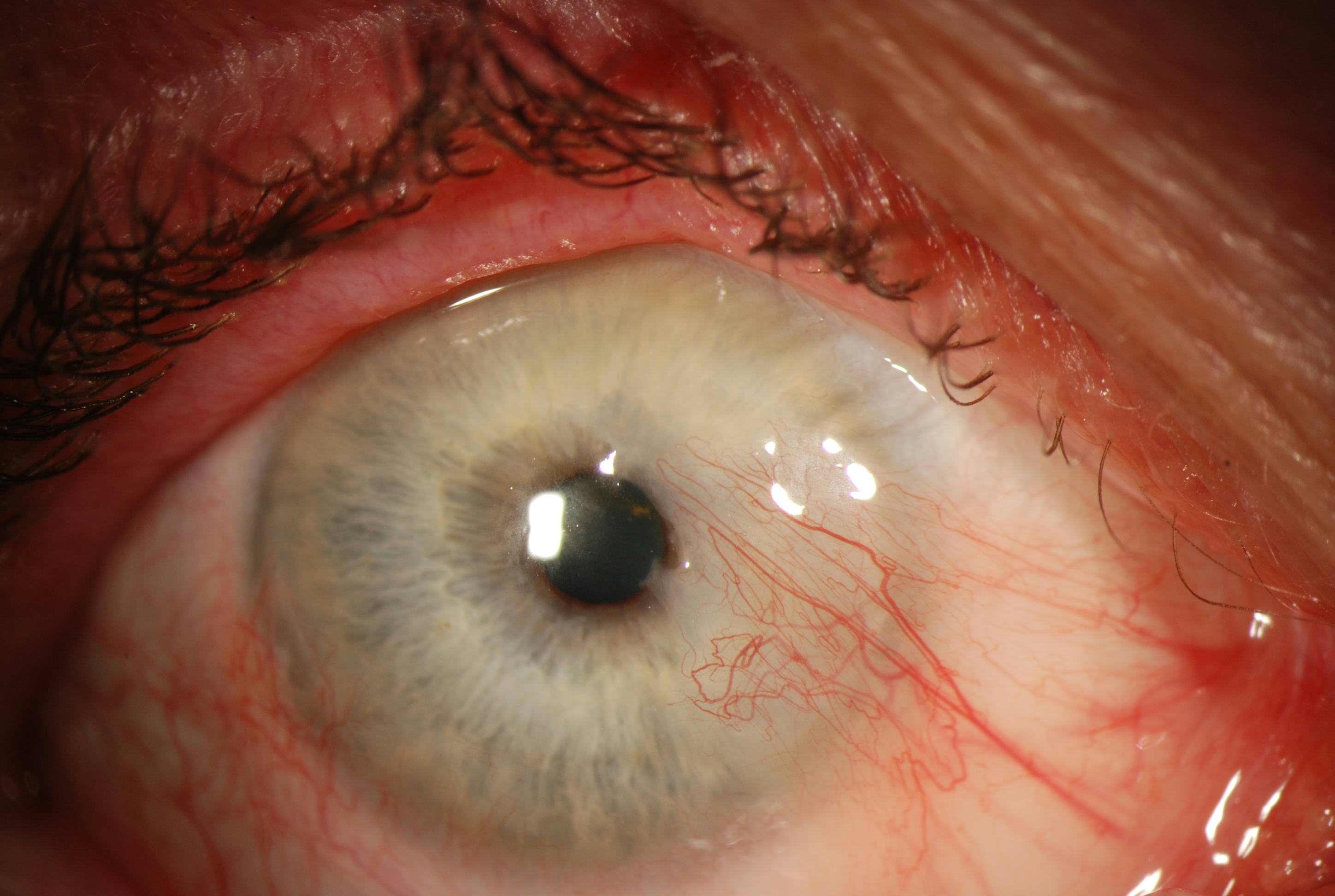
Pterygium
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Pterygium, from the Greek pterygos meaning “wing”, is a common ocular surface lesion originating in the limbal conjunctiva within the palpebral fissure with progressive involvement of the cornea. The lesion occurs more frequently at the nasal limbus than the temporal with a characteristic wing-like appearance.
Etiology
The pathogenesis of pterygia is highly correlated with UV exposure. An increased incidence is noted in latitudes nearer the equator and in individuals with a history of increased UV exposure (outdoor work). Some studies have shown a slightly higher incidence in males than females, which may only reflect a higher rate of UV radiation.
Risk Factors
UV radiation, proximity to the equator, dry climates, outdoor lifestyle[1].
General Pathology
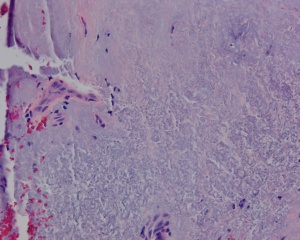
Histologically, pterygia are an accumulation of degenerated subepithelial tissue which is basophilic with a characteristic slate gray appearance on H&E staining. Vermiform or elastotic degeneration refers to the wavy worm-like appearance of the fibers. Destruction of Bowman layer by fibrovascular ingrowth is typical. The overlying epithelium is usually normal, but may be acanthotic, hyperkeratotic, or even dysplastic and often exhibits areas of goblet cell hyperplasia.
The American Academy of Ophthalmology's Pathology Atlas contains two virtual microscopy images of tissue samples with Pterygium[2]:
Pathophysiology
The large number of theories that exist to explain the pathogenesis of pterygium growth underscores the uncertainty of the etiology. The increased prevalence in hot dry climates and regions nearer to the equator suggest a role of environmental factors such as UV radiation and dryness. Actinic changes seen on histopathology similar to actinic keratoses on the skin also supports the role of UV radiation. It has been suggested that radiation activated fibroblasts may result in excessive production of material resulting in pterygia. Other proposed theories include choline deficiency, inflammation, disregulation of angiogenesis, immune system abnormalities, tear film abnormalities, as well as the possible role of a viral stimulus.
Coronea MT proposed that pterygium occur due to albedo concentration in the anterior eye (albedo's hypothesis). Light entering the temporal limbus at 90 degree is concentrated at medial limbus and this is responsible for predominance of medial pterygia.[3]
Primary Prevention
As UV radiation is believed to play an important role in the pathophysiology, avoidance of UV exposure is important to primary prevention. Ocular surface lubrication may also help.
Diagnosis
The diagnosis is made by slit-lamp examination of the wing-shaped limbal growth at the characteristic location within the palpebral fissure. The diagnosis is most often clear clinically, but histopathologic confirmation is performed routinely, as there can be associated dysplasia of the overlying tissue.
High resolution anterior segment optical coherence tomography can be used to differentiate pterygia from ocular surface squamous neoplasia (OSSN). In pterygia, the epithelium is of normal thickness with underlying sub epithelial fibrosis. In comparison in OSSN, the epithelium will appear thickened and hyper-reflective with an abrupt transition from normal epithelium[4].
Physical examination
A complete eye exam should be performed on all patients with apparent pterygia focusing on assessment of visual and refractive impact as well as the exclusion of less common alternate diagnoses.
- Visual acuity with current correction and manifest refraction
- External examination (lids, lashes, lacrimal apparatus)
- Examination of bulbar and palpebral conjunctiva as well as fornices with eyelid eversion
- Slit lamp biomicroscopy of the ocular surface and anterior segment
- Keratometry
- Corneal Topography
- Motility Exam
- The remainder of a comprehensive eye exam to include pupil exam, visual fields, intraocular pressure, and dilated funduscopic exam
Signs
The diagnosis of pterygium is based on the clinical appearance of the lesion. Typical findings include
- Fibrovascular conjunctival growth within the palpebral fissure extending onto the corneal surface
- triangular or trapezoidal shape with the apex, or head, extending onto the cornea
- vascular straightening in the direction of the advancing head of the pterygium on the corneal surface.
- It may affect the nasal and temporal limbus of both eyes or only a single location.
- Raised lesion, white to pink in color depending on vascularity
- Ranges from a fine transparent area with very mild elevation, few vessels, and minimal corneal involvement in the early stages to a thick opaque vascular growth extending to the visual axis in later stages
- Pinguecula are often present in the ipsilateral or contralateral eye
- A pigmented epithelial iron line (Stocker’s line) adjacent to a pterygium is evidence of chronicity.
It is unusual for pterygia to deviate from the characteristic locations of three and nine o’clock within the palpebral fissure. Pterygioid lesions in other locations should elevate suspicion for alternate diagnoses.
Symptoms
Though frequently asymptomatic, pterygia can become inflamed and cause ocular surface irritation. Many patients will disclose their dislike of the appearance of the pterygium when questioned directly. As the lesion progresses, vision may be affected by induction of astigmatism or obscuration of the visual axis.
Clinical diagnosis
Diagnosis is based on the typical clinical appearance. Presence of leukoplakia, pigmentation, irregular feeder vessels, atypical elevation, and rapid growth should increase suspicion for malignancy.
Diagnostic procedures
Assessment of visual acuity, changes in manifest refraction, and corneal topography can aid in determining the visual impact of pterygia. Histopathologic confirmation is routine for excised lesions and can be important in ruling out malignancy. The prevalence of concurrent ocular surface squamous neoplasia within surgically excised pterygia varies from 1.7% in South Florida to as high as 9.8% in Australia[5][6]. A higher index of suspicion for concurrent malignancy is required for patients living in areas with higher UV index.
Differential diagnosis
- Conjunctival Intraepithelial Neoplasia (CIN)
- Fuchs' Superficial Marginal Keratitis
- Limbal dermoid
- Limbal stem cell deficiency
- Neurotrophic keratitis
- Pannus
- Stevens Johnson Syndrome
- Symblepharon secondary to chemical, thermal, or mechanical injury
- Terrien’s marginal degeneration
Management
General treatment
A number of potential therapeutic options exist for the management of pterygia ranging from conservative management with lubrication to surgical excision with conjunctival autografts. Due to the potential for recurrence of a more aggressive lesion, as well as other surgical risks, the surgical removal of pterygia should not be undertaken casually. Surgical excision is indicated if it is causing persistent irritation resistant to medical therapy, obscuring visual axis or causing blurry vision from induced astigmatism, increasing in size, or restricting ocular motility.
Medical therapy
Inflamed pterygia may cause irritation, foreign body sensation, and tearing which, in many cases, can be alleviated with over the counter vasoconstrictor drops, lubricating drops and ointments. Short courses of topical corticosteroids can be used to reduce inflammation, though long term use is not recommended.
Medical follow up
Initially, the corneal extension of the pterygium should be measured and followed every 1 to 2 years to determine the rate of growth toward the visual axis.
Surgery
Excision with conjunctival autograft is considered the current gold standard because of its low rate of recurrence. This approach carries an approximate rate of recurrence of 5-10% with minimal risk of complications. Results reported with a modification the standard conjunctival autograft technique, involving extensive Tenon's resection and suturing of the conjunctival autograft (referred to as P.E.R.F.E.C.T. for pterygium) indicate a further decreased recurrence rate (as low as 1 in 1000 in one series)[7][8][9].
Excision with amniotic membrane graft can be considered as an alternative to conjunctival autograft, though recurrence rates are still higher than rates with conjunctival autograft. The amniotic membrane can be placed with stromal side facing the scleral bed and secured with fibrin glue and/or sutures. This technique may be considered if conjunctiva needs to be spared for future surgery (ex: glaucoma surgery).
Simple excision with bare sclera or conjunctival closure will result in a recurrence rate as high as 80% and is now considered unacceptable.
Excision together with adjunctive therapies such as mitomycin C or 5-fluorouracil can reduce the risk of recurrence to approximately 10%[10]. However, the use of mitomycin C and 5-fluorouracil can increase the risk of corneal or scleral melt postoperatively[11].
Surgical follow up
Topical steroids are often utilized in the initial postoperative period. Patients should be followed to monitor for recurrence. 97% of all recurrences occur in the first year after surgery.
Complications
- Recurrence
- Corneal scarring
- Corneal perforation
- Strabismus
- Non-healing epithelial defect (esp with mitomycin C)
- Scleral melt (esp with mitomycin C)
Prognosis
Recurrence rate can be as low as 1 in 1000 depending on surgical approach. Defects in Bowman layer often result from surgical excision due to the involvement of Bowman layer by the lesion, and mild residual astigmatism may be present despite successful surgery. Visual prognosis is typically very good and only limited by the cornea if the excised lesion involved the visual axis.
Additional Resources
- Boyd K, Lipsky SN. Pinguecula and Pterygium. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/pinguecula-pterygium-list. Accessed March 21, 2019.
References
- ↑ Nemesure B, Wu S, Henis A, Leske MC, Barbados Eye Studies Group. Nine-year incidence and risk factors for pterygium in the Barbados Eye Studies. Ophthalmology 2010;115:2153-2158.
- ↑ Ocular Pathology Atlas. American Academy of Ophthalmology Web site. https://www.aao.org/resident-course/pathology-atlas. Published 2016. Accessed January 4, 2017.
- ↑ Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77(11):734-739. doi:10.1136/bjo.77.11.734
- ↑ Venkateswaran N, Galor A, Wang J, Karp CL. Optical coherence tomography for ocular surface and corneal diseases: a review. Eye Vis (Lond). 2018;5:13. Published 2018 Jun 12. doi:10.1186/s40662-018-0107-0
- ↑ Oellers P, Karp CL, Sheth A, Kao AA, Abdelaziz A, Matthews JL, Dubovy SR, Galor A. Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium. Ophthalmology. 2013 Mar;120(3):445-450. doi: 10.1016/j.ophtha.2012.08.010. Epub 2012 Oct 27. PMID: 23107578; PMCID: PMC3562397.
- ↑ Hirst LW, Axelsen RA, Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009 Jan;127(1):31-2.
- ↑ Hirst LW. Recurrent pterygium surgery using pterygium extended removal followed by extended conjunctival transplant: recurrence rate and cosmesis. Ophthalmology. 2009 Jul;116(7):1278-86.
- ↑ Hirst LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008 Oct;115(10):1663-72. Epub 2008 Jun 16.
- ↑ Hirst LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008 Oct;115(10):1663-72. Epub 2008 Jun 16.
- ↑ Hirst LW. Mitomycin C in the treatment of pterygium. Clin Experiment Ophthalmol. 2006 Apr;34(3):197-8
- ↑ Yanoff M, Duker JS. Ophthalmology. Fifth edition. Elsevier Saunders; 2018.