Cystinosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Cystinosis is a rare lysosomal storage disease in which cystine accumulates in organs and tissues throughout the body. Although renal disease predominates in the early forms of cystinosis, all forms of the disease can result in ophthalmic sequelae, particularly pigmented retinopathy and deposition of cystine crystals in the cornea, with associated photophobia and visual impairment. Patients should be followed closely by nephrology and other specialists in order to manage life threatening renal disease and extra-renal symptoms, but also need regular ophthalmologic evaluation to determine the extent of ocular disease and manage pathology appropriately. The early and late forms of ocular disease can both be treated with cysteamine eye drops to halt progression of ocular manifestations and diminish the presence of cystine crystals in the cornea. Oral cysteamine therapy may decrease the occurrence of retinopathy.
Disease Entity
Epidemiology
There are three main forms of the disease which are categorized as infantile, juvenile, and adult cystinosis. The most commonly diagnosed form of cystinosis is the infantile, or nephropathic form, which has been shown to have an incidence of 1 in every 100,000 to 200,000 children.[1] The adult, non-nephropathic, ocular form of cystinosis is less frequently encountered, but is thought to be more prevalent than documented as it is usually discovered incidentally during ophthalmologic examination, and thus, may otherwise remain undiagnosed.
Pathophysiology
In healthy individuals, the amino acid cystine is the byproduct of protein metabolism within lysosomes. It is typically transported out of the lysosome by a membrane transport protein known as cystinosin. A genetic defect in cystinosin results in the accumulation of lysosomal cystine, and subsequent cystine deposition within tissues. The build up of cystine within cells leads to crystal formation, and resultant organ damage, which occurs most prominently in the kidneys, leading to early renal failure. However, as noted above, crystals can form in other organs including the liver, thyroid, pancreas, muscle, brain, and eyes[2].
Genetics
Cystinosis is inherited in an autosomal recessive fashion. A mutation in the CTNS gene on chromosome 17p13 results in dysfunction of the lysosomal membrane transport protein cystinosin.[3] Over 100 different mutations are known to cause cystinosis, all with variable clinical presentations.[4][5]
Diagnosis
Ophthalmologists can play an important role in the diagnosis of cystinosis, as one of the three main diagnostic criteria is the finding of corneal crystals on slit lamp exam. Cystinosis may also be diagnosed by genetic testing for the CTNS mutation, or by elevated leukocyte cystine content.[2]
Ophthalmologic Features
The most common symptom of cystine accumulation in the structures of the eye is photophobia, although other symptoms such as foreign body sensation, visual impairment, and blepharospasm can be present and confound the picture, as these can be symptoms of congenital glaucoma.[6] Structure-specific involvement is described below:
- Cornea: All forms of cystinosis show early deposition of cystine crystals in the cornea, often as early as 16 months.[7] These crystals were first described in 1941 as small, white, sequin-like crystals in the cornea (Figure 1). Other descriptions label them as needle-shaped and reflective in nature. Cystine crystals are found primarily in the corneal stroma, but can be present in all layers of the cornea. Crystal deposition begins in the anterior periphery of the cornea and extends inward and posteriorly as the disease progresses.[8] As crystal deposition worsens, visualization with the naked eye can show diffuse haziness of the cornea. Research suggests that the extent of corneal involvement may serve to reflect the course and severity of systemic disease.[7]
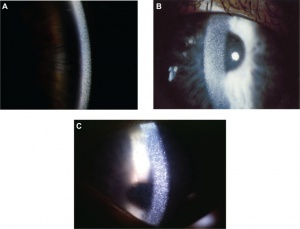
- Retina: Pigmented retinopathy has been shown to be the earliest finding of ocular involvement in cystinosis, appearing as early as 5 months of age in the infantile form of the disease. This most frequently presents as bilateral, peripheral hypopigmentation and mottling of the retinal pigment epithelium. Cystine crystals may also accumulate in the retina. Advanced retinal disease in older, untreated patients is thought to contribute to decreased visual acuity and visual field deficits.[9][10]
- Others: Careful ophthalmologic examination can also reveal cystine crystals within the conjunctiva, iris, anterior lens, choroid, and optic nerve. However, no specific clinical symptoms have been correlated with these findings. Conjunctival crystals may give the conjunctiva a “ground glass” appearance on microscopic examination.[8]
Non-ophthalmologic Features
- Infantile, nephropathic cystinosis: This form of cystinosis often presents in the first year of life as renal Fanconi syndrome, a defect in proximal tubular function that progresses to end stage renal disease by the age of 10 in the absence of treatment. This is frequently accompanied by growth retardation, rickets, hypothyroidism, and failure to thrive. Studies have also shown specific cognitive deficits and difficulties in visuo-spatial processing and behavioral functioning.[11][12][13][14]
- Late-onset, juvenile cystinosis: Presentation is similar to infantile cystinosis, but occurs later in childhood and may progress more slowly, with renal failure occurring in the late teens or early adult years.
- Adult, ocular cystinosis: The adult form is often benign and incidentally discovered during slit lamp examination. It may involve cystine crystal formation within the bone marrow, in addition to the eye, but is typically asymptomatic.
Management
General treatment
Individuals with infantile cystinosis previously did not survive beyond the first or second decade of life, but with advancements in renal dialysis, kidney transplantation, and pharmacologic treatment, individuals are now surviving well into adulthood.[15] The most effective medical treatment for cystinosis is the use of cysteamine, a cystine-depleting drug that acts within the lysosomes of cells and converts accumulated cystine into a form that can be easily excreted from the lysosome in the absence of functional cystinosin. Some hospitals have started multi-disciplinary cystinosis clinics in an effort to approach the disease in a more collaborative way.[16]
Oral therapy
Treatment with oral cysteamine is started as soon as possible after the diagnosis of infantile or juvenile cystinosis. Early intervention is shown to improve renal function, decrease growth restriction, and prevent hypothyroidism.[17] Oral cysteamine also appears to decrease retinopathy, evidenced by data showing that the fraction of patients with retinopathy decreased proportionately to the length of time spent receiving cysteamine therapy.[10]
Topical therapy
While oral therapy proves helpful in diminishing retinopathy, the lack of vasculature in the cornea makes systemic therapy ineffective in this location. As a result, a topical formulation of cysteamine was developed to target corneal cystine crystal deposition. Cysteamine eye drops have since been proven to decrease corneal crystal deposition if used every 1-2 hours throughout the day.[18][19][20][21] Topical cysteamine drops also have the disadvantage of needing to be kept cool and used within a week of opening. These issues make treatment adherence very difficult, especially in the youngest patients, and may also negatively impact patient quality of life. Recent advances in pharmacology have resulted in more stable cysteamine preparations and also a new gel form of topical treatment that requires decreased dosing with similar or possibly improved efficacy when compared to currently available treatments.[22][23][24]
Summary
Cystinosis is an autosomal recessive lysosomal storage disorder that results in the accumulation of the amino acid cystine crystals in many organs throughout the body. Renal damage is prominent in the early forms of cystinosis, but ocular involvement is present in all forms of the disease. Ophthalmic manifestations of all 3 types of cystinosis include crystal deposition in the cornea, conjunctiva, iris, lens, choroid, and retina with early development of pigmented retinopathy and the extent of corneal crystals related to age of onset and the use of disease modifying medications. Topical cysteamine is the treatment of choice for ophthalmologic management, and can effectively reduce corneal cystine crystal accumulation and alleviate symptoms such as photophobia. Ophthalmologic surveillance and management of cystinosis is important in order to monitor disease progression and prevent late visual complications of untreated cystine accumulation.
Additional Resources
- Cystinosis Foundation: http://www.cystinosisfoundation.org/
The Cystinosis Foundation is a non-profit organization with more than 30 years of International experience in supporting and educating families and the medical community through the dissemination of educational literature, funding research, and annual conferences.
- Cystinosis Research Network: https://cystinosis.org/
The Cystinosis Research Network is an all-volunteer, non-profit organization dedicated to supporting and advocating research, providing family assistance and educating the public and medical communities about cystinosis.
- Cystinosis Research Foundation: http://www.cystinosisresearch.org/
The mission of the Cystinosis Research Foundation is to support bench and clinical research that is focused on developing improved treatments and a cure for cystinosis.
References
- ↑ Adamson MD, Andersson HC, Gahl WA. Cystinosis. Seminars in nephrology 1989; 9:147-61.
- ↑ Jump up to: 2.0 2.1 Elmonem MA, Veys KR, Soliman NA, van Dyck M, van den Heuvel LP, Levtchenko E. Cystinosis: a review. Orphanet Journal of Rare Diseases 2016; 11:47.
- ↑ Town M, Jean G, Cherqui S, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 1998; 18:319.
- ↑ Anikster Y, Shotelersuk V, Gahl WA. CTNS mutations in patients with cystinosis. Hum Mutat 1999; 14:454.
- ↑ Attard M, Jean G, Forestier L, et al. Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet 1999; 8:2507.
- ↑ Kaiser-Kupfer MI, Caruso RC, Minkler DS, Gahl WA. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol 1986; 104:706.
- ↑ Jump up to: 7.0 7.1 Gahl WA, Kuehl EM, Iwata F, et al. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab 2000; 71:100.
- ↑ Jump up to: 8.0 8.1 Tsilou E, Zhou M, Gahl WA, Sieving PC, Chan C. Ophthalmologic manifestations and histopathology of infantile nephropathic cystinosis: report of a case and review of the literature. Survey of Ophthalmology 2007; 52:1.
- ↑ Jump up to: 9.0 9.1 Elmonem MA, Veys KR, Soliman NA, van Dyck M, van den Heuvel LP, Levtchenko E. Cystinosis: a review. Orphanet Journal of Rare Diseases 2016; 11:47.
- ↑ Jump up to: 10.0 10.1 Tsilou ET, Rubin BI, Reed G, Caruso RC, Iwata F, Balog J, Gahl WA, Kaiser-Kupfer MI. Nephropathic cystinosis: posterior segment manifestations and effects of cysteamine therapy. Ophthalmology. 2006; 113(6):1002-9.
- ↑ Ballantyne AO, Trauner DA. Neurobehavioral consequences of a genetic metabolic disorder: visual processing deficits in infantile nephropathic cystinosis. Neuropsychiatry, neuropsychology, and behavioral neurology 2000; 13:254-63.
- ↑ Trauner DA, Spilkin AM, Williams J, Babchuck L. Specific cognitive deficits in young children with cystinosis: evidence for an early effect of the cystinosin gene on neural function. The Journal of pediatrics 2007; 151:192-6.
- ↑ Ballantyne AO, Scarvie KM, Trauner DA. Academic achievement in individuals with infantile nephropathic cystinosis. American journal of medical genetics 1997; 74:157-61.
- ↑ Delgado G, Schatz A, Nichols S, Appelbaum M, Trauner D. Behavioral profiles of children with infantile nephropathic cystinosis. Developmental medicine and child neurology 2005; 47:403-7.
- ↑ Gahl WA, Thoene JG, Schneider JA. Cystinosis: A disorder of lysosomal membrane transport. The Metabolic and Molecular Bases of Inherited Diseases. 8th ed. New York: McGraw-Hill; 2001. p. 5085-108.
- ↑ Multi-specialty UCSD Cystinosis Clinic. University of California San Diego - Rady Children’s Hospital. https://cystinosis.org/21-family-support/resources/209-multi-specialty-ucsd-cystinosis-clinic
- ↑ Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med 1993; 328:1157–1162.
- ↑ Kaiser-Kupfer MI, Fujikawa L, Kuwabara T, Jain S, Gahl WA. Removal of corneal crystals by topical cysteamine in nephropathic cystinosis. N Engl J Med 1987; 316:775–779.
- ↑ Kaiser-Kupfer MI, Gazzo MA, Datiles MB, Caruso RC, Kuehl EM, Gahl WA. A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis. Arch Ophthalmol 1990; 108:689–693.
- ↑ Jones NP, Postlethwaite RJ, Noble JL. Clearance of corneal crystals in nephropathic cystinosis by topical cysteamine 0.5%. Br J Ophthlamol 1991; 75:311–312.
- ↑ Bradbury JA, Danjoux J-P, Voller J, Spencer M, Brocklebank T. A randomized placebo-controlled trial of topical cysteamine therapy in patients with nephropathic cystinosis. Eye 1991; 5:755–760.
- ↑ Shams F, Livingstone I, Oladiwura D, Ramaesh K. Treatment of corneal crystal accumulation in patients with cystinosis. Clinical Ophthalmology 2014; 8:2077-84.
- ↑ Tsilou ET, Thompson D, Lindblad AS, et al. A multicentre randomised double masked clinical trial of a new formulation of topical cysteamine for the treatment of corneal cystine crystals in cystinosis. Br J Ophthalmol 2003; 87(1):28–31.
- ↑ Labbé A, Baudouin C, Deschênes G, et al. A new gel formulation of topical cysteamine for the treatment of corneal cystine crystals in cystinosis: the Cystadrops OCT-1 study. Mol Genet Metab 2014; 111:314.

