Fabry Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Fabry disease is an inability to break down globotriaosylceramide due to a deficiency of α-galactoside A. This results in the accumulation of globotriaosylceramide in lysosomes across the entire body. Fabry disease is a type of lysosomal storage disease tht has two recognized forms: classic and atypical. Classic Fabry disease is characterized by dysfunction of the skin, heart, kidney, brain, vascular, ocular, and nervous systems. Atypical Fabry disease usually involves cardiac dysfunction and rarely has any ocular manifestations.
Epidemiology
Fabry disease is an incredibly rare disease affecting approximately 1/40,000 to 1/60,000 individuals.[2] Fabry disease is an X-linked recessive disorder and thus most cases involve males. The disease is seen across all racial and ethnic groups approximately equally.
Disease
Ocular Manifestations
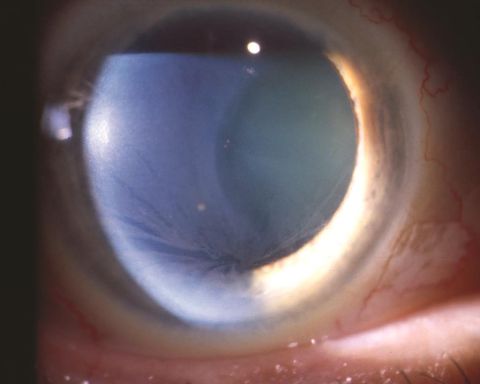
Corneal disease: The classic and most common presentation of corneal disease involves the presence of cornea verticillata. Verticillata can be observed stemming from the central cornea as opacified, snaking lines.[3] Verticillata present early and are seen in up to 70% of patients with Fabry disease. Their presence requires further evaluation if observed in an otherwise asymptomatic patient.
Corneal manifestations can include hyperreflective intracellular inclusions at the level of the basal epithelial cells or epithelial basement membrane. This can include the involvement of Bowman's layer, causing a distorted and uneven appearance. Some studies have suggested the manifestations may be different for females affected, in which the inclusions were finer and more diffuse.[4] The central and peripheral cornea appear to be affected similarly. The opacities appear in a vortex-like pattern, which results from the centripetal migration of deposit-laden limbal stem cells as the corneal epithelium undergoes natural growth and repair.
Conjunctival disease: Unlike corneal involvement, male and female patients do not differ in terms of conjunctival involvement. Intracellular inclusions that are round in shape have been observed. Additionally, vascular abnormalities include vessel tortuosity, microaneurysms, and general dilation. The vessel abnormalities have been postulated to be due to the abnormal storage of globotriaosylceramide in the vascular endothelium causing breakdown and weakening of the vessel walls.[3][5]
Retinal disease: The most common finding is retinal vessel tortuosity.[3] Other forms of retinal vessel disease have also been described, including disc edema. The cause of the tortuosity and microaneurysms has been suggested to be similar to the cause of conjunctival vessel disease. The lysosomal inclusions have been found in endothelial cells and pericytes of retinal vessels.[5]
Lens disease: Anterior capsular or posterior subcapsular cataracts are the most common manifestations.[3][5] Posterior subcapsular cataracts are so common that they are sometimes termed Fabry cataracts.
Other rare findings: retinal artery occlusions, optic disc edema, optic atrophy, lid edema, dry eye syndrome, and visual field defects.
Non-ocular Manifestations
Non-ocular manifestations of Fabry disease are neuropathic pain, dermatologic manifestations such as telangiectasias and angiokeratomas, renal failure, strokes, and deafness. Neuropathic pain is a common association, with typical onset in childhood. Patients frequently do not live beyond the fourth or fifth decade of life.
Etiology
The genes involved in Fabry disease are present on the X chromosome. A study done in Missouri found a mutation rate of one in 2,913 newborns. Not all of these newborns developed Fabry disease, suggesting that there are pathologic and non-pathologic variants.
Risk Factors
The only known risk factor for Fabry disease is a positive family history. There have not been environmental risk factors proposed.
Pathophysiology
Fabry disease is caused by a deficiency in α-galactoside A which cleaves the terminal galactose from globotriaosylceramide. The accumulation of globotriaosylceramide in lysosomes causes death of the cells and breakdown of the tissue.
Histology
Fabry disease is characterized by accumulation of globotriaosylceramide in tissue and this is evident on microscopy. It appears as an osmiophilic substance when dyed with toluidine blue and can also be stained using hematoxylin and eosin.[2] Stains that are more specific for sphingolipid include Luxol fast blue, Oil red O, and Sudan black. Finally, lectins can be used to stain for α- and β-galactosyl residues.[6]
Primary Prevention
Fabry disease is an inherited lack of enzyme production. As such, it cannot currently be prevented. Complications of the disease and progression can be mitigated using enzyme replacement therapy.
Diagnosis
History
Patients will typically present in the first or second decades of life with neuropathic pain. This pain can be in the extremities, abdomen, joints, or chest. The pain description is variable, but often said to be burning in quality. The pain can be chronic or occur in acute attacks. It is exacerbated by conditions causing stress to the body including temperature changes, exercise, alcohol, or fevers.[7] Other symptoms begin later and can include angiokeratomas, heat intolerance, tinnitus, GI symptoms, renal and cardiac dysfunction, TIA/stroke, shortness of breath, and fatigue.
Symptoms
Constitutional: fatigue, dyshidrosis/hypohydrosis
Eyes: cloudiness, decreased vision
HEENT: tinnitus, hearing loss
Cardiovascular: exercise intolerance, palpitations
Pulmonary: shortness of breath, cough
GI: nausea and vomiting after meals, lower abdominal pain, diarrhea or constipation
GU: hematuria, “frothy” urine (proteinuria)
Neurologic: neuropathic pain anywhere in the body, dizziness, TIA, inability to concentrate, headaches
Integumentary: small, raised, dark red spots (angiokeratomas) frequently found around the groin and mouth.[7]
Clinical Diagnosis
Diagnosis of Fabry disease often begins after symptoms have been present for long enough to cause the patient enough discomfort to be seen by a healthcare provider. Many patients are initially misdiagnosed, as the disease can present in a multitude of patterns. A personal history of the symptoms of Fabry disease combined with a family history of early cardiac or renal failure in the setting of similar symptoms is suggestive.
Diagnostic procedures
Biopsy is not required nor is it typically used for diagnosis. However, if the diagnosis is in question, it can be helpful. Specific renal manifestations of Fabry disease can be observed on electron microscopy with the appearance of enlarged secondary lysosomes with lamellated membrane structures. The lamellated appearance has been said to be more diagnostic, but can also be present in patients on amiodarone or chloroquine.[6] Other tissues biopsied will show electron-dense lysosomes in various tissues including blood vessels, eccrine glands, and fibroblasts.[8]
Laboratory test
The physician may order an enzyme assay to assess the amount of α-galactoside A activity. A low level of activity (<1%) is often found in males and homozygous females. This test has been adopted by some states into routine newborn screenings. A positive enzyme assay test is considered diagnostic.
Differential diagnosis
Fabry disease has a wide range of potential presentation and so has a large differential. It is often initially misdiagnosed until other signs become more readily apparent. Some of the diseases mistaken for Fabry disease include rheumatic fever, juvenile arthritis, degenerative neurologic diseases, hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu), Schindler disease, sialidosis, Fox-Fordyce disease, fucosidosis, Meniere disease, MS, IBS, or any disease presenting with renal failure.
Management
Systemic Disease - General treatment
The mainstay of treatment is symptomatic relief. This involves various strategies, including management of respiratory or GI symptoms, management of pain, and protection from advancement of untreated disease.
Systemic Disease- Medical therapy
Enzyme replacement therapy: ERT is considered first line treatment for Fabry disease. Two forms of the enzyme are currently produced (Agalsidase alfa and Migalastat hydrochloride). ERT has been shown to be safe and effective in reducing levels of pain, increasing sweat production, and reducing GI symptoms.[9] [10] ERT is costly to maintain, requiring up to $350,000 dollars per year per patient as recently as 2012.[11] The effect of ERT on ocular findings is less clear, although corneal deposits have been shown to regress in some cases. There is significant difficulty in quantifying the degree of remission as there is currently no standardized way to measure the corneal opacities.[12]
Pain management: Pain should be managed as appropriate for each individual patient. Care should be taken to avoid opiates given the high likelihood of lifetime use and addiction potential. Multimodal pain relief should be pursued whenever possible.
Renal protection: Most patients with renal manifestations are placed on ACE inhibitors to reduce kidney damage as other patients with renal disease are. There is conflicting evidence for the prophylactic use of ACE inhibitors in otherwise non-hypertensive Fabry patients.
Kidney Transplant: For those patients who develop ESRD, kidney transplantation is an option. Roughly 50% of patients with Fabry disease will require kidney transplant by fifty years of age. Graft survival has not been found to differ between Fabry patients and other causes of ESRD, however mortality post-transplant was shown to be higher in Fabry patients than those with other causes of ESRD.[13]
Medical follow up
Frequency of follow up will be decided based upon individual physician preference and severity of disease. Follow up should be multidimensional and involve a team of physicians including neurology, endocrinology, ophthalmology, cardiology, gastroenterology, nephrology, and any other physicians assisting in the management of other disease manifestations.
Surgery
Surgical management of Fabry patients is limited to controlling symptoms after damage has occurred. The most common surgical procedures patients will have include cardiac procedures (CABG, septal myectomy) and renal transplants.
Prognosis
Ocular prognosis: Patients placed on ERT may have a slowed progression of ocular symptoms or even regression. Further studies will need to be done to evaluate the effectiveness of ERT in reversing or halting progression.
General: By the fourth or fifth decade of life, most patients will have renal, cardiac, and neurologic manifestations of disease. The major causes of death in Fabry patients are cardiac and renal complications. The development of ERT has the potential to reduce morbidity and mortality, but long-term data is still being gathered.[14]
Additional Resources
https://rarediseases.info.nih.gov/diseases/6400/fabry-disease
https://www.fabrydisease.org/index.php
References
- ↑ American Academy of Ophthalmology. Fabry disease. https://www.aao.org/image/fabry-disease-6 Accessed December 4, 2019.
- ↑ Jump up to: 2.0 2.1 Yuasa T, Takenaka T, Higuchi K, et al. Fabry Disease. J Echocardiogr 2017;15(4):151.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Nguyen TT, et al. Ophthalmological Manifestations Of Fabry Disease: A Survey Of Patients At The Royal Melbourne Fabry Disease Treatment Centre. Clin Exp Ophthalmol. 2005;33(2):64-168.
- ↑ Mastropasqua L,et al. Corneal And Conjunctival Manifestations In Fabry Disease: In Vivo Confocal Microscopy Study. Am J Ophthalmol 2006;141(4)709-709.e11.
- ↑ Jump up to: 5.0 5.1 5.2 Sodi A, Ioannidis A, Pitz S. Ophthalmological manifestations of Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 26.
- ↑ Jump up to: 6.0 6.1 5 Alroy J,et al. Renal Pathology In Fabry Disease. J Am Soc Nephrol. 2002;13(2(suppl)):S134-S138.
- ↑ Jump up to: 7.0 7.1 Mehta A, Widmer U. Natural history of Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 19.
- ↑ Lidove O, Jaussaud R, Aractingi S. Dermatological and soft-tissue manifestations of Fabry disease: characteristics and response to enzyme replacement therapy. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 24.
- ↑ Schiffmann R, Kopp JB, Austin III HA, et al. Enzyme Replacement Therapy in Fabry Disease: A Randomized Controlled Trial. JAMA. 2001;285(21):2743–2749.
- ↑ Breunig F. Enzyme Replacement Therapy for Fabry Disease: Proving the Clinical Benefit. Nephrol Dial Transplant. 2003;18(1):7–9.
- ↑ Alegra T, Vairo F, de Souza MV, Krug BC, Schwartz IV. Enzyme replacement therapy for Fabry disease: A systematic review and meta-analysis. Genet Mol Biol. 2012;35(4 (suppl)):947–954.
- ↑ Fledelius HC, et al. Ophthalmic Experience over 10 Years in an Observational Nationwide Danish Cohort of Fabry Patients with Access to Enzyme Replacement. Acta Ophthalmol. 2014;93(3):258–264.
- ↑ Shah T, et al. Kidney Transplant Outcomes in Patients With Fabry Disease. Transplantation. 2009;87(2):280–285.
- ↑ Jamboti J, Forrest CH. “Fabry Disease; Early Diagnosis Improves Prognosis but Diagnosis Is Often Delayed.” J Nephropathol. 2017;6(3):30–133.