Nystagmus
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Nystagmus is defined by rhythmic, abnormal eye movements with a "slow" eye movement driving the eye off the target, followed by a second movement that brings the eye back to the target. The movement can be horizontal, vertical, or torsional, or a combination of these movements. Nystagmus can be jerk (named for fast phase) or pendular, variable amplitude and frequency, and can be worsened or improved by gaze position, fixation, or covering 1 eye (latent). This is in contrast to "saccadic intrusions" or "saccadic oscillations," which are defined as "fast, back-to-back (without intersaccadic interval)" eye movements driving the eye off the visual target. The neurological origin of the saccadic intrusions/oscillations is different from that of nystagmus and is covered in a different section. In this article, different types of nystagmus, their etiologies, and their treatment modalities are discussed.
Epidemiology
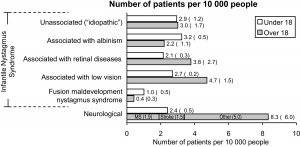
According to Sarvanathan et al[1] who published the only epidemiological study of nystagmus in the general population (see Figure 1), the prevalence of pathologic nystagmus is estimated to be 24 per 10,000, with a slight predilection toward European ancestry. The prevalence of infantile/pediatric nystagmus is said to be 17 per 10,000 by the same author. It has been reported to be as low as 6.7 per 100,000.[2] The frequency of acquired nystagmus is estimated at 17% in children in contrast to 40% in adults. All forms of presumed acquired nystagmus need further diagnostic workup to determine the etiology. Nystagmus is considered to be acquired in patients presenting at or after the age of 6 months, especially with asymmetric nystagmus (one eye with greater amplitude and/or frequency than the fellow eye), preservation of optokinetic nystagmus (in infantile idiopathic nystagmus, characteristic reversal of normal optokinetic nystagmus can be demonstrated), presence of a relative afferent pupillary defect, papilledema, or neurologic signs or symptoms.[3]
Clinical Types and Terminology
Nystagmus comes from the Greek word "nystagmos," meaning "drowsiness," and "nustazein," meaning "to nod off or be sleepy." It can be described as periodic, involuntary movements of one or both eyes in a fast or slow oscillatory motion. By definition, nystagmus starts by a slow movement of the eye away from the visual target. The second movement brings the eye back to the visual target. If the second movement is slow, the nystagmus is said to be pendular. If this second movement is quick, the nystagmus is called jerk nystagmus. By convention, the direction of jerk nystagmus (eg, right-beating nystagmus) is named after the fast phase of nystagmus. In a right-beating nystagmus, the fast phase is to the patient's right.
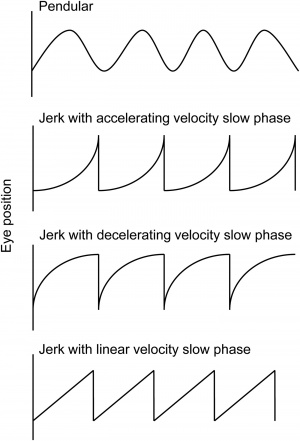
Nystagmus waveforms are named for their slow phase velocity profile (See Figure 2). The pendular form has no fast phase and is best depicted by the first wave of Figure 2. The exponential increasing velocity type is associated with congenital nystagmus. The exponential decreasing velocity waveform is commonly seen in gaze-evoked nystagmus, which can be a physiologic finding. The linear waveform is typical of vestibular nystagmus. Dissociated nystagmus refers to the 2 eyes having nystagmus with the same direction but with differing amplitudes. Disconjugate nystagmus occurs when the 2 eyes have different directions of oscillation, one example of which is seesaw nystagmus. An interesting type of jerk nystagmus is the Periodic Alternating Nystagmus (PAN), which is characterized by a cycle of unidirectional jerk nystagmus followed by a dampening or cessation of the abnormal eye movement, then jerk nystagmus occurring in the opposite direction. In order to observe PAN, the examiner should consider observing the patient for at least several minutes.
Classification
Physiologic
Physiologic nystagmus (nystagmus that is characteristic of normal oculomotor function) includes optokinetic nystagmus, vestibular ocular reflex, caloric nystagmus, and post-rotatory nystagmus. As they do not represent pathologic states, they will only be briefly discussed here.
Vestibulo-ocular reflex
Vestibulo-ocular reflex (VOR) is the reflexive movement of the eye that keeps the visual image stable on the retina during brief, high-frequency rotation of the head. VORs are controlled by the vestibular system of the inner ears, namely the semicircular canals, utricle, and saccule.
Optokinetic Nystagmus
Optokinetic nystagmus (OKN) is a physiologic movement of the eyes in response to large, moving visual fields (eg, when one is looking out the window of a moving train).[4] The initial movement is a smooth pursuit movement followed by contraversive saccade back to primary gaze or direction of visual interest. The OKN system cannot be isolated from VOR for clinical demonstration, but the use of an optokinetic drum gives an approximation of OKN in action. Asymmetry of the OKN response to the rotating drum may suggest lesions of the cerebrum, typically a large lesion of the parietal or parieto-occipital cortex and associated with homonymous hemianopia. This is in contrast to the lesions of the occipital lobe, which also produce homonymous hemianopia but without the OKN asymmetry.[5]
Caloric Nystagmus
Caloric nystagmus is a type of VOR (vestibulo-ocular reflex) that is elicited by stimulating the horizontal semicircle with either warm or cold water in the ear canal to create a convection current in the endolymph of the semicircle. In normal subjects, when cold water is placed in one ear, the eyes will slowly turn toward the ear with the horizontal fast phase away from the ear. The absence of caloric nystagmus may indicate brain death.[6]
Post-rotatory
Post-rotatory nystagmus is a reflexive, transient, conjugate, jerk nystagmus that occurs after the whole body of a subject is passively rotated about the z-axis then decelerated to rest. The nystagmus that occurs in this situation has the fast phase in the opposite direction of the previous rotation and is accompanied by a somatogyral illusion (sensation of rotating in the opposite direction of the original rotation). This nystagmus is due to the movement of the cupulas in response to rotation and deceleration from rotation.[7]
Early Onset (Childhood) Nystagmus
Infantile idiopathic nystagmus
Infantile idiopathic nystagmus (IIN), also known as congenital (motor) nystagmus, is the most common type of nystagmus seen in young patients followed by congenital sensory nystagmus.[8] Congenital (motor) nystagmus (eg, IIN) is by definition idiopathic (eg, without a known cause or associated afferent pathway disease) and is therefore a diagnosis of exclusion. It is present from infancy but usually recognized a few months into life[9] and may even be evident only after the child has reached several years of age. IIN is almost always bilateral, conjugate, and occurs in the horizontal plane, even in upgaze and downgaze, with little variability, which is in contrast to the highly variable presentation of spasmus nutans. Characteristics of congenital nystagmus can be concisely presented by the mnemonic “CONGENITAL” (table shown below):
Both jerk and pendular types are seen in IIN, although pendular nystagmus can change to jerk waveform in right and left gaze. Note that congenital pendular nystagmus, a rare entity, is almost always horizontal, which is in contrast to acquired pendular nystagmus that can take horizontal, vertical, and torsional planes with resultant elliptical waveforms.[10] Importantly, patients usually do not have oscillopsia[11][12] despite the presence of retinal slip exceeding 100 degrees/sec, which may be due to down-regulation of cortical activity in the area of MT/V5 bilaterally--an important part of the visual cortex for motion processing.[13] The rarity of oscillopsia in IIN is a testament to the "efficacy of the mechanism by which the visual system compensates for nearly incessant retinal image motion."[14] There may be a family history of the disorder, with X-linked mutations accounting for the most common mode of inheritance.[15] In 2006, Tarpey and colleagues discovered the first gene causing IIN—the FRMD7 gene—so obtaining history and examining family members may be of yield. [16]
Two additional important signs of IIN are:
- Reversal of normal optokinetic nystagmus upon presentation of the rotating OKN drum[17]
- Exponential increase of slow phase eye movement (see Figure 2, second waveform)
The visual acuity is proportional to the foveation time, and in most patients, the visual acuity is at or greater than 20/40.[18] It is thought that IIN patients use "foveation strategy" such as "foveating saccades" to improve vision by maximizing the duration of the slow phase.[19] Strabismus is present in about 15% of the patients with IIN. Good stereopsis is often present.
Visual attention and fixation amplifies (worsens) the nystagmus in IIN, but convergence on a near target dampens the amplitude and sleep abolishes it altogether.[17] IIN usually has a null point at which the abnormal eye movement has a lower intensity than in other directions of gaze. If the null point is not the primary gaze position, the patient may develop a head position/head turn in effort to reduce nystagmus. In these cases, extraocular muscle surgery can be performed to shift the null point to primary position and therefore minimize the amplitude of nystagmus at primary gaze position.
Sensory nystagmus
Sensory nystagmus, also known as nystagmus associated with afferent visual system abnormalities, is usually seen in the first 3-4 months and has the same oculomotor features as infantile nystagmus, but it is due to anatomic disorders of the eye that, by limiting the proper visual sensory input to the eye, limit the visual development of the patient. The causes of sensory nystagmus are many—but a few common etiologies can be remembered by the 5 A’s mnemonic: Aplasia (hypoplasia) of the optic nerve (optic nerve hypoplasia), Leber congenital amaurosis, aniridia, Achromatopsia, and ocular albinism. Other causes include impairment of the cortical system as seen in preterm infants with periventricular leukomalacia and those with traumatic brain injury or metabolic disorders. These entities must be ruled out in patients undergoing evaluation of their nystagmus by searching for impairment of visual tracking and optic atrophy. Neuroimaging may be recommended.
Latent Nystagmus (fusional maldevelopment nystagmus)
Latent nystagmus, also known as fusional maldevelopment nystagmus, is a benign, early onset conjugate horizontal jerk-nystagmus that only manifests after occlusion of one eye. By definition, the nystagmus does not manifest under binocular conditions. The direction of the nystagmus is toward the uncovered eye, which means that the direction changes depending on which eye is occluded. Latent nystagmus is typically associated with congenital esotropia[20] and dissociated vertical deviation. Because of the induced nystagmus upon monocular occlusion, the visual acuity of these patients should be checked with partial optical blurring such as with a high-plus lens filter rather than with complete monocular occlusion.
Spasmus Nutans
Spasmus nutans (SN) is classically characterized by the triad of binocular small-amplitude pendular nystagmus, head nodding, and abnormal head posture or torticollis manifesting itself in the first year of life. Some authors suggest that the head nodding in SN is a compensatory mechanism for oscillopsia rather than a separate pathological manifestation.[21] Spasmus nutans in itself is a relatively benign condition that resolves by the end of the first decade of life though with often reduced visual acuity with or without significant refractive error.[22] The pendular nystagmus of SN is notable in that it is highly variable in amplitude and phase direction--at times becoming disconjugate, dissociated, purely monocular, then to conjugate over the course of minutes--which is in contrast to the stable nystagmus of IIN. The markedly asymmetric and sometimes monocular involvement of SN may be indistinguishable from the possibly life-threatening manifestations of optic pathway gliomas that cause monocular nystagmus of childhood. Neuroimaging should be considered.
Some authors reported the association between retinal dystrophies and suggested that electroretinographic studies be considered to assess for such disorders in patients presenting with suspected SN.[23]
Monocular nystagmus of childhood
Monocular nystagmus of childhood is nystagmus that involves the same eye at all times in a child. The waveforms have small amplitudes and can be vertical or elliptical. Heimann-Bielschowsky phenomenon is a type of monocular nystagmus that occurs due to longstanding poor vision in one eye with amblyopia, optic neuropathy, or dense cataract. It can often be inhibited by convergence or fixation. There are reports of improved monocular nystagmus after extraocular muscle surgery in the case of strabismic amblyopia causing the Heiman-Bielschowsky phenomenon.[24][25] When an infant presents with signs of afferent pathway disease (eg, optic disc atrophy, relative afferent pupillary defect, and monocular nystagmus) however, neuroimaging should be considered.[26] See Figure 3 for an example of a child who presented with monocular nystagmus and a chiasmic-hypothalamic mass.[27]
Acquired Forms of Nystagmus
Nystagmus due to Defective Gaze-holding
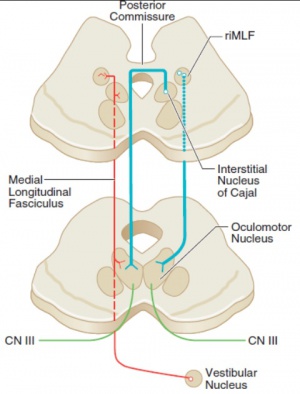
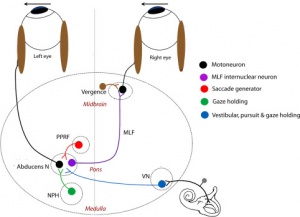
The neural mechanism for maintaining eccentric gaze involves a number of areas of the brainstem called the neural integrator. The horizontal gaze neural integrator consists of the nucleus prepositus hypoglossi and medial vestibular nuclei. Vertical and torsional gaze holding is maintained by the interstitial nucleus of Cajal. Other components of the neural integrator include the flocculus and nodulus of the cerebellum. All of these components are necessary to sustain eccentric gaze. When any part of the mechanism fails, defective gaze-holding manifests as nystagmus. See Figure 4 for illustration of the neural integrators.
Gaze-evoked Nystagmus and Rebound Nystagmus
Gaze-evoked nystagmus has jerk waveform movement that occurs in lateral gaze or upgaze. After each eccentric gaze, the eyes move toward the primary position followed by a saccade toward the eccentric direction, leading to right jerk nystagmus in right gaze and left jerk nystagmus in left gaze, etc. This format follows Alexander's law, which states that nystagmus increases in amplitude and frequency as the patient looks in the direction of the fast phase. Unlike end-gaze nystagmus (conjugate, in both right and left directions of gaze, transient, low amplitude of under 4-degrees, more prominent with age, benign), gaze-evoked nystagmus is sustained, larger in amplitude, possibly asymmetric, and is often associated with down-beat nystagmus.
Gaze-evoked nystagmus is a sign of neural integrator dysfunction. Advanced age can cause degenerative impairment of the neural integrator, leading to an often symmetric, horizontal gaze-evoked nystagmus. Unsustained gaze-evoked nystagmus of short duration, with a low amplitude and frequency, elicited at extreme horizontal fields of gaze, especially in an elderly patient, is usually physiologic and does not require further investigation. In contrast, primary position nystagmus, sustained (>20 seconds), or asymmetric gaze evoked nystagmus are usually pathologic however, and should prompt further investigation.[29][30] Etiologies may include intoxication (eg, sedatives, anticonvulsants, alcohol, illicit drug or hypnotic use), trauma, stroke, demyelination, Chiari malformation, or tumor. See Figure 5.

Rebound nystagmus is a variant of gaze-evoked nystagmus. When the subject resumes primary gaze after a period of eccentric gaze holding the eyes drift back toward the prior eccentric direction of gaze with saccade back to primary gaze. The nystagmus is transient (usually less than 30 seconds). Rebound nystagmus is often associated with cerebellar disease such as those associated with gaze-evoked nystagmus.
Gaze-evoked and rebound nystagmus require attention and evaluation to find the underlying cause. The nystagmus itself generally does not require treatment.
Peripheral Vestibular Nystagmus
Peripheral, as opposed to central, vestibular nystagmus arises from end-organ dysfunction. A table comparing the clinical characteristics and common etiologies of peripheral versus central vestibular nystagmus is shown below.[32][33][34]
The vestibular system includes the inner ear elements such as the semicircular canals, otolithic structures, and the vestibular nerve. See Figure 6 for a diagram of the involved neural networks. Because of the disruption of the vestibular input into the neural integrator that routes the signal to the contralateral paramedian pontine reticular formation (PPRF), the resulting nystagmus has slow phase toward the side of the problematic vestibular system. The nystagmus follows Alexander's law. Generally, peripheral vestibular nystagmus follows a horizontal-torsional pattern, which is in contrast to the purely vertical or torsional nystagmus seen in central vestibular nystagmus.
Because of the involvement of the vestibular system, patients often present with symptoms of inner ear disease such as vertigo, nausea, vomiting, oscillopsia, tinnitus, and sometimes even hearing loss. The symptoms usually improve over time, though they may be recurrent. The type associated with tinnitus and hearing loss is called Ménière disease,[35] while the type associated with vertigo in certain postures is known as benign paroxysmal positional vertigo (BPPV). The Dix-Hallpike maneuver is useful in the diagnosis of BPPV, and the Epley maneuver is used to treat BPPV, although it has a high disease recurrence rate.
A unique characteristic of peripheral vestibular nystagmus is the dampening effect on the nystagmus by visual fixation, which is in contrast to central vestibular nystagmus. Treatment of the different types of peripheral vestibular nystagmus may vary from observation to otolaryngologic surgery.[35]
Central Vestibular Nystagmus
Central forms of vestibular nystagmus arise from dysfunction in one of the many interconnections between the central vestibular structures and the neural integrators. In contrast to peripheral vestibular nystagmus, centrally derived nystagmus is not classically inhibited by visual fixation and is typically confined to one plane (eg, purely vertical or torsional). Neuroimaging is crucial in determining the location of the etiologic structural lesions, although metabolic and biochemical pathologies should also be considered. Generally, if patients present with small amplitude, eccentric nystagmus, the patient may be visually asymptomatic but may still have other associated brainstem or cerebellar signs.[36] Selected nystagmus and their most common corresponding etiologic structural lesions are presented in the table below.[32][37][38]
Bruns Nystagmus
Bruns nystagmus is a combination of peripheral and central vestibular nystagmus due to the involvement of 2 different neural pathways--(1) cerebellar flocculi and (2) peripheral vestibular components of the cerebellum—and usually manifests as a symptom of cerebellar pontine angle tumors (eg, acoustic neuromas or meningiomas) greater than 3.5 cm.[39] Initially, with only peripheral involvement (eg, vestibular nerve impairment), the first phase of oscillatory movement is toward the side of the lesion, initiating the second phase in the opposite direction, which is fast and corrective—rapid, small amplitude nystagmus away from the side of the lesion. As the lesion expands (eg, enlargement of the tumor, compression of the ipsilateral brainstem) involving the central nervous system, a second nystagmus may manifest. With compression of the brain, specifically the cerebellar flocculus, the ability to hold ipsilateral eccentric gaze becomes impaired—the nystagmus is slower, with an increase in amplitude and change in direction towards the side of the lesion—slow, large amplitude nystagmus toward the side of the lesion. Thus, this specific type of nystagmus consists of 2 simultaneous nystagmuses: (1) coarse, large amplitude, low frequency evoked on gaze ipsilateral to the lesion (2) fine, low amplitude, high frequency evoked on gaze contralateral to the lesion. [39][40]
Downbeat Nystagmus
Downbeat nystagmus (downward fast phase) is the most common of the central vestibular nystagmuses. Its jerk nystagmus waveform begins with upward drift of the eyes corrected with a downward saccade. This form of nystagmus follows Alexander's law and hence is accentuated by downgaze and also by lateral-down gaze, but it is also amplified by convergence and lying prone.[41] Concomitant gaze-evoked nystagmus and rebound nystagmus may be observed.[36] Generally, patients are symptomatic from vertical oscillopsia. The differential for down-beating nystagmus is broad, but structural lesions can be ruled out with neuroimaging. Cervico-medullary junction is the most probable location of a structural lesion.
- Tumors at the foramen magnum
- Arnold-Chiari malformation Type I
- Demyelination
- Stroke
- Cranial trauma
- Drug toxicity (lithium, anticonvulsants)
- Platybasia
- Spinocerebellar degeneration
- Brainstem encephalitis
- Paraneoplastic syndrome
- Impaired nutrition (eg, Wernicke encephalopathy, parenteral feeding, magnesium deficiency)
- Antibody to glutamic acid decarboxylase (GAD)
- Idiopathic
There are several antibodies that have been associated with downbeat nystagmus in a growing number of cases, mainly antibodies to voltage-gated calcium channels and to glutamic acid decarboxylase (GAD).[42][43] Glutamic acid is converted to GABA, a central player in neuronal signaling and transmission in the CNS, by GAD. Downbeat nystagmus is caused by imbalance of the activities of the anterior and posterior canals caused by lesions in the vestibulocerebellum (specifically the flocculi) and the medulla. The pathophysiology behind this association is the GABAergic neuronal regulation of communication between Purkinje cells and floccular neurons. Antibodies to GAD prevent the conversion of glutamic acid to GABA, reducing the innervation of floccular neurons to the anterior vestibular canals, thereby resulting in downbeat nystagmus.[43][44] There were several case studies that reported that steroids and intravenous immunoglobulin (IVIG) may be beneficial for anti-GAD-associated downbeat nystagmus or cerebellar ataxia.[44][45][46]
Base-out prism and pharmacologic therapies with clonazepam, baclofen, gabapentin,[47] memantine, or aminopyridine have been suggested but usually with mixed success[48][49][50] and with side effects like conversion to upbeat nystagmus[51] or seizures.[52] (See the section on pharmacologic therapy).
Upbeat Nystagmus
Upbeat nystagmus is purely vertical conjugate nystagmus that manifests itself in primary gaze with slow downdrift of the eyes, corrected by fast upward saccade. Patients may experience vertical oscillopsia. Structural lesions in the brainstem or in the anterior cerebellar vermis can cause this type of nystagmus. Causes include demyelinating disease,[53] stroke, tumors, cerebellar degeneration, and tobacco smoking.[54]
Torsional Nystagmus
Eyes with torsional nystagmus have fast phase intorsion or extorsion that is usually conjugate and symmetric. Purely torsional nystagmus without horizontal or vertical components indicates a defect in the brainstem, while torsional with a vertical component indicates a lesion in the midbrain. See the table below for comparison of the 2 different types of torsional nystagmus.
| Purely torsional | Torsional with either downward or upward component | |
|---|---|---|
| Localization | Brainstem (pontomedullary junction) contralateral to fast phase | Midbrain |
| Ocular tilt reaction direction | Ipsi-lesional | Contra-lesional |
The differential includes stroke, demyelinating disease, and Chiari malformation. Neuroimaging is important for localization of structural lesions. See pharmacological therapy for treatment options.
Seesaw Nystagmus and Hemi-Seesaw Nystagmus
Seesaw nystagmus, a subtype of torsional nystagmus, is descriptively named for the pendular, disconjugate movement of eyes in which one elevates/intorts while the fellow eye depresses/extorts. Hemi-seesaw nystagmus is similar but with jerk waveform rather than pendular. See the table below for common causes.
| Seesaw Nystagmus | Hemi-seesaw Nystagmus | |
|---|---|---|
| Common cause | Large parasellar tumor (craniopharyngioma, pituitary adenoma[55]) | Midbrain hemorrhage, medullar infarcts, Chiari malformation |
Congenital achiasma has been rarely associated with seesaw nystagmus.[56] Other ocular associations with seesaw nystagmus include retinitis pigmentosa[57] and albinism.[58]
Periodic Alternating Nystagmus
This rare type of nystagmus is a strictly horizontal, conjugate, jerk nystagmus that periodically alternates its direction of fast phase. One half cycle of 30 to 90 seconds will involve right-beating nystagmus followed by the same duration of left-beating nystagmus. The 2 halves of each cycle are divided by a transition period of minimal to no nystagmus or small-amplitude vertical nystagmus. Because of the multi-minute duration of each cycle, the observer should consider watching the patient's eye for at least several minutes to detect the change in direction. The patient may also alternate his/her head turn to the eye position to null point (right head turn during right-beating cycle) in accordance with Alexander's law.
Periodic alternating nystagmus (PAN) can be congenital or acquired, although the congenital form is less predictably periodic compared to the acquired form.[36] Associated central nervous system pathologies may involve Chiari malformation, multiple sclerosis, and stroke, but phenytoin use[59] and bilateral significant visual loss[60] should also be considered as the cause of PAN.
See Clinical Types and Terminology for a video example of PAN.
Acquired Pendular Nystagmus
Unlike congenital pendular nystagmus, acquired pendular nystagmus often entails slow-phase eye movements in horizontal, vertical, and torsional planes with resultant elliptical or circular nystagmus. The movements may be monocular, or if bilateral, conjugate or disconjugate, and may also be dissociated. The most common cause of acquired pendular nystagmus is multiple sclerosis. If the vision is asymmetric between the 2 eyes, the poorer-seeing eye has greater amplitude and frequency of nystagmus compared to the fellow eye.[10]
Oculopalatal myoclonus or tremor
If acquired pendular nystagmus has concomitant palatal myoclonus (oscillation of the palate), it is called oculopalatal myoclonus or oculopalatal tremor. The eye movements are continuous, with both torsional and vertical components with frequency of 1-3 Hz. Additional muscles (pharynx, face, vocal cords, respiratory muscles, and even trunk and extremities) may be involved in this involuntary rhythmic movement that persists during sleep.
Magnetic resonance imaging (MRI) of the brain would show hypertrophic degeneration of the inferior olivary nucleus in the medulla (radiologic pimento sign).[61] This degeneration occurs as a result of brain infarct or hemorrhage, but the onset of the oculopalatal tremor is delayed for months or years after the initial insult, due to neural deafferentation.[36]
Oculo-masticatory myorhythmia
The combination of pendular vergence nystagmus and associated contractions of the masticatory/facial/pharyngeal muscles are pathognomonic for Whipple disease.[62] The horizontal oscillation of each eye with oculomasticatory myorhythmia is out of phase and hence produces convergence-divergence nystagmus at about 1 cycle per second.[36] Patients commonly have systemic manifestation of the disease including fever, abdominal pain, diarrhea, cognitive dysfunction, weight loss, and arthralgia. Definitive diagnosis can be made through biopsy of the duodenum that shows periodic acid-Schiff staining of foamy macrophages in the villae. Antibiotic therapy should be instituted for this lethal disease, which has a high rate of recurrence for central nervous system (CNS)-involving manifestations.[63] Polymerase chain reaction (PCR) of Tropheryma whippelii RNA can be a useful laboratory test.
Dissociated Nystagmus
Dissociated nystagmus is characterized by a difference or dissimilarity of the direction, extent and/or periodicity of the ocular oscillations between the 2 eyes. The most common etiology of this type of nystagmus is a lesion of the MLF (medial longitudinal fasciculus) and subsequent internuclear ophthalmoplegia (INO). This results in impaired horizontal movements with slowed or weakened adduction of the affected eye (ipsilateral to MLF lesion) and abduction nystagmus of the unaffected eye (contralateral to MLF lesion).[64] The pathogenesis of this condition is the development of an adaptive response and increased neural pulsing/signaling to compensate for weakened adduction on lateral gaze/medial rectus muscle. Hering’s law of equal innervation follows that the increased neural pulsing/signaling should also be accompanied by equal pulsing/signaling to the contralateral yoke muscle, which in this case creates excessive saccadic movements on contralateral gaze/lateral rectus muscle.[65][66]
Toxin/Illicit Drug-induced Nystagmus
Classically, phencyclidine (PCP) intoxication is associated with nystagmus, specifically rotatory or torsional nystagmus.[67] Other common drugs/toxins that may be associated with vertical, horizontal, rotatory, or mixed nystagmus include anticonvulsants (phenytoin, carbamazepine, valproic acid, lamotrigine, topiramate), ethanol, amphetamines, barbiturates, benzodiazepines, 3,4-Methylenedioxymethamphetamine (MDMA), also known as “ecstasy”), salicylates, selective serotonin reuptake inhibitors (SSRI), lithium, dextromethorphan, ketamine and lysergic acid diethylamide (LSD).[68][69]
Physical examination
When assessing a patient with nystagmus, the examiner should consider assessing ocular stability/motility in primary gaze first followed by observation of the eye movement in cardinal gazes. A full description of the nystagmus should be gathered from examination, concisely presented in the form of the mnemonic DWARF—Direction, Waveform, Amplitude, Reducing direction, Frequency. In addition, the following characteristics should be identified:
- Monocular or binocular involvement
- Conjugacy (do both eyes move together?)
- Direction of movement (horizontal, vertical, torsional, or mixed)
- Stability of direction of movement (is the phase gaze always to the right?)
- Continuous or intermittent
- Amplitude (how big are the movements?)
- Frequency (how often are the movements happening?)
- Presence of null point (the direction of gaze or distance of fixation at which nystagmus is minimal to nil)
- Presence of slow phase (if there is no slow phase, the eye movement disorder is considered a saccadic intrusion)
- General condition of the patient (is the patient comatose?)
- Associated symptoms such as vertigo, nausea, and oscillopsia
Management
Options for managing nystagmus can vary from observation to aggressive surgeries depending on the nature and cause of the nystagmus.
Pharmacological therapy
For the treatment of congenital nystagmus (both in idiopathic and secondary forms), pharmacologic therapies with gabapentin and memantine have been studied in randomized clinical trials of adult patients. Statistically significant improvement in visual acuity and nystagmus amplitude were measured in patients in the treatment arm compared to those in the placebo arm.[70] Clonazepam and baclofen have also been tried with mixed results.[71]
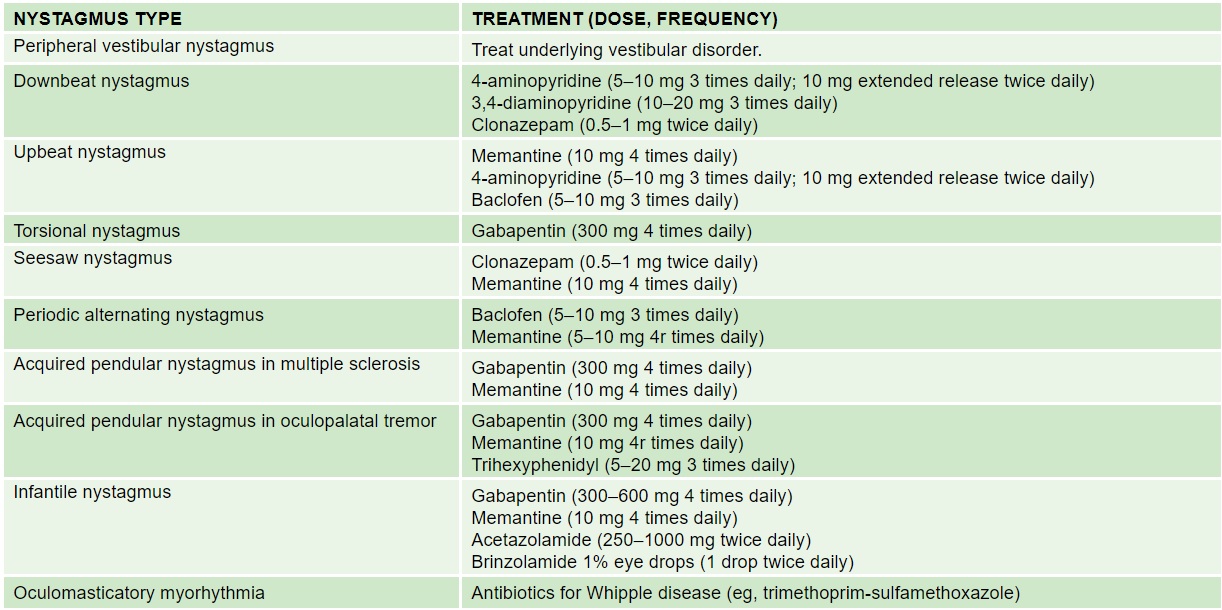
See the following summary of recommended pharmacologic treatment for the various clinical types of nystagmus.[36] Note that none of these medications have been studied in children.
Botulinum Toxin Injection
Trials of botulinum toxin injection into the retrobulbar space have been done to treat symptomatic nystagmus. While some patients reported improvement in oscillopsia,[72][73] side effects including ptosis, diplopia, and paradoxical worsening of oscillopsia due to limitation of VOR[74] have limited the utility of botulinum toxin as a treatment option.
Optical Treatments

Correction of refractive error, which can be high in patients with nystagmus, is required.[76] Contact lenses may be particularly useful in infantile nystagmus.[77] Traditional amblyopia therapy may be employed in cases of latent nystagmus. If the nystagmus dampens with convergence (such as infantile idiopathic nystagmus and monocular nystagmus of childhood, base-out prism to induce convergence may help.[78]
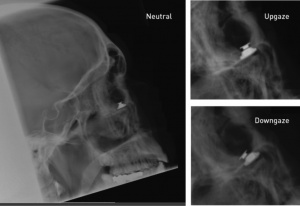
A prototype of an electro-optical, portable, image-shifting device has been developed to treat pendular nystagmus.[75] The utility and efficacy of the device is unknown. (See Figure 7.)
Surgery
Extraocular muscle surgery as a treatment for nystagmus is mostly for infantile nystagmus. The Anderson-Kestenbaum procedure mechanically shifts the null point from a horizontal cardinal position to primary position.[80] An equivalent procedure may be performed for patients with vertical nystagmus.[81] More recently, four-muscle tenotomy and reattachment without transposition has been found to be effective as well.[82]
A case of a pilot, experimental intervention using magnetic oculomotor prosthesis has also been reported with objective improvement of visual acuity and reduction of acquired nystagmus.[79] (See Figure 8.)
Additional Resources
- Boyd K, DeAngelis KD. Nystagmus. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/nystagmus. Accessed March 19, 2019.
- McLean RJ, Windridge KC, Gottlob I. Living with nystagmus: a qualitative study. Br J Ophthalmol. 2012 Jul;96(7):981-6. doi: 10.1136/bjophthalmol-2011-301183. Epub 2012 Apr 19. PMID: 22517800.
References
- ↑ Sarvananthan N, Surendran M, Roberts EO, Jain S, Thomas S, Shah N, et al. The prevalence of nystagmus: the Leicestershire nystagmus survey. Invest Ophthalmol Vis Sci, 50 (2009), pp. 5201-5206
- ↑ Nash DL, Diehl NN, Mohney BG. Incidence and Types of Pediatric Nystagmus. Am J Ophthalmol. 2017 Oct;182:31-34.
- ↑ Ehrt O. Infantile and acquired nystagmus in childhood. Eur J Paediatr Neurol. 2012 Nov;16(6):567-72.
- ↑ Büttner U, Kremmyda O. Smooth pursuit eye movements and optokinetic nystagmus. Dev Ophthalmol. 2007;40:76-89.
- ↑ Optokinetic Nystagmus Dysfunction. In: Basic and Clinical Science Course (BCSC) Section 5: Neuro-Ophthalmology. Academy of Ophthalmology; 2015:192-193
- ↑ Golia AG, Pawar M. The diagnosis of brain death. Indian J Crit Care Med. 2009 Jan-Mar; 13(1): 7–11.
- ↑ Howard IP, Zacher JE, Allison RS. Post-rotatory nystagmus and turning sensations after active and passive turning. J Vestib Res. 1998 Jul-Aug;8(4):299-312.
- ↑ Papageorgiou E, McLean RJ, Gottlob I. Nystagmus in childhood. Pediatr Neonatol. 2014 Oct;55(5):341-51.
- ↑ Abadi RV, Dickinson CM. Waveform characteristics in congenital nystagmus. Doc Ophthalmol, 64 (1986), pp. 153-167
- ↑ Jump up to: 10.0 10.1 Acquired Pendular Nystagmus. In: Basic and Clinical Science Course (BCSC) Section 5: Neuro-Ophthalmology. Academy of Ophthalmology; 2015:237.
- ↑ LeighRJ, Dell'OssoLF, YaniglosSS, ThurstonSE. Oscillopsia, retinal image stabilization and congenital nystagmus. Invest Ophthalmol Vis Sci. 1988;29:279–282.
- ↑ LeeAG, BrazisPW. Localizing forms of nystagmus: symptoms, diagnosis, and treatment. Curr Neurol Neurosci Rep. 2006;6:414–420.
- ↑ Schlindwein P, Schreckenberger M, Dieterich M. Visual-motion suppression in congenital pendular nystagmus. Ann New York Acad Sci, 1164 (2009), pp. 458-460. Basic and Clinical Aspects of Vertigo and Dizziness
- ↑ Cham KM, Anderson AJ, Abel LA. Factors influencing the experience of oscillopsia in infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3424-31.
- ↑ Kerrison JB, Giorda R, Lenart TD, Drack AV, Maumenee IH. Clinical and genetic analysis of a family with X-linked congenital nystagmus (NYS1). Ophthalmic Genet, 22 (2001), pp. 241-248
- ↑ Tarpey P, Thomas S, Sarvananthan N, Mallya U, Lisgo S, Talbot CJ, Roberts EO, Awan M, Surendran M, McLean RJ, Reinecke RD, Langmann A, Lindner S, Koch M, Jain S, Woodruff G, Gale RP, Bastawrous A, Degg C, Droutsas K, Asproudis I, Zubcov AA, Pieh C, Veal CD, Machado RD, Backhouse OC, Baumber L, Constantinescu CS, Brodsky MC, Hunter DG, Hertle RW, Read RJ, Edkins S, O'Meara S, Parker A, Stevens C, Teague J, Wooster R, Futreal PA, Trembath RC, Stratton MR, Raymond FL, Gottlob I. Mutations in FRMD7, a newly identified member of the FERM family, cause X-linked idiopathic congenital nystagmus. Nat Genet. 2006 Nov;38(11):1242-4. Epub 2006 Oct 1. Erratum in: Nat Genet. 2011 Jul;43(7):720. Bastawrous, Andrew [added]. PubMed PMID: 17013395; PubMed Central PMCID: PMC2592600.
- ↑ Jump up to: 17.0 17.1 Infantile Nystagmus Syndrome. In: Basic and Clinical Science Course (BCSC) Section 5: Neuro-Ophthalmology. Academy of Ophthalmology; 2015:229.
- ↑ Thomas S, Proudlock FA, Sarvananthan N, Roberts EO, Awan M, McLean R, et al. Phenotypical characteristics of idiopathic infantile nystagmus with and without mutations in FRMD7. Brain, 131 (2008), pp. 1259-1267.
- ↑ L.F. Dell'Osso, R.B. Daroff. Congenital nystagmus waveforms and foveation strategy. Doc Ophthalmol, 39 (1975), pp. 155-182.
- ↑ Simonsz HJ, Kolling GH. Best age for surgery for infantile esotropia. Eur J Paediatr Neurol. 2011 May;15(3):205-8
- ↑ Weissman BM, Dell'Osso LF, Abel LA, Leigh RJ. Spasmus nutans. A quantitative prospective study. Arch Ophthalmol. 1987;105:525–528.
- ↑ Kiblinger GD, Wallace BS, Hines M, Siatkowski RM. Spasmus nutans-like nystagmus is often associated with underlying ocular, intracranial, or systemic abnormalities. J Neuroophthalmol. 2007 Jun;27(2):118-22.
- ↑ Smith DE, Fitzgerald K, Stass-Isern M, Cibis GW. Electroretinography is necessary for spasmus nutans diagnosis. Pediatr Neurol. 2000 Jul;23(1):33-6.
- ↑ Smith JL, Flynn JT, Spiro HJ. Monocular vertical oscillations of amblyopia. The Heimann-Bielschowsky phenomenon. J Clin Neuroophthalmol. 1982 Jun;2(2):85-91.
- ↑ ElKamshoushy A, Sprunger DT. Recession of three muscles to reduce ocular oscillations in patients with Heimann-Bielschowsky phenomenon. J AAPOS. 2011 Feb;15(1):67-8
- ↑ Jacob FD, Ramaswamy V, Goez HR. Acquired monocular nystagmus as the initial presenting sign of a chiasmal glioma. Can J Neurol Sci. 2010 Jan;37(1):96-7.
- ↑ Toledano H, Muhsinoglu O, Luckman J, Goldenberg-Cohen N, Michowiz S. Acquired nystagmus as the initial presenting sign of chiasmal glioma in young children. Eur J Paediatr Neurol. 2015 Nov;19(6):694-700.
- ↑ Ropper AH, Samuels A, Kelin JP, Prasad S. Adams and Victor's Principles of Neurology. 2015. Ch 13: Disorders of Ocular Movement and Pupillary Function.
- ↑ Strupp M, Kremmyda O, Adamczyk C, et al. Central ocular motor disorders, including gaze palsy and nystagmus. J Neurol. 2014;261 Suppl 2:S542-558.
- ↑ Shallo-Hoffmann J, Schwarze H, Simonsz HJ, Muhlendyck H. A reexamination of end-point and rebound nystagmus in normals. Invest Ophthalmol Vis Sci. 1990;31(2):388-392.
- ↑ Udaka YT, Shayan K, Chuang NA, Crawford JR. Atypical presentation of atypical teratoid rhabdoid tumor in a child. Case Rep Oncol Med. 2013;2013:815923.
- ↑ Jump up to: 32.0 32.1 Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998;339(10):680-685.
- ↑ Fife TD, Tusa RJ, Furman JM, et al. Assessment: vestibular testing techniques in adults and children: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2000;55(10):1431-1441.
- ↑ Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348(11):1027-1032.
- ↑ Jump up to: 35.0 35.1 Espinosa-Sanchez JM, Lopez-Escamez JA. Menière's disease. Handb Clin Neurol. 2016;137:257-77.
- ↑ Jump up to: 36.0 36.1 36.2 36.3 36.4 36.5 36.6 36.7 Fong AMF. A Practical Approach to Nystagmus and Saccadic Oscillations. Focal Points by American Academy of Ophthalmology. 2014.
- ↑ Stahl JS, Averbuch-Heller L, Leigh RJ. Acquired nystagmus. Arch Ophthalmol. 2000;118(4):544-549.
- ↑ Tarnutzer AA, Straumann D. Nystagmus. Curr Opin Neurol. 2018;31(1):74-80.
- ↑ Jump up to: 39.0 39.1 Venkateswaran R, Gupta R, Swaminathan RP. Bruns nystagmus in cerebellopontine angle tumor. JAMA Neurol. 2013;70(5):646-647.
- ↑ Lloyd SK, Baguley DM, Butler K, Donnelly N, Moffat DA. Bruns' nystagmus in patients with vestibular schwannoma. Otol Neurotol. 2009;30(5):625-628.
- ↑ Strupp M, Kremmyda O, Adamczyk C, Böttcher N, Muth C, Yip CW, Bremova T. Central ocular motor disorders, including gaze palsy and nystagmus. J Neurol. 2014 Sep;261 Suppl 2:S542-58.
- ↑ Tilikete C, Vighetto A, Trouillas P, Honnorat J. Potential role of anti-GAD antibodies in abnormal eye movements. Ann N Y Acad Sci. 2005;1039:446-454.
- ↑ Jump up to: 43.0 43.1 Antonini G, Nemni R, Giubilei F, et al. Autoantibodies to glutamic acid decarboxylase in downbeat nystagmus. J Neurol Neurosurg Psychiatry. 2003;74(7):998-999.
- ↑ Jump up to: 44.0 44.1 Chen Y, Morgan ML, Palau AE, Mudd JA, Lee AG, Barton JJ. Downbeat down south. Surv Ophthalmol. 2015;60(2):177-181.
- ↑ Dalakas MC. The role of IVIg in the treatment of patients with stiff person syndrome and other neurological diseases associated with anti-GAD antibodies. J Neurol. 2005;252 Suppl 1:I19-25.
- ↑ Dayalu P, Teener JW. Stiff Person syndrome and other anti-GAD-associated neurologic disorders. Semin Neurol. 2012;32(5):544-549.
- ↑ Averbuch-Heller L, Tusa RJ, Fuhry L, Rottach KG, Ganser GL, Heide W, Bu¨ttner U, Leigh RJ. A double-blind controlled study of gabapentin and baclofen as treatment for acquired nystagmus. Ann Neurol. 1997;41:818–825
- ↑ Strupp M, Schuler O, Krafczyk S, Jahn K, Schautzer F, Buttner U, Brandt T. Treatment of downbeat nystagmus with 3,4-diaminopyridine: a placebo-controlled study. Neurology. 2003;61:165–170.
- ↑ Kalla R, Glasauer S, Büttner U, Brandt T, Strupp M. 4-aminopyridine restores vertical and horizontal neural integrator function in downbeat nystagmus. Brain. 2007 Sep; 130(Pt 9):2441-51.
- ↑ Claassen J, Spiegel R, Kalla R, Faldon M, Kennard C, Danchaivijitr C, Bardins S, Rettinger N, Schneider E, Brandt T, Jahn K, Teufel J, Strupp M, Bronstein A. A randomised double-blind, cross-over trial of 4-aminopyridine for downbeat nystagmus--effects on slow phase eye velocity, postural stability, locomotion and symptoms. J Neurol Neurosurg Psychiatry. 2013 Dec; 84(12):1392-9.
- ↑ Helmchen C, Sprenger A, Rambold H, Sander T, Kompf D, Straumann D. Effect of 3,4-diaminopyridine on the gravity dependence of ocular drift in downbeat nystagmus. Neurology. 2004;63:752–753
- ↑ Thurtell MJ, Leigh RJ. Therapy for nystagmus. J Neuroophthalmol. 2010 Dec;30(4):361-71.
- ↑ Tilikete C, Milea D, Pierrot-Deseilligny C. Upbeat nystagmus from a demyelinating lesion in the caudal pons. J Neuroophthalmol. 2008 Sep;28(3):202-6.
- ↑ Sibony PA, Evinger C, Manning KA. Tobacco-induced primary-position upbeat nystagmus. Ann Neurol. 1987 Jan;21(1):53-8.
- ↑ Yat-Ming Woo P, Takemura S, Ming-Yan Cheong A, Chi-Ho Chu A, Chan Y, Wong HT, Chan KY. Pendular Seesaw Nystagmus in a Patient With a Giant Pituitary Macroadenoma: Pathophysiology and the Role of the Accessory Optic System. J Neuroophthalmol. 2017 Nov 9.
- ↑ Davies-Thompson J, Scheel M, Jane Lanyon L, Sinclair Barton JJ. Neuropsychologia. 2013 Jun;51(7):1260-72. Functional organisation of visual pathways in a patient with no optic chiasm.
- ↑ Bergin DJ, Halpern J. Congenital see-saw nystagmus associated with retinitis pigmentosa. Ann Ophthalmol. 1986 Dec;18(12):346-9. PMID: 3813357.
- ↑ Hoyt CS, Gelbart SS. Vertical nystagmus in infants with congenital ocular abnormalities. Ophthalmic Paediatr Genet. 1984 Dec;4(3):155-61.
- ↑ Campbell WW Jr. Periodic alternating nystagmus in phenytoin intoxication. Arch Neurol. 1980 Mar;37(3):178-80.
- ↑ Jay WM, Williams BB, De Chicchis A. Periodic alternating nystagmus clearing after cataract surgery. J Clin Neuroophthalmol. 1985 Sep;5(3):149-52.
- ↑ Eggenberger E, Cornblath W, Stewart DH. Oculopalatal tremor with tardive ataxia. J Neuroophthalmol. 2001 Jun;21(2):83-6.
- ↑ Revilla FJ, de la Cruz R, Khardori N, Espay AJ. Teaching NeuroImage: Oculomasticatory myorhythmia: pathognomonic phenomenology of Whipple Disease. Neurology. 2008 Feb 5;70(6):e25.
- ↑ 48 Ratnaike RN. Whipple's disease. Postgrad Med J. 2000 Dec;76(902):760-6.
- ↑ Virgo JD, Plant GT. Internuclear ophthalmoplegia. Pract Neurol. 2017;17(2):149-153.
- ↑ Baloh RW, Yee RD, Honrubia V. Internuclear ophthalmoplegia. I. Saccades and dissociated nystagmus. Arch Neurol. 1978;35(8):484-489.
- ↑ Zee DS, Hain TC, Carl JR. Abduction nystagmus in internuclear ophthalmoplegia. Ann Neurol. 1987;21(4):383-388.
- ↑ Dominici P, Kopec K, Manur R, Khalid A, Damiron K, Rowden A. Phencyclidine Intoxication Case Series Study. J Med Toxicol. 2015;11(3):321-325.
- ↑ Wheeler SD, Ramsay RE, Weiss J. Drug-induced downbeat nystagmus. Ann Neurol. 1982;12(2):227-228.
- ↑ Corbett JJ, Jacobson DM, Thompson HS, Hart MN, Albert DW. Downbeating nystagmus and other ocular motor defects caused by lithium toxicity. Neurology. 1989;39(4):481-487.
- ↑ McLean R, Proudlock F, Thomas S, Degg C, Gottlob I. Congenital nystagmus: randomized, controlled, double-masked trial of memantine/gabapentin. Ann Neurol. 2007 Feb;61(2):130-8.
- ↑ Rucker JC. Current Treatment of Nystagmus. Curr Treat Options Neurol 2005;7:69-77
- ↑ Helveston EM, Pogrebniak AE. Treatment of acquired nystagmus with botulinum A toxin. Am J Ophthalmol. 1988;106:584–586.
- ↑ Leigh RJ, Tomsak RL, Grant MP, Remler BF, Yaniglos SS, Lystad L, Dell’Osso LF. Effectiveness of botulinum toxin administered to abolish acquired nystagmus. Ann Neurol. 1992;32:633–642.
- ↑ Acheson JF, Bentley CR, Shallo-Hoffmann J, Gresty MA. Dissociated effects of botulinum toxin chemodenervation on ocular deviation and saccade dynamics in chronic lateral rectus palsy. Br J Ophthalmol. 1998;82:67–71.
- ↑ Jump up to: 75.0 75.1 Smith RM, Oommen BS, Stahl JS. Image-shifting optics for a nystagmus treatment device. J Rehabil Res Dev. 2004 May;41(3A):325-36.
- ↑ Hertle RW. Examination and refractive management of patients with nystagmus. Surv Ophthalmol, 45 (2000), pp. 215-222
- ↑ Allen ED, Davies PD. Role of contact lenses in the management of congenital nystagmus. Br J Ophthalmol, 67 (1983), pp. 834-836
- ↑ Serra A, Dell’osso LF, Jacobs JB, Burnstine RA. Combined gaze-angle and vergence variation in infantile nystagmus: two therapies that improve the high-visual-acuity field and methods to measure it. Invest Ophthalmol Vis Sci. 2006; 47:2451–2460.
- ↑ Jump up to: 79.0 79.1 Nachev P, Rose GE, Verity DH, Manohar SG, MacKenzie K, Adams G, Theodorou M, Pankhurst QA, Kennard C. Magnetic Oculomotor Prosthetics for Acquired Nystagmus. Ophthalmology. 2017 Oct;124(10):1556-1564.
- ↑ Kumar A, Shetty S, Vijayalakshmi P, Hertle RW. Improvement in visual acuity following surgery for correction of head posture in infantile nystagmus syndrome. J Pediatr Ophthalmol Strabismus. 2011 Nov-Dec;48(6):341-6.
- ↑ Taylor JN, Jesse K. Surgical management of congenital nystagmus. Aust N Z J Ophthalmol. 1987 Feb;15(1):25-34.
- ↑ Greven MA, Nelson LB. Four-muscle tenotomy surgery for nystagmus. Curr Opin Ophthalmol. 2014 Sep;25(5):400-5.