Enucleation
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Enucleation is the surgical procedure that involves removal of the entire globe and its intraocular contents, with preservation of all other periorbital and orbital structures. Enucleation is in contrast to evisceration, in which the ocular contents are removed from an intact sclera, and exenteration, in which the entire orbital contents, including the globe and soft tissues, are removed.
Background
The surgical removal of the eye was first reported in the 1500s as a procedure known as extirpation.[1] Unlike an enucleation, the conjunctiva and extraocular muscles were not spared. By the mid 1800s, an enucleation without implant placement was described in the literature. The first reports of implant insertion following enucleation were described in 1886 and 1887, with variable success in implant retention.[1]
Indications
The following are indications for enucleation:
- Intraocular malignancy or high suspicion for intraocular malignancy (most commonly uveal melanoma and retinoblastoma)
- Trauma without visual potential
- Blind, painful eye
- Severe infection without visual potential
- Sympathetic ophthalmia
- Microphthalmos
The role of primary enucleation in acute trauma remains controversial, particularly when a patient's mental status may be altered and/or they are unable to consent. Many surgeons advocate for primary closure of an open globe with later consideration for an enucleation if the eye remains no light perception or becomes painful without vision. Enucleation of an eye can be associated with significant psychological trauma, and primary globe repair gives the patient time to consider their options and the pros and cons of enucleation after the initial trauma. In addition, this allows any altered mental status to resolve and provides autonomy to elect this operation in the future. The rare risk of sympathetic ophthalmia in the uninvolved eye must be considered and discussed with the patient. In select cases where the eye is determined to have no visual potential, when repair or at least primary closure of the globe is determined to be impossible, and/or the medical comorbidities of the patient are significant, the surgeon may elect to perform primary enucleation.
Advantages
In contrast to evisceration, enucleation allows for histologic examination of an intact globe and optic nerve. This is particularly important in settings of biopsy-proven or suspected intraocular malignancy, in which it is essential to determine the margins of the malignancy and invasion of the optic nerve, if any.
When comparing the aesthetics of an enucleated socket to an eviscerated socket, one retrospective study showed no statistically significant difference between enucleation and evisceration patients graded by both patients and masked observers.[2]
Enucleation classically has been thought to decrease the risk of sympathetic ophthalmia as it avoids exposure to uveal antigens that may occur during an evisceration.[3] However, more recent studies have reported no cases of sympathetic ophthalmia following evisceration.[4][5] In addition, the classic teaching was that a severely traumatized eye with no vision potential should be removed with 14 days to prevent sympathetic ophthalmia. However, this rule has been shown to be arbitrary and not scientifically supported[6]
Disadvantages
A reduction in implant motility is often noted in enucleations. Compared to patients who underwent evisceration, one study found enucleation patients to have statistically significant poorer implant motility.[2] However, no difference in prosthesis motility was noted between evisceration and enucleation patients.
In a 2003 survey of all board-certified ocularists in the United States, 92% reported preferring evisceration to enucleation for a patient who required removal of the eye.[7] Eighty-two percent of survey respondents believed that evisceration afforded the best ocular motility and best overall cosmetic outcome, and 94% percent believed that complications of enophthalmos and/or deep superior sulcus were more common after enucleations.
Surgical Technique
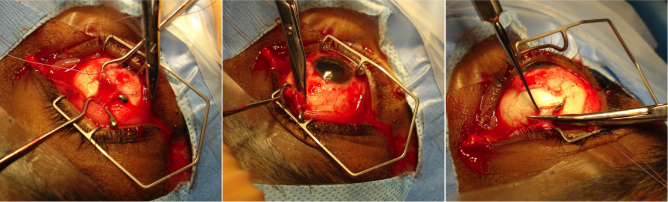
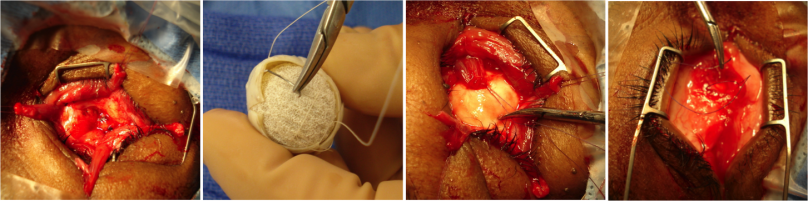
An enucleation is often performed as an outpatient procedure under general anesthesia. A retrobulbar block of local anesthetic with epinephrine is administered to aid in hemostasis and postoperative pain management. After a time-out is performed to confirm the correct operative eye with the entire operating room team, the face is prepared and draped in sterile fashion. A limbal conjunctival peritomy is performed with Wescott scissors for 360 degrees. Blunt dissection in the sub-Tenon's plane is then carried out in each of the oblique quadrants. Each rectus muscle is then identified, isolated with a muscle hook, secured with suture, and cut at the insertion to the globe. The superior and inferior oblique muscles are isolated and generally transected, though some surgeons prefer to tag these as well. Conversely, some surgeons prefer to secure the muscles with suture after the eye has been removed.[8]
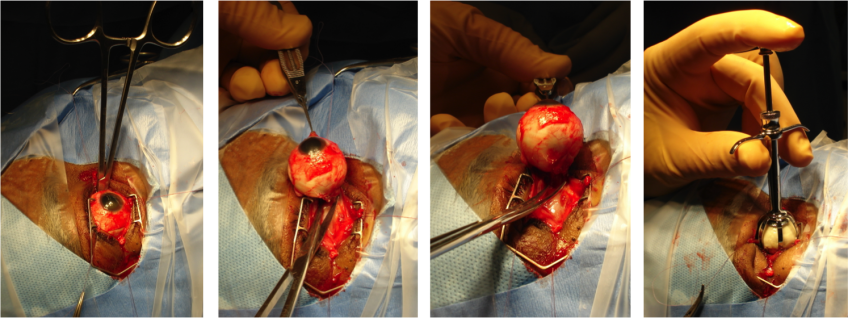
Once the globe is determined to rotate freely, the optic nerve is identified, strummed, and cut with enucleation scissors or an enucleation snare wire. Some surgeons prefer to first clamp the optic nerve with a curved hemostat prior to transection to encourage further hemostasis. An attempt should be made to cut a long segment of the optic nerve, particularly in situations of intraocular malignancy where histologic examination of the optic nerve is crucial. Additional hemostasis is then achieved with direct pressure in the intraconal space and cautery of the optic nerve if needed.
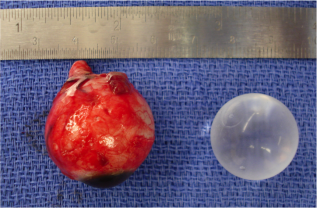
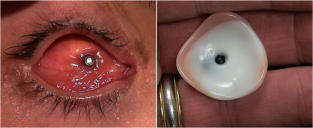
An implant is then placed in the intraconal space to replace volume lost by the enucleated globe, achieve cosmetic symmetry with the fellow socket, and allow for motility of the prosthesis. To determine appropriate diameter of the implant, use of the formula axial length-2 mm has been shown to provide for adequate replacement of lost volume and minimize superior sulcus deformity and enophthalmos.[9] A sizing set can also be used to determine the appropriate size intraoperatively to ensure there is not too much tension when closing.
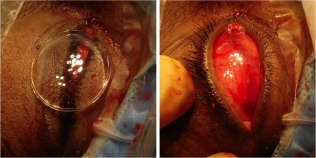
In certain circumstances including severe infection, a surgeon may choose not to place an implant at the time of enucleation and elect to place an implant in a second surgery. The extraocular muscles are generally attached to each other anterior to the implant or sutured directly to a porus or wrapped implant. A two layered closure is then carried out with absorbable sutures, first of Tenon’s capsule and then of the conjunctiva. A three layered closure can be performed as well closing posterior Tenon's first. Antibiotic ointment is applied, a clear plastic conformer is placed over the closed conjunctiva, and a pressure patch is placed over the socket for 2-3 days depending on surgeon preference. A temporary tarsorrhaphy may be placed as well to be removed in 5-7 days once again depending on surgeon preference.
Implants
There are several types of implants that may be utilized in an enucleation. Implants are made in distinct sizes, and intraoperative selection of the appropriately sized implant is determined by the size of the patient’s orbit and the size of the implant necessary to achieve symmetry with the fellow eye. Implants may be porous or nonporous. Porous implants allow for anchoring of the extraocular muscles with proliferation of fibrovascular tissues into the implant itself. These include hydroxyapatite, porous polyethylene, and proplast. Some considerations regarding time of implant can include presence of infection and/or fracture(s).
Hydroxyapatite implants were first introduced in 1989.[10] Because of their rough surface, they are typically wrapped with material such as donor sclera, acellular dermis, or pericardium. Additional wrapping materials include autologous tissue grafts, such as temporalis fascia or fascia lata and synthetic meshes. The extraocular muscles may be then sutured to the wrapping material for enhanced motility of the implant. Porous polyethylene implants were later developed as an alternative to hydroxyapatite. They have a smoother surface and do not require wrapping. Other advantages of porous polyethylene over hydroxyapatite implants include cheaper cost and ability to suture the extraocular muscles directly to the implant.[3]
Implants may be pegged, in which a hole is drilled into the implant where a peg can be placed that attaches to the prosthesis. Pegging is typically performed six to twelve months postoperatively and allows for increased motility of the prosthesis.[3] Compared to unpegged porous implants, pegged orbital implants were found in one study to produce a statistically significant improvement in horizontal but not vertical motility.[11] Pegged implants were found to retain 87% of motility of the fellow eye while unpegged implants were found to retain only 50% of motility of the fellow eye.
In one series of 802 patients who underwent evisceration, enucleation, or placement of a secondary implant where a hydroxyapatite implant was used, 156 of 353 (44%) of patients with pegged implants had a complication. The most common complication in this series was peg extrusion in 20% of cases, which was shown to occur less frequently with titanium pegs as opposed to other types of pegs.[12] In another retrospective series of patients with pegged hydroxyapatite implants, complications were noted in 38% (62/165) of patients. Complications of pegging included discharge (most common), pyogenic granulomas, loss of peg, poor transfer of movement, and audible click.[13] Two patients (3%) had implant infection that required removal of the implant. Because of these complications, pegging has fallen out of favor and is now utilized less commonly.
Nonporous implants do not allow for proliferation of tissues into the implant. Therefore, they may have decreased motility and greater risk of implant migration, yet several studies indicate no problems with implant migration with appropriate technique. [14][15][16]Types of nonporous implants include glass, silicone, acrylic, and polymethylmethacrylate (PMMA).[3] The rectus muscles still may be sutured over the implant to impart motility to the implant and prosthesis, or suture to a wrapped material. Nonporous implants may be advantageous in the setting of increased infection risk, including in patients with comorbidities such as diabetes, and in setting of trauma.
In a survey published in 2004 of active members of the American Society of Ophthalmic Plastic and Reconstructive Surgery (ASOPRS) regarding management trends and preferences in primary enucleations and eviscerations, the most popular and commonly used implant was porous polyethylene in 43% of cases, followed by hydroxyapatite (27%) and nonporous implants (20%).[17] The most commonly cited rationale for implant choice was outcome. Pegs were utilized in only 8% of cases. A majority of implants were not wrapped, but, of those that were, donor sclera was the most commonly utilized wrapping material.
An autologous dermis fat graft may be alternatively used for primary or secondary socket reconstruction. (See https://eyewiki.aao.org/Dermis_Fat_Graft).
Postoperative Management
Patients are typically patched for a brief period postoperatively and instructed to return to clinic one week after the procedure. Prescriptions are given for analgesics and antiemetics. Some surgeons also elect to prescribe prophylactic antibiotics, although there is no evidence that antibiotics reduce the risk of implant infection.[18][19][20]
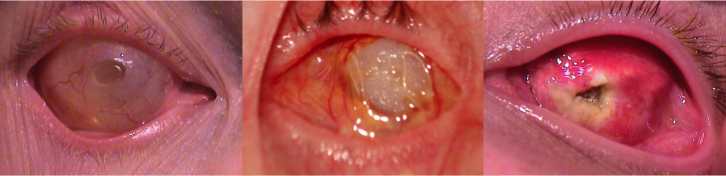
Once the conjunctiva closure has healed, generally about 4-8 weeks postoperatively, patients are referred to an ocularist for fitting of an ocular prosthetic fitting. Patients will require regular follow up with both an oculoplastic surgeon and an ocularist to maintain the health of their socket.
Complications
Complications of an enucleation include the following:
Intraoperative:
- Removal of the wrong eye
- Damage to or loss of extraocular muscles
- Hemorrhage
- Perforation of eye
Postoperative:
- Infection
- Hemorrhage
- Wound dehiscence
- Extrusion of the conformer
- Contraction of the fornices
- Exposure of the implant
- Extrusion of the implant
- Migration of the implant
- Ptosis
- Pain
- Ectropion
- Entropion
- Hollow or deep superior sulcus
- Poorly fitting prosthesis
- Enophthalmos
- Socket contracture
- Orbital cellulitis
Acknowledgements
Thank you to Oculoplastics Associates of Texas, Dallas, TX for providing article images.
References
- ↑ 1.0 1.1 Sami D, Young S, Petersen R. Perspective on orbital enucleation implants. Surv Ophthalmol 2007;52(3):244-65.
- ↑ 2.0 2.1 Nakra T, Simon GJ, Douglas RS, Schwarcz RM, McCann JD, Goldberg RA. Comparing outcomes of enucleation and evisceration. Ophthalmology 2006;113(12):2270-5.
- ↑ 3.0 3.1 3.2 3.3 Moshfeghi DM, Moshfeghi AA, Finger PT. Enucleation. Surv Ophthalmol 2000;44(4):277-301.
- ↑ Levine MR, Pou CR, Lash RH. The 1998 Wendell Hughes Lecture. Evisceration: is sympathetic ophthalmia a concern in the new millennium? Ophthal Plast Reconstr Surg 1999;15(1):4-8.
- ↑ Zheng C, Wu AY. Enucleation versus evisceration in ocular trauma: a retrospective review and study of current literature. Orbit 2013;32(6):356-61
- ↑ Jordan, David R. M.D., F.A.C.S., F.R.C.S.C.*; J. Dutton, Jonathan M.D., Ph.D., F.A.C.S.†. The Ruptured Globe, Sympathetic Ophthalmia, and the 14-Day Rule. Ophthalmic Plastic and Reconstructive Surgery 38(4):p 315-324, July/August 2022. | DOI: 10.1097/IOP.0000000000002068
- ↑ Timothy NH, Freilich DE, Linberg JV. Evisceration versus enucleation from the ocularist's perspective. Ophthal Plast Reconstr Surg 2003;19(6):417-20; discussion 20.
- ↑ Jordan DR, Stoica B, Dutton JJ. The Hook and Release Technique During Enucleation Surgery. Ophthalmic Last Reconstr Surg 2018;34(1):31-36.
- ↑ Kaltreider SA, Lucarelli MJ. A simple algorithm for selection of implant size for enucleation and evisceration: a prospective study. Ophthal Plast Reconstr Surg 2002;18(5):336-41.
- ↑ Custer PL. Enucleation: past, present, and future. Ophthal Plast Reconstr Surg 2000;16(5):316-21.
- ↑ Guillinta P, Vasani SN, Granet DB, Kikkawa DO. Prosthetic motility in pegged versus unpegged integrated porous orbital implants. Ophthal Plast Reconstr Surg 2003;19(2):119-22.
- ↑ Yoon JS, Lew H, Kim SJ, Lee SY. Exposure rate of hydroxyapatite orbital implants a 15-year experience of 802 cases. Ophthalmology 2008;115(3):566-72 e2.
- ↑ Jordan DR, Chan S, Mawn L, et al. Complications associated with pegging hydroxyapatite orbital implants. Ophthalmology 1999;106(3):505-12.
- ↑ Nunery WR, Cepela MA, Heinz GW, Zale D, Martin RT. Extrusion rate of silicone spherical anophthalmic socket implants. Ophthalmic Plast Reconstr Surg 1993;9(2):90-5.
- ↑ Massry GG, Holds JB. Evisceration with scleral modification. Ophthalmic Plast Reconstr Surg 2001;17(1):42-7.
- ↑ Wladis EJ, Aakalu VK, Sobel RK, Yen MT, Bilyk JR, Mawn LA. Orbital Implants in Enucleation Surgery: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018 Feb;125(2):311-317. doi: 10.1016/j.ophtha.2017.08.006. Epub 2017 Sep 9. PMID: 28899574.
- ↑ Su GW, Yen MT. Current trends in managing the anophthalmic socket after primary enucleation and evisceration. Ophthal Plast Reconstr Surg 2004;20(4):274-80.
- ↑ Pariseau B, Fox B, Dutton JJ. Prophylactic Antibiotics for Enucleation and Evisceration: A Retrospective Study and Systematic Literature Review. Ophthal Plast Reconstr Surg 2018;34(1):49-54.
- ↑ Fay A, Nallasamy N, Pemberton JD, Callahan A, Wladis EJ, Nguyen J, Durand ML; New England Oculoplastics Society Study Group.. Ophthal Plast Reconstr Surg 2013;29(4):281-5.
- ↑ Fay A, Nallasamy N, Allen RC, Bernardini FP, Bilyk JR, Cockerham K, Cruz AA, Devoto M, Dolman PJ, Dutton JJ, Jordan DR, Kersten R, Kim YD, Lucarelli MJ, McNab AA, Mombaerts I, Mourits M, Nerad J, Perry JD, Rose G, Saeed P, Seah LL, Selva D, Sivak-Callcott J, Strianese D, Verity DH; Orbital Society. Perioperative Prophylactic Antibiotics in 1,250 Orbital Surgeries. Ophthalmic Plast Reconstr Surg. 2020 Jul/Aug;36(4):385-389.