Medulloepithelioma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Medulloepithelioma is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
- C69.40 - Malignant neoplasm of unspecified ciliary body
- C69.41 - Malignant neoplasm of right ciliary body
- C69.42 - Malignant neoplasm of left ciliary body
- D31.40 - Benign neoplasm of unspecified ciliary body
- D31.41 - Benign neoplasm of right ciliary body
- D31.42 - Benign neoplasm of left ciliary body
Disease
Medulloepithelioma is an embryonal neuroectodermally derived tumor with an intraocular predilection for the non-pigmented ciliary epithelium (NPCE)[1][2]. Besides the ciliary epithelium, medulloepithelioma has been noted in the CNS and even orbit[3]. Ciliary body medulloepithelioma is the most common tumor arising from the non-pigmented ciliary epithelium and second most common pediatric intra-ocular tumor behind retinoblastoma[2].
Etiology
Most intraocular medulloepitheliomas occur sporadically and are not associated with congenital malformations or cytogenetic abnormalities. However, an association exists between medulloepithelioma and Pleuropulmonary Blastoma Family Tumor and Dysplasia Syndrome (PPB-FTDS) in about 5% of cases. This syndrome is associated with the DICER-1 gene, which is a member of the ribonuclease III family and is inherited in an autosomal dominant fashion[4][5][6][7]. About 3% of DICER-1 positive patients will manifest a ciliary body medulloepithelioma[7][8]. Tumors associated with this mutation include cystic nephromas, pleuropulmonary blastoma, ovarian tumors and thyroid hyperplasia[4][5][6]. DICER-1 mutations have been associated with a higher rate of retinal abnormalities[8]. KMT2D may represent another somatic mutation mutually exclusive to DICER-1 implicated in ciliary body medulloepithelioma pathogenesis[9]. Rare occurrences of medulloepithelioma in patients with retinoblastoma have also been reported, although the exact mechanism of the association remains to be elucidated[10].
Risk Factors
There are no large population-based studies on the incidence or prevalence of medulloepitheliomas. The literature largely consists of isolated case reports and case series. The Armed Forces Institute of Pathology (AFIP) reported the largest case series consisting of 56 histologically proven cases of medulloepitheliomas[11] . They reported a range of age of presentation from 6 months to 41 years, with a mean age of 3.8 years. The typical reported age of presentation was between 2 to 10 years, with most cases manifesting within the first decade[12]. However, there have been reports of isolated cases documented in adulthood. Based on the reports from AFIP and other case-series, there is no predilection towards any specific race or either gender[13][14][15]. Zimmerman et al. reported comorbid persistent hyperplastic primary vitreous in up to 20% of cases[11].
General Pathology and Pathophysiology
Medulloepitheliomas are thought to arise from neuroepithelial cells. In the eye, they commonly arise from within the non-pigmented ciliary epithelium. They may also be found intracranially in periventricular areas[2][16][17]. Its unique microstructural appearance arises from pseudostratified primitive neuroepithelial cells forming neural tube-like structures with distinct internal and external limiting membranes visualized with periodic acid-Schiff (PAS) staining[16][17][18][19]. Previously classified as primitive neuroectodermal tumors (PNET), the current WHO classification system for CNS embryonal tumors designates the category encompassing medulloepithelioma as “embryonal tumor with multilayered rosettes (ETMR)” and further designates genetic markers for amplification of the C19MC region of chromosome 19 or DICER-1 mutations[20][21]. In the absence of C19MC, tumors may be designated as medulloepithelioma provided they meet histological requirements[20]. Although histopathologically similar, intra-ocular medulloepithelioma does not express C19MC amplification suggesting a different origin from CNS medulloepithelioma and different behavior pattern portending better prognosis[22].
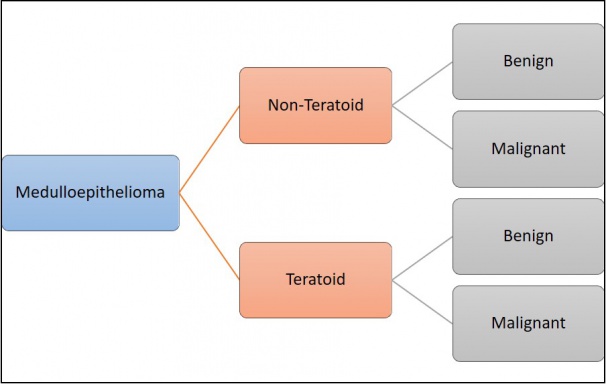
According to the Zimmerman classification, medulloepitheliomas can be histopathologically classified into teratoid and non-teratoid types, and each of these can be further sub-classified into benign or malignant types (Figure 1)[11].The non-teratoid type consists of cells that resemble the ciliary epithelium. The teratoid type may demonstrate cells of diverse origins, including cartilage, rhabdomyoblasts and brain. Both teratoid and non-teratoid types may contain cysts that contain a hyaluronidase-sensitive mucopolysaccharide that is secreted by tumor cells. Large case series have reported that 50-63% of tumors were nonteratoid and 38-50% were teratoid[23]. Flexner-Wintersteiner and Homer Wright rosettes may also be visualized[12][24].
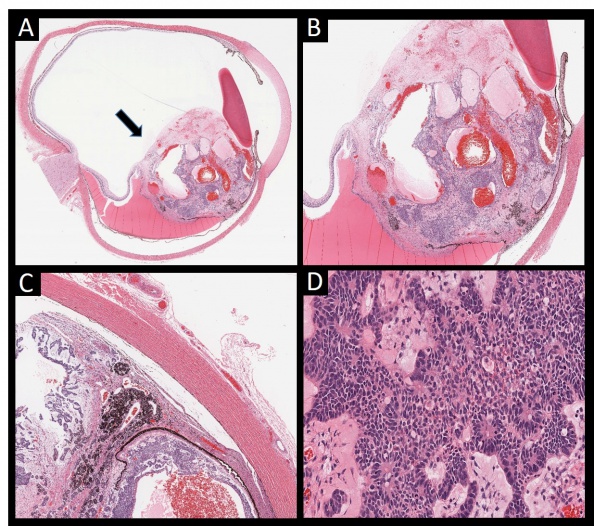
Benign or malignant classification depends upon factors that conventionally signify malignant potential:
- Retinoblastoma-like elements
- Sarcoma-like elements
- Poor cellular differentiation and cellular pleomorphism
- Abnormal mitotic activity
- Extension into surrounding ocular structures (optic nerve, sclera, uvea, cornea)
Most medulloepitheliomas tend towards malignancy. Although metastasis is rare, mortality is often associated with intracranial extension[7][25]. Benign and malignant lesions have been further sub-classified by the Zimmerman classification into solid, papillary and pleomorphic types[12][26][27]. Unfortunately, histological classification of ciliary body medulloepithelioma does not consistently correlate with clinical behavior[25].
Primary prevention
There are currently no preventative measures for Medulloepithelioma.
Diagnosis
Diagnosis of Medulloepithelioma is based on ocular exam findings and ancillary testing. Definitive diagnosis is made by histopathological analysis of tissue sample.
History
Medulloepithelioma can affect individuals of all races and genders. Most cases are reported within the first decade. Patients usually present with secondary complaints such as visual loss or pain in either eye. A careful family history is also helpful given the known association of medulloepithelioma with the familial cancer syndrome PPB-FTDS.
Clinical Findings
Due to the slow-growing nature of medulloepithelioma, patients frequently do not experience symptoms until tumor size is clinically observable or large enough to induce secondary symptoms. Initial clinical findings may include decreased vision, elevated intraocular pressure (IOP), angle closure, leukocoria, and eye redness. Causes of visual loss include cataracts, lens subluxation, lens coloboma, retrolental membranes, or neovascular glaucoma[15][27].
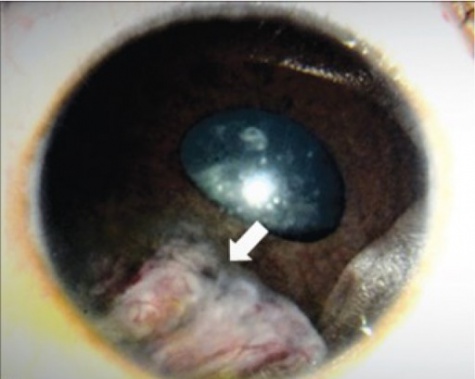
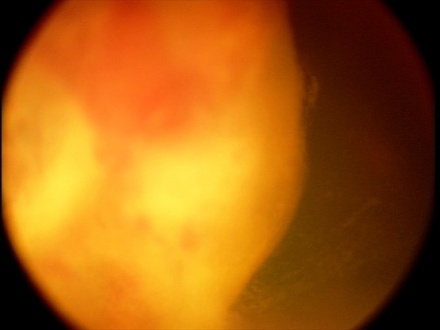
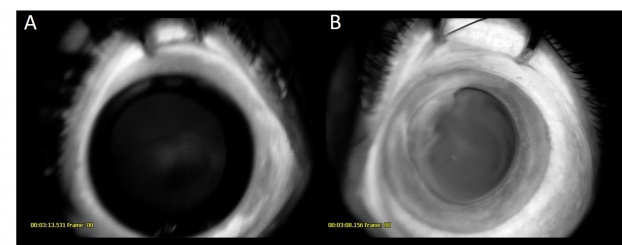
The tumor can typically be found in the ciliary body on slit lamp exam or gonioscopy and appears whitish-pink with chalky calcified opacities (Figure 3)[27] . However, cases of these tumor arising from the optic nerve have been described[28][29][30](Figure 3). Multiple grayish-white areas of cartilage or cysts may be seen within the tumor[26] (Figure 4). These cysts may dislodge and be observed floating in the anterior chamber or vitreous in up to 50% of patients[12]. One characteristic early feature, a congenital lens notch, results from the absence of zonules adjacent to the tumor.
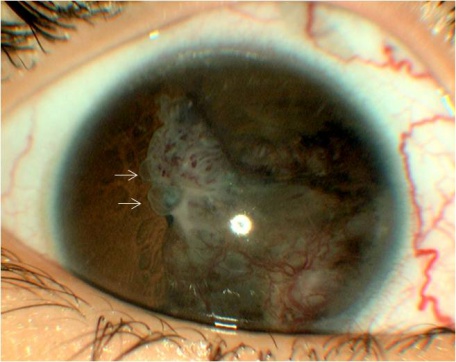
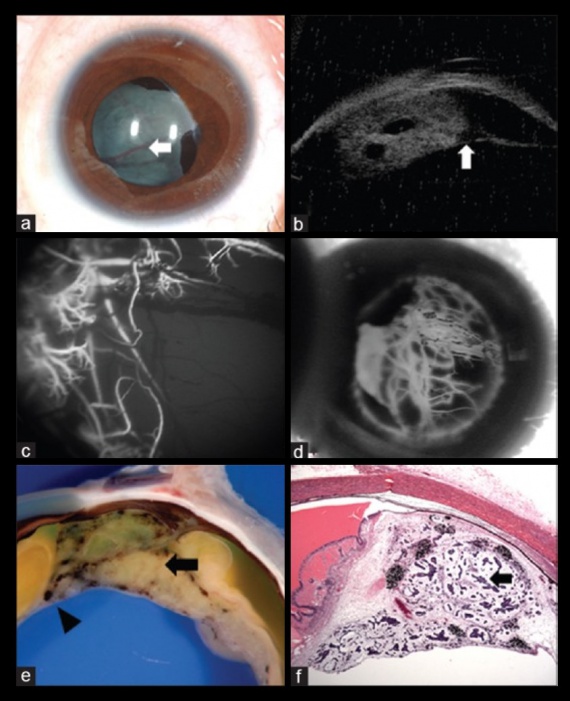
Other anterior segment findings include ectropion uveae, corectopia or iris neovascularization (Figure 5a). Neoplastic tissue growing over the anterior hyaloid and the posterior lens capsule may form a vascular retrolental membrane present in up to 60% of patients[11][12][26]. The body of the mass may also extend into the vitreous (Figure 6).
Medulloepithelioma may produce secondary effects such as unilateral cataracts and neovascular glaucoma, both of which may be seen in up to 50% of cases[11][12][23]. Secondary glaucoma is almost always due to iris neovascularization and subsequent angle closure, but direct tumor invasion into the angle may serve as another mechanism[31]. Medical treatment of secondary glaucoma should be prioritized over surgical until regression of the primary tumor is achieved[31].
Other less frequent masquerading manifestations include uveitis, hyphema, vitreous hemorrhage, retinal detachment, and extraocular extension of the tumor. The presence of these features can often delay diagnosis of the tumor. Such delay may portend poor prognosis including orbital invasion, distant metastases and even death[32][33].
Presenting phenotype may differ between congenital and childhood presentations of ciliary body medulloepithelioma. Neonatal medulloepithelioma may present in more advanced stages with leukocoria, buphthalmos, larger posterior chamber mass, and elevated IOP. Childhood medulloepithelioma may demonstrate more neovascular features, secondary glaucoma, and lens subluxation with a ciliary body mass[34].
Symptoms
Patients with medulloepithelioma present with the following symptoms (Table)[15]:
Clinical diagnosis
The diagnosis of medulloepithelioma may be suspected using a combination of patient's history, clinical exam findings, and with the help of various imaging modalities. Definitive diagnosis is made using histopathological analysis of tissue sample.
Diagnostic procedures
Ultrasound B-scan
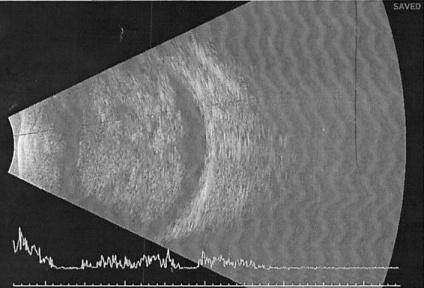
Ultrasound B-Scan shows a pattern of heterogenous areas of high internal reflectivity (Figure 7). Clear cysts may also be visualized within the mass (Figure 8b). Areas of cartilage within the tumor can produce dense echoes that may mimic the echoes produced by calcifications in retinoblastoma lesions[35]. For smaller tumors ultrasound biomicroscopy is also an effective way to determine the location and size of the tumor (Figure 8b).
Fluorescein Angiography
Fluorescein angiography demonstrates vessels arising within the tumor peripherally. These lesions demonstrate early hyperfluorescence and gradual late staining of the mass[36]. (Figure 5 c-d and Figure 8)
Magnetic Resonance Imaging (MRI)
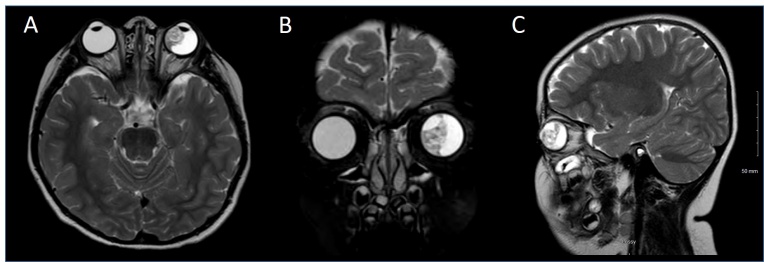
MRI is useful as a preoperative investigative modality. The tumor typically appears as a retrolental mass that is either isointense or hyperintense to the vitreous and enhances with gadolinium contrast (Figure 9). Contrast enhancement usually appears homogeneous, but may appear heterogeneous due to cysts. On T1 weighted imaging, the masses appear hypointense and on T2 weighted imaging they appear hyperintense. Lens notching or subluxation is highly suggestive of medulloepithelioma[27][37]. Extra scleral, or extraocular invasion of the tumor may also be identified on this imaging modality.
Differentiating medulloepithelioma from other intraocular tumors, particularly retinoblastoma can prove to be quite difficult on clinical assessment and imaging alone[38]. Needle biopsy can make a definitive diagnosis[39]. The histopathology of medulloepithelioma is distinctive as described above.
Laboratory testing
Laboratory testing is not routinely used for diagnosis or management.
Differential diagnosis
The differential diagnosis of medulloepithelioma is similar to that of leukocoria on presentation. Most notably it includes retinoblastoma, Coats disease, persistent hyperplastic primary vitreous and uveal melanoma. Additionally, differential diagnosis may vary based on demographics when considering ciliary body masses not causing leukocoria.
Leukocoria Differential:
- Retinoblastoma: Medulloepithelioma can be frequently misdiagnosed as retinoblastoma. Retinoblastoma is characterized by the presence of calcification, which may be confused with the presence of intratumoral cartilage in medulloepithelioma. Medulloepitheliomas can be differentiated based on their location in the ciliary body. The presence of a retrolental cyclitic membrane in medulloepithelioma serves as an important differentiating feature. Fluorescein angiography in medulloepitheliomas will demonstrate haphazard filling of vessels across the hyaloid face, whereas in retinoblastoma the vessels appear to be regular and organized. In addition, classic retinoblastoma lacks intratumoral cysts, which are a hallmark of medulloepithelioma.
- Coats Disease: Coats Disease is characterized by the presence of lipoproteinaceous exudates in the subretinal space differentiating it from medulloepithelioma[40].In addition, no calcifications are visualized in Coats Disease on imaging.
- Juvenile Xanthogranuloma (JXG): Juvenile Xanthogranuloma is characterized by a fleshy tumor restricted to the iris (unlike medulloepithelioma) associated with spontaneous hyphema.
- Persistent Hyperplastic Primary Vitreous (PHPV): The retrolental cyclitic membrane in medulloepithelioma may be confused with PHPV. The cyclitic membrane represents proliferation of a neoplastic tissue from the main tumor across the anterior vitreous face. Unlike PHPV this is not associated with a stalk extending to the optic disc.
Pediatric Ciliary Body Mass Differential[7][27]:
- Anterior Retinoblastoma
- Ciliary Body Cyst
- Leiomyoma
- Juvenile Xanthogranuloma
Adult Ciliary Body Mass Differential[7][15]:
- Ciliary Epithelial (Pigmented or Nondpigmented) Adenoma/Adenocarcinoma
- Mesoectodermal Leiomyoma
- Neurilemmoma
- Metastasis
- Ciliochoroidal Melanoma
- Granuloma (including intraocular toxocariasis)
Management
Enucleation is the standard therapy for advanced ciliary body medulloepithelioma[26]. In cases with orbital involvement an extended enucleation or exenteration may be required. Local resection may be attempted for smaller tumors occupying less than 3-4 clock hours, but high rates of recurrence resulting in secondary enucleation have been previously reported[11][12][26]. Other options for smaller tumors include cryotherapy or radiotherapy. Plaque brachytherapy has been used in small to medium sized tumors with some success[41][42][43][44]. The process is shorter, and produces better outcomes than local resection. Standard external beam radiotherapy is less effective and has been used primarily as adjuvant therapy. Intracameral and intravitreal chemotherapy were shown to achieve tumor regression in one case report[45]. In a case of orbital medulloepithelioma, an intraconal tumor was first treated with chemoradiation prior to resection, which avoided enucleation[3]. The role of systemic chemotherapy remains to be fully explored in patients with medulloepithelioma, but a combination of vincristine, etoposide, and carboplatin has been reported in one case series to prevent recurrence and metastasis for advanced medulloepithelioma patients[46].
Extra-ocular Considerations
Patients with DICER-1 mutations may require routine chest x-ray screening, CT, and ultrasonographic screening (thyroid, abdominal, and pelvic) for extra-ocular manifestations of PPB-FTDS. DICER-1 positive patients without ocular involvement should receive annual ophthalmic examination yearly from age 3 to at least age 10 . Genetic testing should be offered to "at-risk" family members[1][7][8].
Prognosis
Given the rarity of the disease, there have been few studies on the long-term prognosis in these patients. Tumors that remain confined to the globe have excellent prognosis, with a 5-year survival rate of 90-95% after enucleation[12][42]. Broughton and Zimmerman et al. reported tumor-related deaths in 4 of 33 patients (12%), all these deaths occurred in patients with malignant tumors demonstrating extraocular extension. Thus extraocular spread proves to be a major predictor of mortality in these patients. However, cases have been reported of successful management of patients with orbital extension and metastatic disease with adjuvant chemotherapy, radiotherapy and surgery[40].
References
- ↑ 1.0 1.1 Schultz KAP, Williams GM, Kamihara J, Stewart DR, Harris AK, Bauer AJ, Turner J, Shah R, Schneider K, Schneider KW, Carr AG, Harney LA, Baldinger S, Frazier AL, Orbach D, Schneider DT, Malkin D, Dehner LP, Messinger YH, Hill DA. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin Cancer Res. 2018 May 15;24(10):2251-2261. Doi: 10.1158/1078-0432.CCR-17-3089. Epub 2018 Jan 17. PMID: 29343557; PMCID: PMC6260592.
- ↑ 2.0 2.1 2.2 de Kock L, Priest JR, Foulkes WD, Alexandrescu S. An update on the central nervous system manifestations of DICER1 syndrome. Acta Neuropathol. 2020 Apr;139(4):689-701. Doi: 10.1007/s00401-019-01997-y. Epub 2019 Apr 5. PMID: 30953130.
- ↑ 3.0 3.1 Gallo RA, Shoag J, Johnson TE, Solomon DA, Perry A, Podda A, Lee JY, Rong AJ. Eye-sparing Treatment of Localized Orbital Medulloepithelioma With Neoadjuvant Chemoradiation. Ophthalmic Plast Reconstr Surg. 2021 Jan-Feb 01;37(1):e13-e16. Doi: 10.1097/IOP.0000000000001703. PMID: 32427730.
- ↑ 4.0 4.1 Cai S, Zhao W, Nie X, et al. Multimorbidity and Genetic Characteristics of DICER1 Syndrome Based on Systematic Review. Journal of pediatric hematology/oncology. 2017;39(5):355-361.
- ↑ 5.0 5.1 Ramasubramanian A, Correa ZM, Augsburger JJ, Sisk RA, Plager DA. Medulloepithelioma in DICER1 syndrome treated with resection. Eye (London, England). 2013;27(7):896-897.
- ↑ 6.0 6.1 Durieux E, Descotes F, Nguyen AM, Grange JD, Devouassoux-Shisheboran M. Somatic DICER1 gene mutation in sporadic intraocular medulloepithelioma without pleuropulmonary blastoma synrome. Human pathology. 2015;46(5):783-787.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Schultz KAP, Stewart DR, Kamihara J, et al. DICER1 Tumor Predisposition. 2014 Apr 24 [Updated 2020 Apr 30]. In: Adam MP, Everman DB, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022. Available from: https://www-ncbi-nlm-nih-gov.proxygw.wrlc.org/books/NBK196157/
- ↑ 8.0 8.1 8.2 Hurlyn LA, Turriff A, Harney LA, et al. Dicer1 Syndrome: Characterization of the ocular phenotype in a family-based cohort study. Ophthalmology. 2019 Feb; 126(2): 296-304. https://doi.org/10.1016/j.ophtha.2018.09.038
- ↑ Sahm F, Jakobiec FA, Meyer J, Schrimpf D, Eberhart CG, Hovestadt V, Capper D, Lambo S, Ryzhova M, Schüller U, Zheludkova O, Kumirova E, Lichter P, von Deimling A, Jones DT, Pfister SM, Kool M, Korshunov A. Somatic mutations of DICER1 and KMT2D are frequent in intraocular medulloepitheliomas. Genes Chromosomes Cancer. 2016 May;55(5):418-27. Doi: 10.1002/gcc.22344. Epub 2016 Feb 4. PMID: 26841698.
- ↑ Minoda K, Hirose Y, Sugano I, Nagao K, Kitahara K. Occurrence of sequential intraocular tumors: malignant medulloepithelioma subsequent to retinoblastoma. Japanese journal of ophthalmology. 1993;37(3):293-300.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Broughton WL, Zimmerman LE. A clinicopathologic study of 56 cases of intraocular medulloepitheliomas. American journal of ophthalmology. 1978;85(3):407-418.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 Kaliki S, Shields CL, Eagle RC, Jr., et al. Ciliary body medulloepithelioma: analysis of 41 cases. Ophthalmology. 2013;120(12):2552-2559.
- ↑ Sansgiri RK, Wilson M, McCarville MB, Helton KJ. Imaging features of medulloepithelioma: report of four cases and review of the literature. Pediatric radiology. 2013;43(10):1344-1356.
- ↑ Shields JA, Eagle RC, Jr., Ferguson K, Shields CL. TUMORS OF THE NONPIGMENTED EPITHELIUM OF THE CILIARY BODY: The Lorenz E. Zimmerman Tribute Lecture. Retina (Philadelphia, Pa). 2015;35(5):957-965.
- ↑ 15.0 15.1 15.2 15.3 Tadepalli SH, Shields CL, Shields JA, Honavar SG. Intraocular medulloepithelioma – A review of clinical features, DICER 1 mutation, and management. Indian journal of ophthalmology. 2019;67(6):755-762.
- ↑ 16.0 16.1 Takei, H., Florez, L., Moroz, K. and Bhattacharjee, M.B. (2007), Medulloepithelioma: Two unusual locations. Pathology International, 57: 91-95. https://doi-org.proxygw.wrlc.org/10.1111/j.1440-1827.2006.02062.x
- ↑ 17.0 17.1 Becker LE, Sharma MC, Rorke LB. Medulloepithelioma. In: Kleihues P, Cavenee WK, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press, 2000; 124–6.
- ↑ Saunders T, Margo CE. Intraocular medulloepithelioma. Archives of pathology & laboratory medicine. 2012;136(2):212-216.
- ↑ Bailey PCushing HA classification of the gliomataBailey PCushing HClassification of Tumors of the Glioma on a Histogenetic Basis, with a Correlated Study of Prognosis.1926546
- ↑ 20.0 20.1 Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016 Jun;131(6):803-20. Doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9. PMID: 27157931.
- ↑ Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021 Aug 2;23(8):1231-1251. Doi: 10.1093/neuonc/noab106. PMID: 34185076; PMCID: PMC8328013.
- ↑ Korshunov A, Jakobiec FA, Eberhart CG, Hovestadt V, Capper D, Jones DT, Sturm D, Stagner AM, Edward DP, Eagle RC, Proia AD, Koch A, Ryzhova M, Ektova A, Schüller U, Zheludkova O, Lichter P, von Deimling A, Pfister SM, Kool M. Comparative integrated molecular analysis of intraocular medulloepitheliomas and central nervous system embryonal tumors with multilayered rosettes confirms that they are distinct nosologic entities. Neuropathology. 2015 Dec;35(6):538-44. doi: 10.1111/neup.12227. Epub 2015 Jul 16. PMID: 26183384.
- ↑ 23.0 23.1 Saunders T, Margo CE. Intraocular medulloepithelioma. Archives of pathology & laboratory medicine. 2012;136(2):212-216.
- ↑ Stagner AM, Jakobiec FA. Updates on the Molecular Pathology of Selected Ocular and Ocular Adnexal Tumors: Potential Targets for Future Therapy. Semin Ophthalmol. 2016;31(1-2):188-96. Doi: 10.3109/08820538.2015.1115257. PMID: 26959146.N
- ↑ 25.0 25.1 Verdijk RM. On the Classification and Grading of Medulloepithelioma of the Eye. Ocul Oncol Pathol. 2016 Apr;2(3):190-3. Doi: 10.1159/000443963. Epub 2016 Feb 13. PMID: 27239464; PMCID: PMC4881278.
- ↑ 26.0 26.1 26.2 26.3 26.4 Shields JA, Eagle RC, Jr., Shields CL, Potter PD. Congenital neoplasms of the nonpigmented ciliary epithelium (medulloepithelioma). Ophthalmology. 1996;103(12):1998-2006.
- ↑ 27.0 27.1 27.2 27.3 27.4 Vajaranant TS, Mafee MF, Kapur R, Rapoport M, Edward DP. Medulloepithelioma of the Ciliary Body and Optic Nerve: Clinicopathologic, CT, and MR Imaging Features. Neuroimaging Clinics of North America. 2005;15(1):69-83.
- ↑ Corrêa ZM, Augsburger JJ, Spaulding AG. Medulloepithelioma of the optic disc. Hum Path. 2011;42(12):2047-51.
- ↑ Bakhshi S, Bhat GM, Sen S, Sharma S, Sharma DN. Malignant medulloepithelioma of optic nerve head. J Pediatr Hematol Oncol. 2008;30(6):478-80.
- ↑ Chidambaram B, Santosh V, Balasubramaniam V. Medulloepithelioma of the optic nerve with intradural extension–report of two cases and a review of the literature. Childs Nerv Syst. 2000;16(6):329-33.
- ↑ 31.0 31.1 Camp DA, Yadav P, Dalvin LA, Shields CL. Glaucoma secondary to intraocular tumors: mechanisms and management. Curr Opin Ophthalmol. 2019 Mar;30(2):71-81. Doi: 10.1097/ICU.0000000000000550. PMID: 30562240.
- ↑ Zhou L, Meng Y, Yang H, et al. [A teratoid malignant medulloepithelioma of the optic nerve]. Yanke xue bao = Eye science. 2008;24(1):68-70.
- ↑ Sosinska-Mielcarek K, Senkus-Konefka E, Jaskiewicz K, Kordek R, Jassem J. Intraocular malignant teratoid medulloepithelioma in an adult: clinicopathological case report and review of the literature. Acta ophthalmologica Scandinavica. 2006;84(2):259-262.
- ↑ Barchitta M, De Francesco S, Menicacci C, Girolamo MM, Salvoldi F, Hadjistilianou D. Medulloepithelioma in newborn: Advanced clinical status. Eur J Ophthalmol. 2022 Nov;32(6):NP73-NP77. Doi: 10.1177/11206721211019579. Epub 2021 May 31. PMID: 34053319.
- ↑ Hennis HL, Saunders RA, Shields JA. Malignant teratoid medulloepithelioma of the ciliary body. Journal of clinical neuro-ophthalmology. 1990;10(4):291-292.
- ↑ Sharma P, Shields CL, Turaka K, Eagle RC, Jr., Shields JA. Ciliary body medulloepithelioma with neoplastic cyclitic membrane imaging with fluorescein angiography and ultrasound biomicroscopy. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249(8):1259-1261.
- ↑ Galluzzi P, Casseri T, Cerase A, Guglielmucci D, Toti P, Hadjistilianou T. Conventional, diffusion, and permeability findings in ocular medulloepithelioma. Neuroradiology. 2018;60:1213-1222.
- ↑ Lee J, Choung HK, Kim YA, Kim N, Khwarg SI. Intraocular medulloepithelioma in children: clinicopathlologic features itself hardly differentiate it from retinoblastoma. Int J Ophthalmol. 2019;12(7):1227-1230.
- ↑ Potter PD, Shields CL, Shields JA, Flanders AE. The role of magnetic resonance imaging in children with intraocular tumors and simulating lesions. Ophthalmology. 1996;103(11):1774-1783.
- ↑ 40.0 40.1 Viswanathan S, Mukul D, Qureshi S, Ramadwar M, Arora B, Kane SV. Orbital medulloepitheliomas – with extensive local invasion and metastasis: a series of three cases with review of literature. International journal of pediatric otorhinolaryngology. 2008;72(7):971-975.
- ↑ Davidorf FH, Craig E, Birnbaum L, Wakely P, Jr. Management of medulloepithelioma of the ciliary body with brachytherapy. American journal of ophthalmology. 2002;133(6):841-843.
- ↑ 42.0 42.1 Ang SM, Dalvin LA, Emrich J, Komarnicky L, Shields JA, Shields CL. Plaque Radiotherapy for Medulloepithelioma in 6 Cases From a Single Center. Asia-Pacific journal of ophthalmology (Philadelphia, Pa). 2019;8(1):30-35.
- ↑ Attiku Y, Rishi P, Biswas J, Krishnakumar S. Plaque radiation for ciliary body medulloepithelioma presenting with neovascular glaucoma and vitreous hemorrhage in 13-year-old Asian girl. American Journal of Ophthalmology Case Reports. 2020;18:100719.
- ↑ Poon DS, Reich E, Smith VM, Kingston J, Reddy MA, Hungerford JL, Sagoo MS. Ruthenium-106 Plaque Brachytherapy in the Primary Management of Ocular Medulloepithelioma. Ophthalmology. 2015 Sep;122(9):1949-51. doi: 10.1016/j.ophtha.2015.03.002. Epub 2015 Apr 8. PMID: 25863421.
- ↑ Stathopoulos C, Gaillard MC, Schneider J, Munier FL. Successful treatment of ciliary body medulloepithelioma with intraocular melphalan chemotherapy: a case report. BMC Ophthalmology. 2020;20(239).
- ↑ Sheriff, I.H.N., Karaa, E.K., Chowdhury, T. et al. Systemic adjuvant chemotherapy for advanced malignant ocular medulloepithelioma. Eye (2022). https://doi.org/10.1038/s41433-022-01936-4