-red fovea with a white or pale macula
- Corneal opacification
- Brown discoloration of the anterior lens capsule
C. Mucolipidoses
Autosomal recessive
1. ML 1 (sialidosis I)
- Defective enzyme: Neuraminidase
- Accumulation: Sialic acid
- Stromal opacities that represent intralysosomal inclusions
- Other ocular findings include conjunctival and retinal vascular tortuosity and macular cherry-red spots
2. ML II (I-cell)
- Defective enzyme: Transferase
- Accumulation: Multiple acid hydrolases
- Mild granular stromal opacifications, megalocornea
- Other ocular findings include glaucoma and orbital hypoplasia
- Conjunctival biopsy may reveal birefringent lysosomal inclusions
- Death is usually between the ages of 2-8
3. ML III (pseudo-Hurler)
- Defective enzyme: Transferase
- Accumulation: Many
- Mild symptoms, normal life expectancy
- Mild corneal findings that are usually not visually significant
4. ML IV
- Defective enzyme: Mucolipin-1
- Accumulation: Lipids & mucopolysaccharides
- Diffuse corneal clouding & corneal surface irregularities
- Other ocular findings include optic nerve atrophy, retinal degeneration, and cataract
- Treatment
- Epithelial debridement can help temporarily with corneal haze
- Limbal allograft transplantation
D. Galactosialidosis
- Autosomal recessive
- Accumulation: sialyloligosaccharides.
- Diagnosis: Cultured cells
- Mild corneal clouding
- Systemic findings: Seizures, hearing loss, ataxia, facial dysmorphism
E. Cystinosis
- Autosomal recessive
- Defective enzyme: Cystinosin
- Accumulation: Cystine
- Diagnosis: WBC cystine, conjunctival biopsy to measure the free cystine level
- Screening test: Renal function
- Corneal findings are the same for all 3 subtypes
- Needle-shaped crystals in the cornea can be detected on slit-lamp biomicroscopy
- Crystal can be found in all layers of the cornea (starts in the anterior peripheral stroma
- Increased cornea thickness (due to accumulation of crystals)
- Punctate keratopathy
- Band keratopathy
- Peripheral corneal neovascularization
- Recurrent corneal erosions
- Ocular symptoms
- Severe photophobia and blepharospasm
- Loss of color & contrast sensitivities
- Ocular imaging
- AS-OCT and confocal microscopy may help visualize the corneal crystals and monitor progression
- Subtype
- Nephropathic (most common & severe form)
- Cornea findings appear from the 1st year of life
- Retinal depigmentation appears by 3-7 years of age
- Glaucoma
- Renal Fanconi syndrome, end-stage renal disease (ESRD) by 10 years of age
- Intermediate (juvenile-onset or adolescent)
- Corneal crystals are evident
- No retinopathy
- Slower progression to ESRD
- Non-nephropathic aka benign or adult-type
- Nephropathic (most common & severe form)
- Treatment
- Renal transplantation may prolong life but does not decrease crystal accumulation
- PKP, crystals can reappear in the corneal graft.
- Cysteamine has revolutionized the treatment of cystinosis
- Depletes cells of cystine (reacts with cystine, which allows it to leave the lysosome)
- The systemic form can stabilize renal function and the retinopathy, but there is little effect on the corneal crystals
- Topical cysteamine (0.55% cysteamine hydrochloride solution) 10-12 times a day has been shown to dissolve corneal crystals and improves ocular symptoms
Disorders of lipid & lipid metabolism
Characterized by premature onset of coronary artery disease & PVD
A. Hyperlipoproteinemias
1. Type I (hyperchylomicronemia)
- Markedly elevated triglycerides & Chylomicrons
- Acute pancreatitis & hepatosplenomegaly
- Lipemia retinalis, no corneal arcus
2. Type II (hyperbetalipoproteinemia)
- Elevated betalipoprotein & LDL
- Xanthelasma and arcus
3. Type III (familial dysbetalipoproteinemia)
- Corneal arcus, lipemia retinalis, xanthelasma
4. Type IV (hyperprebetalipoproteinemia)
- Corneal arcus, lipemia retinalis, xanthelasma
5. Type V (hyperprebetalipoproteinemia and hyperchylomicronemia)
- Elevated triglycerides, cholesterol, chylomicrons, VLDL
- Corneal arcus, lipemia retinalis, xanthelasma
B. Schnyder corneal dystrophy
- Autosomal dominant
- Corneal findings
- Bilateral lipid and cholesterol crystals deposition in the corneal stroma, full-thickness involvement can occur. 50% of patients have crystals.
- Central corneal disciform opacity is noted in the 1st to 2nd decades of life
- Corneal arcus & diffuse corneal haze is noted after age of 40
- Crystals can be visualized on AS-OCT
- If suspected, obtain a lipid panel
C. Hypolipoproteinemias
1. Lecithin-cholesterol acyltransferase (LCAT) deficiency
- Autosomal recessive
- Markedly reduced cholesterol esters & lysolecithin; increase in unesterified cholesterol, triglycerides, and phospholipids
- Corneal arcus
- Corneal stromal haze due to focal deposits of lipid
- Angioid streaks, peripapillary hemorrhages, and retinal venous dilation
2. Tangier disease (familial HDL deficiency)
- Absence of HDL; accumulation of cholesterol in various tissues
- Dense posterior corneal stromal opacities that are not usually visually significant
- No corneal arcus
- Corneal neuropathy
3. Fish eye disease
- Autosomal recessive
- LCAT is only partially impaired. Reduction of HDL but near-normal levels of cholesteryl esters
4. Familial hypobetalipoproteinemia
- Retinal findings only, no corneal disease
5. Bassen-Bronzweig disease
- Retinal findings only, no corneal disease
Disorders of Amino Acid, Nucleic Acid, and Protein Metabolism
A. Tyrosinemia II
- Autosomal recessive
- Enzyme deficiency: Hepatic tyrosine aminotransferase
- Diagnosis
- Increased levels of tyrosine in the blood and urine
- Confocal microscopy can demonstrate crystalline deposits in the cornea
- Systemic symptoms
- Painful skin blisters
- Seizures
- Developmental delay
- Ocular findings & symptoms
- Bilateral pseudodendritic lesions
- Corneal & conjunctival plaques
- Corneal ulcerations
- Epiphora, photophobia
- Chronic keratitis can result in corneal neovascularization and scarring
- Treatment
- PKP, lesions can recur in the graft
- Tyrosine and phenylalanine restricted diet in infancy is the most effective therapy, which can clear the corneal lesions and ameliorate systemic symptoms
B. Alkaptonuria
- Autosomal recessive
- Enzyme deficiency: Homogentisic acid oxidase
- Accumulation: Homogentisic acid (an intermediate breakdown product in the catabolism of phenylalanine and tyrosine)
- Ocular findings
- Blue-black pigmentation of the conjunctival and intrapalpebral scleral, usually at the insertion of the horizontal rectus muscles
- Brownish-black “oil droplet” pigmentation of the cornea
- Vision is usually not affected
- Systemic findings
- Arthropathy, ochronotic calculi, cardiovascular ochronosis, aortic valve stenosis
C. Amyloidosis
- Local & systemic deposition of amyloid, a protein with a set of unique properties
- Homogenous granular to filamentous eosinophilia with hematoxylin and eosin stain
- Metachromasia with crystal violet
- UV fluorescence (yellow-green) with thioflavin T
- Orange-red staining with Congo red, which exhibits two additional properties- birefringence & dichroism
- Ocular symptoms
- Cornea is involved in systemic and localized amyloidosis (either secondary or primary amyloid deposition)
1. Primary cornea amyloidosis
- Gelatinous droplike dystrophy
- Lattice corneal dystrophy types I & III
- No systemic manifestations
2. Systemic primary amyloidosis
- Lattice dystrophy type II
- Familial amyloidotic polyneuropathy type V or Meretoja syndrome
- Rare dominantly inherited conditions
- Appears in early adulthood
- Rarely described in patients who are not Finnish
- There is a similar condition that is nonfamilial
- Mutation of GLN (gene located on chromosome 9) encoding the actin severing protein Gelsolin
- Triad of symptoms
- Ocular (LCD II, glaucoma)
- Lattice, progressive loss of corneal sensory nerves, dry eyes
- Epithelial erosions later in life
- Visual acuity is usually retained until the 6th decade but visual loss after 60 years of age is common
- Neurological (cranial, peripheral, and autonomic neuropathies)
- Dermatological manifestations
- Ocular (LCD II, glaucoma)
- Other symptoms
- Renal and cardiac involvement
- Treatment
- PKP, can be complicated by neurotrophic persistent epithelial defect. Integrity is dependent on an intact nerve supply to the cornea which is absent in amyloidosis type V
- Excimer laser phototherapeutic keratectomy has been successful in patients who have had recurrences after lamellar keratoplasty
3. Localized secondary corneal amyloidosis
- Localized, follows chronic diseases such as neoplasms, infections, connective tissue disorders, and trauma4.
4. Secondary systemic amyloidosis with corneal manifestations
- Myeloproliferative disorders such as monoclonal gammopathy
- Heavy chain amyloidosis
D. Gout
- Accumulation of urea (urate crystals) in tissue
- Most common in middle aged men
- Etiologies
- Myeloproliferative disorder
- Alcohol
- Chemotherapy
- Obesity
- Ocular findings
- Urate keratopathy- chalky deposits or fine refractile crystals in the corneal stroma
- Band keratopathy
- Scleritis or episcleritis
- Conjunctival vesicles
- Dilated and tortuous conjunctival vessels
- Treatment
- Short-term: colchicine, indomethacin, and phenylbutazone
- Long-term: probenecid, salicylates, allopurinol
- Superficial keratectomy for band keratopathy
E. Porphyria
- Accumulation of porphyrins (pigment involved in heme synthesis) due to abnormally high production
- Enzyme deficiency uroporphyrinogen decarboxylase
1. Porphyria cutanea tarda
- Autosomal dominant or sporadic
- Systemic findings include skin abnormalities (fragility, redness, vesicles), liver disease, and hypertrichosis
2. Congenital erythropoietic porphyria
- Autosomal recessive
- Diagnosis: Excreted porphyrins
- Presents soon after birth
- Cornea & conjunctiva findings
- Chemosis
- Corneal & conjunctival vesicles
- Corneal perforation
- White tan crystals in the deep stroma
- Pingueculas and pterygium
- Scleritis & scleral necrosis
- Treatment
- Artificial tears
- Avoid sunlight
- Phlebotomy
- Subcutaneous desferrioxamine
- Topical steroids and cyclosporine A
- Penetrating keratoplasty
References
- ↑ Ramani PK, Parayil Sankaran B. Tay-Sachs Disease. [Updated 2023 Jan 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564432/
- ↑ Sandhoff Disease. Rare Diseases. https://rarediseases.info.nih.gov/diseases/2521/sandhoff-disease. Accessed [July 3, 2023]
- ↑ Sandhoff Disease. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/disorders/sandhoff-disease. Accessed [July 3, 2023]
- ↑ Xiao C, Toro C, Tifft C. GM2 Activator Deficiency. 2022 Aug 25. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK583219/
- ↑ Tripathy K, Patel BC. Cherry Red Spot. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539841/
- ↑ Farber Disease. Rare Diseases. https://rarediseases.info.nih.gov/diseases/6426/farbers-disease. Accessed [July 3, 2023]
- ↑ Stone WL, Basit H, Master SR. Gaucher Disease. [Updated 2022 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448080/
- ↑ Gaucher Disease. Rare Diseases. https://rarediseases.info.nih.gov/diseases/8233/gaucher-disease. Accessed [July 3, 2023]
- ↑ Bajwa H, Azhar W. Niemann-Pick Disease. [Updated 2023 Mar 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556129/
- Krachmer J, Mannis M, Holland E: CORNEA, 2nd ed.Elsevier Mosby, 2017, 620-644.
- Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D. Diabetic Keratopathy: Insights and Challenges. Surv Ophthalmol 2020.
- External Disease and Cornea, Section 8. Basic and Clinical Science Course, AAO, 2006./li>