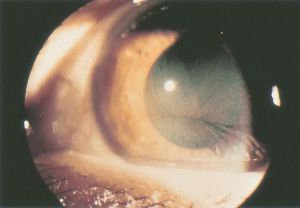
Cornea Verticillata
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.

Disease Entity
Cornea verticillata (also called vortex keratopathy, whorl keratopathy, or Fleischer vortex) describes a whorl-like pattern of golden brown or gray opacities in the corneal epithelium. It is termed cornea verticillata from the Latin noun “verticillus,” meaning "whorl”. Usually asymptomatic, it is caused by the deposition of medications, metabolic substrates, or disease byproducts in the basal epithelial layer of the cornea.
Etiology
Cornea verticillata is most commonly associated with amiodarone and Fabry disease.[1]
Pharmacological etiologies:
It can be caused by a variety of oral medications such as amiodarone, hydroxychloroquine, chloroquine, indomethacin, and phenothiazines.[2] Topical Rho- kinase inhibitors have also been shown to cause cornea verticillata.[3] Other agents that have been less commonly associated with cornea verticillata include gentamicin, tamoxifen, meperidine, chlorpromazine, atovaquone, suramin, tilorone, perhexiline maleate, and the tyrosine kinase inhibitors vandetanib, and osimertinib.[2][4][5] More recently, netarsudil- induced verticillata has been described in a patient who complained of glare and hazy vision, which resolved upon cessation.[6]
Non-pharmacological etiologies:[2][7][8]
- Multiple myeloma
- Multiple sulfatase deficiency
- Generalized gangliosidosis
- Neurotrophic keratitis
- Lisch corneal dystrophy
- Epidemic keratoconjunctivitis
- Iron deposition (i.e., after radial keratotomy)
- Stromal deposition (gold, silver, antacid, retinoid depositions)
Pathophysiology
The whorl-like pattern of cornea verticillata results from the centripetal migration of deposit-laden limbal stem cells as the corneal epithelium undergoes natural growth and repair.[2][9]
The medications that produce cornea verticillata share cationic, amphiphilic properties that allow them to penetrate lysosomes in the basal epithelial layer of the cornea, where they bind to cellular lipids. These medication-lipid complexes are resistant to enzymatic degradation and accumulate as deposits in the cornea.[2] Specifically, amiodarone inhibits lysosomal phospholipase A2 resulting in susceptibility of the inner membrane of the cell to degradation by proteases.[10][11] Suramin inhibits iduronate sulfatase, resulting in accumulation of glycosaminoglycans.[11]
Cornea verticillata is seen in 90% of patients with Fabry disease, a rare X-linked recessive lysosomal storage disorder, that is also associated with progressive nephropathy and peripheral neuropathy.[12] However, female carriers may only present with corneal findings.[10] Unlike that seen with medications, the cornea verticillata in Fabry disease is caused by a deficiency in alpha-galactosidase A, a lysosomal enzyme. This results in the accumulation of glycosphingolipids in lysosomes throughout body tissues, including the cornea.[2][5][12]
Diagnosis
Symptoms
Patients with cornea verticillata typically have no visual complaints or eye discomfort. Rarely, patients may see blue-green rings in their vision or halos around lights.[2]
Signs
Cornea verticillata is recognizable as fine golden-brown or gray opacities in the basal epithelium that branch out from a central whorl, usually across the inferior cornea. The deposits do not stain and are almost always bilateral.[2][13]
Occasionally, slight differences on slit-lamp microscopy can be seen in the opacities due to medications compared to those seen in Fabry disease. Drug-related opacities may appear as horizontal lines with fine branching at the extremities[14], whereas Fabry disease-related opacities may appear as curving lines that form whorls before becoming almost straight at the periphery of the cornea.[13]
The etiology of drug-induced forms of cornea verticillata can typically be identified with a thorough review of the patient’s medical history and medications. If attempting to differentiate between drug-induced cornea verticillata and that caused by Fabry disease, the microstructure of the deposits can be viewed with confocal laser scanning microscopy.[15] However, this is not a routine part of the diagnostic evaluation.
Management and Follow-up
There is no recommended treatment for cornea verticillata. The deposits are typically not visually significant, and typically resolve with cessation of the responsible agent. No alteration in medication regimen or further work-up is required for an isolated finding of cornea verticillata.[2] Several case reports have described the resolution of cornea verticillata with the use of topical heparin.[16]
If cornea verticillata is associated with a drug that is known to produce retinal toxicities—most notably hydroxychloroquine, chloroquine, chlorpromazine, and tamoxifen—patients should be routinely monitored with automated visual fields plus spectral-domain optical coherence tomography (SD-OCT).[17] The presence of cornea verticillata does not correlate with retinal toxicity.[18] The possibility of optic neuropathy should also be considered if patients taking amiodarone or tamoxifen present with reduced vision. For these patients, a reduction in dose or a switch to a different medication may be necessary.[2][19]
References
- ↑ Koh S, Hamano T, Ichii M, Yatsui K, Maeda N, Nishida K. Transient Cornea Verticillata of Unknown Etiology. Cornea. 2019; 38 (5): e16–e17. doi: 10.1097/ICO.0000000000001913.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Raizman M. B., Hamrah P., Holland E. J., Kim T., Mah F. S., Rapuano C. J., & Ulrich R. G. (2017). Drug-induced corneal epithelial changes. Survey of ophthalmology, 62(3), 286–301. 10.1016/j.survophthal.2016.11.008.
- ↑ Wu JH, Chang SN, Nishida T, Kuo BI, and Lin JW. Intraocular pressure- lowering efficacy and ocular safety of Rho- kinase inhibitor in glaucoma: a meta- analysis and systemic review of prospective randomized trials. Graefe's Arch. Clin. Exp. Ophthalmol. Sept 7 2021. Pub online.
- ↑ Shah GK, Cantrill HL, Holland EJ. Vortex keratopathy associated with atovaquone. Am J Ophthalmol, 120 (5) (1995), pp. 669-671.
- ↑ Jump up to: 5.0 5.1 D'Amico DJ, Kenyon KR. Drug-induced lipidoses of the cornea and conjunctiva. Int Ophthalmol, 4 (1–2) (1981), pp. 67-76.
- ↑ Rivera, S.S., Radunzel, N. & Boese, E.A. Symptomatic Netarsudil-Induced Verticillata. JAMA Ophthalmol 141, e232949 (2023).
- ↑ King JA, Affeldt JC, Agarwal MR. Etiologic profile of vortex keratopathy. Investigative Ophthalmology and Visual Science. 2003;44(13):1381.
- ↑ Chong EM, Campbell JR, Bourne WM. Vortex keratopathy in a patient with multiple myeloma. Cornea, 16 (5) (1997), pp. 592-594.
- ↑ Bron AJ. Vortex patterns of the Corneal epithelium. Trans ophthalmol SOC UK. 1973; 93(0):455-72
- ↑ Jump up to: 10.0 10.1 Sahyoun JY, Sabeti S, Robert MC. Drug-induced corneal deposits: an up-to-date review. BMJ Open Ophthalmol 2022;7(1):e000943. DOI: 10.1136/bmjophth-2021-000943.
- ↑ Jump up to: 11.0 11.1 Sahyoun, J.Y., Sabeti, S. & Robert, M.C. Drug-induced corneal deposits: an up-to-date review. BMJ Open Ophthalmol 7, e000943 (2022).
- ↑ Jump up to: 12.0 12.1 Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med, 8 (9) (2006), pp. 539-548.
- ↑ Jump up to: 13.0 13.1 Sodi A, Ioannidis A, Pitz S. Ophthalmological manifestations of Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 26.Available from: https://www.ncbi.nlm.nih.gov/books/NBK11599/.
- ↑ Mäntyjärvi M, Tuppurainen K, Ikäheimo K. Ocular side effects of amiodarone. Surv Ophthalmol, 42 (4) (1998), pp. 360-366.
- ↑ Wasielica-Poslednik J, Pfeiffer N, Reinke J, Pitz S. Confocal laser-scanning microscopy allows differentiation between Fabry disease and amiodarone-induced keratopathy. Graefes Arch Clin Exp Ophthalmol, 249 (11)(2011), pp. 1689-1696.
- ↑ Frings A, Schargus M. Recovery From Amiodarone-Induced Cornea Verticillata by Application of Topical Heparin. Cornea. 2017 Nov;36(11):1419-1422.
- ↑ Chhablani J. Drug induced maculopathy. American Academy of Ophthalmology, EyeWiki. December 2018. https://eyewiki.aao.org/Drug_induced_maculopathy. Accessed June 19, 2019.
- ↑ Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology, 123 (6) (2016), pp. 1386-1394.
- ↑ Ding HJ, Denniston AK, Rao VK, Gordon C, Hydroxychloroquine-related retinal toxicity, Rheumatology, Volume 55, Issue 6, June 2016, Pages 957–967, https://doi.org/10.1093/rheumatology/kev357.