Retinoblastoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Retinoblastoma is an intraocular malignancy with primitive neuroendocrine origins that primarily affects young children.
Incidence
Retinoblastoma is the most common intraocular malignancy afflicting children. The estimated incidence varies by country from 3.4 to 42.6 cases per million live births. Within the United States, the incidence is 11.8 cases per million live births among children less than 5 years of age, and retinoblastoma represents 6.1% of all cancers in this age group.[1] Retinoblastoma typically affects young children with the highest incidence in patients less than 4 years old.[2] It occurs equally in males and females. On presentation, approximately 60% of cases are unilateral, and the remaining 40% are bilateral.[3] Patients diagnosed with retinoblastoma are categorized by whether the mutation is germline or somatic. Patients with bilateral presentation are presumed to have germline mutations, and although most unilateral cases have somatic mutations, 15% of unilateral cases will still have germline mutations.[2]
Genetics and Terminology
For decades, it has been known that there is a genetic component to this condition. The retinoblastoma susceptibility gene RB1 is a tumor-suppressing gene with a regulatory function in the cell division cycle. The RB1 gene encodes a protein Rb which, when activated, targets the E2F family of transcription factors and prevents the transcription of genes necessary for G1 to S phase transition.[4] It is located on subband 13q14.2. Both alleles of the retinoblastoma gene have to be inactivated for tumor development.[2]
Knudson described the "two-hit" mechanism for tumorigenesis in patients with retinoblastoma. Two active copies of the retinoblastoma gene are normally carried in human cells. Both copies must be mutated to lead to the development of retinoblastoma. The initial mutation inactivates one copy of the gene. This mutation may occur in somatic or germline cells. The second mutation occurs in somatic cells. In the majority of cases, this occurs through loss of heterozygosity.
If the first mutation occurs in germline cells - egg or sperm - before conception, the patient has heritable retinoblastoma because the patient has "inherited" the first Rb mutation from mother or father and subsequently the patient may pass on this Rb mutation through the patient's own germline cells. Heritable forms of retinoblastoma may be passed from parents to children through germline mutations. Germline cases represent just over 1/3 of retinoblastoma cases.[2] Patients with germline disease tend to have bilateral and multifocal tumors but may more rarely have unilateral disease. They also have a significantly increased risk for secondary tumors including primitive neuroendocrine tumors in the brain (so called “trilateral retinoblastoma” with pinealoblastoma). Finally, their offspring will have an increased risk of developing retinoblastoma, as this trait is transmitted in an "autosomal dominant" fashion with high penetrance (90%).[3] If the first and subsequently second RB gene mutations occur in a somatic cell of the developing retina and cause intraocular retinoblastoma while that patient's germline cells have normal RB genes, such a patient's retinoblastoma is not heritable and not germline but rather somatic and sporadic.
Most unilateral tumors are sporadic and nonhereditary, but this is not always the case. About 15% of unilateral cases occur in individuals who have germline mutations.[2] In addition, some patients may present initially with unilateral disease but subsequently progress to bilateral involvement. Thus, determining whether the initial mutation is germline is important in the clinical management of these patients, and regular and thorough examinations of both eyes are mandatory. Genetic testing for retinoblastoma patients is important to determine the status of the RB1 gene in the germline.
Genetic testing for RB1 mutations is recommended for all cases of retinoblastoma if possible. Bilateral cases will most likely be germline positive for RB1 mutations or more rarely demonstrate mosaicism where some somatic cells are normal and others carry RB1 mutations. Unilateral cases are more often sporadic and somatic retinoblastoma without germline RB1 mutations. However as mentioned above, about 15% of unilateral cases have a germline RB1 mutation. Patients with genetically detected RB1 mutations have greater risk of other malignancies later in life and require more vigilant screening for new retinoblastoma activity in both eyes as well as periodic MRI screening for primitive neuroendocrine tumor (PNET) activity; these patients with genetically detected RB1 mutations also have a 50% chance of passing the RB1 mutation as heritable retinoblastoma for each child they may conceive. For germline positive cases, family members may also be considered for testing to assess retinoblastoma associated risk in each family member. Patients without genetically detected RB1 mutations may have risks similar to the general population of other cancers later in life; these patients with a negative RB1 genetic test do not require retinoblastoma re-examinations of the fellow known normal eye with such frequency as those with positive RB1 genetic tests but should have routine eye exams per standard care.
Classification System
There are two commonly use classification systems for intraocular retinoblastoma. In the 1950's, the Reese-Ellsworth classification system (see Table 1) was developed to predict the prognosis after treatment with radiation:
| Group 1: Very Favorable |
|---|
| a. Solitary tumor less than 4-disc diameter (DD) in size, at or behind equator.
b. Multiple tumors, none over 4 DD in size, all at or behind equator. |
| Group 2: Favorable |
| a. Solitary tumor, 4 to 10 DD in size, at or behind equator.
b. Multiple tumors, 4 to 10 DD in size, behind equator. |
| Group 3: Doubtful |
| a Any tumor anterior to equator.
b. Solitary tumor, larger than 10 DD, behind equator. |
| Group 4: Unfavorable |
| a. Multiple tumors, some larger than 10 DD in size.
b. Any lesion extending anteriorly to the ora serrata. |
| Group 5: Very Unfavorable |
| a. Massive tumor involving over half the retina.
b. Vitreous seeding. |
In the 1990s, the preferred treatment modality shifted from external beam radiation to chemotherapy due to the increased risk of secondary tumors following radiation in patients with retinoblastoma. Clinicians found the Reese-Ellsworth classification system no longer accurately reflected prognosis with the newer treatment modalities. Thus, the International Intraocular Retinoblastoma Classification (IIRC) scheme, was developed. Shields and colleagues later developed a modified scheme, International Classification of Retinoblastoma (ICRB) (see Table 2), which differed from the IIRC classification in the definitions of the advanced groups (i.e., Group D and E). In a non-comparative interventional case series, the ICRB classification better predicted those with intraocular retinoblastoma who were likely to be cured without the need for enucleation or external-beam radiation treatment:
| Group A | Small intraretinal tumors (< 3mm) away from foveola and disc. |
|---|---|
| Group B | Tumors > 3mm, macular or juxtapapillary location, or with subretinal fluid. |
| Group C | Tumor with focal subretinal or vitreous seeding within 3mm of tumor. |
| Group D | Tumor with diffuse subretinal or vitreous seeding > 3mm from tumor. |
| Group E | Extensive retinoblastoma occupying >50% of the globe with or without neovascular glaucoma, hemorrhage, extension of tumor to optic nerve or anterior chamber. |
Pathology
Retinoblastoma cells are small and stain blue with hematoxylin and eosin (H&E) stain. Rings of cells surrounding an empty lumen are known as Flexner-Wintersteiner rosettes. They are characteristic but not mandatory to make a diagnosis of retinoblastoma. Homer Wright pseudorosettes, a ring of cells with an eosinophilic fibrillary center, are also commonly found. Fleurettes are retinoblastoma cells that have undergone greater photoreceptor differentiation. Calcification is common in these tumors. Necrosis is also very common and occurs when the tumor outgrows its vascular supply. Necrotic cells appear pink on H&E staining.
Diagnosis
Screening
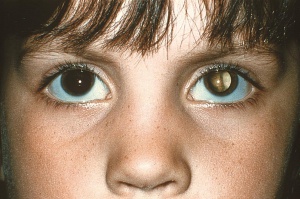
The American Academy of Pediatrics policy statement on Red Reflex Examinations in Neonates, Infants, and Children[5] recommends that all neonates, infants, and children should have an examination of the red reflex before discharge from the neonatal nursery and at all subsequent routine health supervision visits. The red reflex test is performed in a dimly lit or dark room with a direct ophthalmoscope or a retinoscope from about 1-1.5 feet from the patient. Leukocoria (whitening of the red reflex) is the most common presentation of retinoblastoma, and all infants or children with an abnormal red reflex require immediate referral to an ophthalmologist skilled in pediatric examinations.
Newborn screening is of greatest utility in offspring and siblings of patients with retinoblastoma. Offspring and siblings of affected patients require regular screening examinations in childhood. Genetic counseling for families with known retinoblastoma can help to determine whether other family members are at risk for developing disease. Future parents with a family history of retinoblastoma should be strongly advised to undergo genetic testing for RB1 mutations. Familial retinoblastoma exhibits an autosomal inheritance pattern; if one parent harbors a mutant RB1 allele, there is a 45% chance that will inherit a mutated allele.[6] Early prenatal screening methods include preimplantation genetic testing, early prenatal testing, and late prenatal testing. The choice and associated invasiveness of these techniques is dependent on the gestational age. Preimplantation genetic testing occurs in conjunction with in-vitro fertilization (IVF) and can accurately detect the presence of mutant RB1 alleles prior to embryo transfer, allowing for couples to select an embryo without the inherited mutation for implantation.[6] Cell-free fetal DNA (cffDNA) testing is a non-invasive test that can be performed as early as 8 weeks of gestation and is a definitive diagnosis. Fetal DNA originating from placental trophoblasts is isolated from maternal blood samples and is examined for the presence of RB1 mutant alleles.[6] Chorionic villi sampling (CVS) and amniocentesis, which can be performed in the second trimester, are invasive methods for prenatal testing. CVS and amniocentesis involve the trans-abdominal insertion of a needle into the uterus under ultrasound guidance to sample the placental tissue or amniotic fluid, respectively. CVS may also be performed via a trans-cervical approach. Both procedures carry the risk of pregnancy loss, bleeding, infection, rupture of membranes, or uncertain results and many families may elect to defer prenatal testing. Finally, late prenatal screening may be performed using fetal ultrasound or MRI. However, these methods can only confirm RB1 if it appears in utero.
Clinical Presentation
Early diagnosis of retinoblastoma can maximize the patient's visual prognosis as well as survival rate. Retinoblastoma must always be in the differential diagnosis for any child who presents with strabismus, leukocoria, a red eye, or a cellulitis-like picture. Retinoblastoma is the most important differential diagnosis for a child with leukocoria. Leukocoria, or the loss of the red reflex, is secondary to this tumor filling the globe. Strabismus can also be a presenting sign of retinoblastoma so all patients undergoing a strabismus evaluation should also receive a dilated funduscopic examination.
In rare cases, retinoblastoma can present with pain and inflammation and have a similar presentation to endophthalmitis, uveitis, hemorrhage or preseptal or orbital cellulitis. This more often occurs when the tumor has spread outside the globe, portending a poor prognosis. Retinoblastoma can demonstrate local spread along the optic nerve directly into the orbit. It can also metastasize hematogenously to bone, liver, brain and other organs.
History
A careful history of present illness, family history, and thorough ophthalmic examination (as well as appropriate ancillary studies) are critical for prompt diagnosis. The clinician must specifically inquire about a family history of blindness, eye tumors, childhood malignancies, or enucleations. A family history of other cancers such as sarcomas may also be suggestive. The parent should be asked if either leukocoria and/or strabismus have been observed. If known, the duration of time from discovery of the leukocoric reflex to presentation in the ophthalmologist's office should be documented. The results of any ancillary testing done prior to the ophthalmologist’s initial consultation with the family should be obtained.
Physical Examination
Age-appropriate visual acuity testing should be performed monocularly. External examination should rule out proptosis and signs of orbital cellulitis. Slit lamp examination should look for ciliary injection, pseudohypopyon, iris neovascularization, or signs of secondary glaucoma. The presence or absence of an afferent pupillary defect should be determined. As mentioned earlier, a dilated funduscopic examination is critical. If there is any suspicion for retinoblastoma, the patient should undergo an examination under anesthesia with careful scleral depression to evaluate the entire retina is necessary to confirm the diagnosis of retinoblastoma as well as to determine the exact location and extent of the tumor(s) and the tumor staging. Photographic documentation for future comparison is recommended. Complete retinal examination of both eyes is necessary to rule out bilateral disease.[7]
Fundus Examination
Retinoblastoma classically presents with one or multiple nodular, white, or cream-colored masses often associated with increased vascularization. There are two primary clinical patterns of retinoblastoma growth.[3] If the tumor grows anteriorly into the vitreous it is known as an endophytic tumor. This form of retinoblastoma often shows vitreous seeding, which causes vitreous haze and opacities. This can eventually progress to involve the anterior chamber and can cause secondary glaucoma and inflammation. When there is involvement of opacities in a shallow anterior chamber, it is helpful to recognize that the seeds are above the iris on slit lamp evaluation. When the tumor shows a growth pattern more posteriorly that extends beneath the retina (subretinal) it is described as an exophytic tumor. These tumors can cause exudative retinal detachments and may be associated with significant subretinal seeding. Examination under anesthesia with extended ophthalmoscopy is required for patients with retinoblastoma for diagnosis and staging; retinal photography is also important for documentation, the education of the patient and family, and for future comparison.
Diagnostic procedures
Additional testing should include A and B-scan ultrasound.[7] Ultrasound is often useful for diagnosing and characterizing retinoblastoma. The appearance of a hyperechoic intraocular mass with hyper-reflective foci and associated posterior shadowing consistent with calcium is essentially diagnostic for retinoblastoma in a child. It can also help define tumor height and thickness and confirm associated retinal detachment and calcification.
Though computerized tomography (CT) scan can help demonstrate the presence or absence of calcium deposits and help define the size of the tumor, it should generally be avoided with preference for an MRI when available. CT scan exposes patients with germline mutations to radiation increasing the risk of secondary malignancies. Magnetic resonance imaging (MRI) is often performed to evaluate optic nerve involvement, extraocular extension, and to consider for the possibility of concomitant primitive neuroectodermal tumor (trilateral retinoblastoma with pinealoblastoma). As mentioned, MRI is currently the preferred imaging modality for most clinicians because of radiation associated with CT scans. High doses of radiation can be harmful to all young children but especially to this patient population, where heritable and germline retinoblastoma patients are especially at increased risk for secondary tumors. Bone marrow examination or lumbar puncture may also be performed in patients where there is concern regarding the extent of disease, particularly with extraocular extension to rule out cerebrospinal fluid (CSF) or bone marrow metastases.
Differential Diagnosis
Leukocoria should immediately make the clinician think of retinoblastoma. However, the differential diagnosis includes other entities such as a cataract, Coats’ disease, retinopathy of prematurity, toxocariasis, choroidal coloboma, vitreous hemorrhage, myelinated retinal nerve fibers, and other retinal tumors such as astrocytic hamartoma. Corneal opacities can also produce a white reflex, but this can be easily differentiated by more thorough clinical exam. Differentiating retinoblastoma from conditions such as Coats’ disease, persistent fetal vasculature (PFV), or toxocariasis can be a clinical challenge.
Coats' disease often has a more yellow appearance with yellow subretinal exudates compared to retinoblastoma. On fluorescein angiography, Coats' has more characteristic telangiectatic and dilated vessels, regions of non-perfusion, and aneurysms with leakage; in contrast, retinoblastoma has less leakage and more often shows irregular perfusion abnormalities consistent with tumor vascular patterns, such as dilated vessels with non-perfusion. In Coats’ disease, abnormally dilated, tortuous vessels may be associated with significant exudation causing a mass-like lesion of lipid with associated telangiectatic neovascularization. The exudate in Coats’ disease is more yellow than white because of the presence of cholesterol. Furthermore, Coats’ disease is typically unilateral and predominantly affects boys between 6 to 8 years of age, which is older than the typical patient with retinoblastoma.
PFV is a congenital condition, and leukocoria becomes noticed clinically early in life. It is typically a unilateral condition, and eyes tend to be microphthalmic. A cataract is often present and may be associated with elongated ciliary processes. Retinal detachment in PFV is more often tractional and tent shaped whereas retinoblastoma retinal detachments are more likely exudative with subretinal fluid and possible seeds. If present, these features can help differentiate PFV from retinoblastoma.
Ocular toxocariasis can cause a white, peripheral retinal mass with a similar appearance to retinoblastoma. Toxocariasis is usually unilateral and associated with more signs of inflammation than retinoblastoma such as injection, pain, photophobia, and anterior chamber and/or vitreous cells. Patients should be questioned about contact with puppies and eating dirt. In addition, a history of fever, eosinophilia, pneumonitis, or hepatosplenomegaly would be suggestive of systemic manifestations of peripheral larva migrans. Serum titers positive for toxocara canis would further confirm the diagnosis.
A and B-scan ultrasound is helpful to differentiate retinoblastoma from these conditions. Ultrasonography can detect calcification, which would favor a diagnosis of retinoblastoma. It can identify a retrolental mass and a shorter anterior-posterior dimension compared to the unaffected eye, which would favor PFV. Fluorescein angiography would show dilated vessels in retinoblastoma with variable degrees of non-perfusion and less leakage than Coats'. In contrast, Coats’ disease classically shows focal telangiectasias with profuse leakage into the subretinal space and subretinal yellow appearing exudates.
Retinoblastoma can appear like several conditions with vitreous seeding being mistaken for inflammation or even Coats' disease. A good rule of thumb is to avoid entering an eye for a biopsy. It is always helpful to have additional opinions.
Management
General treatment
Spontaneous regression of retinoblastoma has been reported but is exceedingly rare.[8] The priorities in the treatment of retinoblastoma are to preserve life, preserve globe, and preserve vision, in that specific order. Minimizing side effects and complications of treatment are also of paramount importance in these very young patients. Enucleation remains the definitive treatment of intraocular retinoblastoma, particularly in many patients who present with unilateral disease. However, the loss of an eye is associated with significant social stigma in certain cultures. In addition, bilateral enucleation is a devastating option for bilaterally affected patients. Treatment modalities that may be successful in globe salvage include systemic chemotherapy with focal consolidation, intra-arterial chemotherapy, intravitreal chemotherapy, and focally destructive therapy (cryopexy, laser photocoagulation, hyperthermia and plaque irradiation).[9] Radiation therapy is avoided, if possible, but still has a role to preserve vision in recurrent tumors.
Local invasion of the optic nerve and choroid (i.e., advanced intraocular retinoblastoma) are the most important risk factors for development of metastatic disease and are therefore indications for adjuvant systemic chemotherapy.[10] However, these factors cannot be accurately assessed without histopathologic assessment of the enucleated eye. While globe salvage therapy has rapidly supplanted enucleation in the management of intraocular retinoblastoma, approximately 10% to 15% of advanced intraocular tumors harbor high-risk features that may go undetected with globe-sparing treatments.[11] Unlike other cancers, retinoblastoma cannot be biopsied directly due to the risk of seeding and extraocular extension. Thus, there is an increasing interest for diagnostic testing using “liquid biopsies” of patient aqueous humor to determine which eyes can be safely preserved versus which should enucleated. Berry and colleagues have utilized next generation sequencing to assay tumor derived nucleic acid fragments in the aqueous humor without the need for a primary tumor biopsy or enucleation.[12] The same group found that aqueous humor sampling was superior to conventional peripheral blood sample testing in aiding diagnosis through the identification of tumor-derived cell-free DNA.[13] While this technique has yet to be broadly used in the clinical setting, it raises exciting possibilities to retinoblastoma management and prognostication. Such an approach would allow for molecular assessment of tumor progression, metastatic surveillance, and response to therapy.
Medical Treatment of Intraocular Retinoblastoma
Intravenous Chemotherapy (IVC) or Chemoreduction with Focal Consolidation
Prior to the 1990s, external beam radiation (EBRT) played a central role in the treatment of retinoblastoma. Due to disappointingly high recurrence rates, chemotherapy was limited largely to the treatment of metastatic cases. However, with long term follow-up clinicians began realizing how significant an impact EBRT had on the prevalence of secondary tumors, especially in patients with germline mutations. It is estimated that 38.2% of patients with hereditary retinoblastoma will develop a secondary malignancy with an associated long-term mortality rate of 26%.[14][15] The risk of second malignancy is increased more than threefold by EBRT, especially if the patient is less than 1 year of age.[16] The growing reluctance to use EBRT coincided with the rise of more effective chemotherapeutic regimens.
Systemic chemotherapy is administered with focal consolidative therapy with laser, thermotherapy, and/or cryotherapy. This treatment approach has been coined "chemoreduction" because the goal is to shrink the tumor. Shrinking the tumor increases the success of focal therapies, which are less successful with thicker tumors.[17] Focal therapy is directly destructive to tumor cells and also breaks down the blood ocular barrier and increases penetration of chemotherapeutic agents into the eye.[18] Today, systemic chemotherapy, applied in conjunction with local therapy, is one of the main globe salvaging options in retinoblastoma management.[19]
In patients with advanced bilateral retinoblastoma, traditionally the more severely affected eye has been enucleated, while the less severely affected eye has undergone chemoreduction with or without external beam irradiation. In cases where only one eye harbors tumor, enucleation is usually considered when the tumor is large and there is poor vision potential. As chemoreduction has proven its efficacy, clinicians are expanding its clinical indications to allow more eyes to be saved.
The Children’s Oncology Group (COG) has had multiple trials evaluating chemotherapy regimens in conjunction with focal consolidation. Since group A eyes are often easily managed with focal therapies alone, no COG trial exists for this group. For group B tumors, a two-agent protocol involving vincristine and low-dose carboplatin along with local therapy showed 65% event free survival and a rate of 15% enucleation[20]. In COG ARET0231, Group C and D tumors were treated with three agent chemotherapy involving vincristine, high-dose carboplatin, and etoposide along with local therapy as well as subtenon injection of carboplatin. These trials have also reached stopping points. Children with tumor massively involving the optic nerve, orbit, brain or present at distant sites are currently being enrolled in a COG group F trial.
Intra-arterial Chemotherapy (IAC)
More localized delivery of medication may help decrease the systemic side effects of chemotherapeutic agents while still allowing the success clinicians have seen with chemoreduction techniques. The quest for local delivery for chemotherapeutics is not new. From intravitreal injection, subconjunctival injection, to more complex delivery systems incorporating fibrin sealant, clinicians have been evaluating the possibility for local delivery of antineoplastics around the eye for decades. In addition to improving intravitreal penetration, these approaches would minimize systemic toxicity and possibly be more available in the developing world where retinoblastoma remains a lethal disease.
Since about 2009, significant attention has been focused on intra-arterial delivery of chemotherapeutic agents. Based on early attempts in Japan to directly cannulate the carotid artery in order to deliver antineoplastic drugs to the eye, Abramson and colleagues recently modified the Japanese protocol to cannulate the ophthalmic artery.[21] This technique has been called supra-selective intra-arterial chemotherapy (IAC). Using the chemotherapeutic agent, Melphalan, Abramson and colleagues have reported good response rates. Variability exists in protocols including use of triple agent therapy with melphalan, topotecan and carboplatin, and the use of tandem therapy for patients with bilateral disease. [22] To compare the clinical efficacy of IAC to IVC, Chen and colleagues conducted a meta-analysis of twenty-six studies and showed that the overall success rate, defined as the avoidance of external beam radiation therapy or enucleation, was higher than IAC than with IVC.[23] However, IAC and IVC were not significantly different regarding recurrence and metastasis rates. Side effects have also been described including sectoral occlusive choroidopathy, and concerns exist about the potential to cause strokes with this method of delivery.[24] The total dose of whole-body radiation given with multiple fluoroscopies also remains undefined. A randomized clinical trial has been recommended to determine the efficacy and safety of this approach, as well as to determine the optimum chemotherapeutic agent. Such a trial is currently under consideration by the COG cooperative group of the National Cancer Institute.
Intravitreal Chemotherapy (IVitC)
Eyes with refractory vitreous seeds after systemic intravenous chemotherapy or intra-arterial chemotherapy have been successfully treated with intravitreal chemotherapy (IVitC) by retinoblastoma experts around the world. Intravitreal injections, often with 20 to 30 µg of melphalan or 20 µg of topotecan, given through the pars plana of eyes with small 30-32-gauge needles along with double or triple freeze thaw therapy to the injection site, have succeeded in the hands of retinoblastoma experts at controlling the majority of cases of vitreous seeds that otherwise have resisted or recurred after other forms of treatment. As penetrating the eye wall in cases of active retinoblastoma may cause the spread of retinoblastoma beyond the eye, extreme care is taken to select an appropriate injection site free of tumor activity and to apply multiple rounds of freeze-thaw treatment to the injection site to kill any retinoblastoma cells that may attempt to travel along the needle injection track. Complications may include cataract, hemorrhage, retinal detachment, and infection. Procedural complications may include cataract, vitreous hemorrhage, retinal detachment, severe hypotony, and infection while chemotoxicity can manifest as retinal pigment epithelial and choroidal atrophy, iris atrophy, posterior synechiae, and uveal inflammation (particularly with high dose melphalan. Published reports of case series of treated eyes with this IVitC technique by retinoblastoma experts worldwide have document vitreous seed control in the majority of eyes without major adverse events and without metastatic spread. The combination IVC and/or IAC along with IVitC as needed for active vitreous seeds may spare globes that otherwise would require enucleation for uncontrolled retinoblastoma activity.[25][26][27]
Radiation Treatment
External beam radiation and plaque radiotherapy are secondary treatment options following the primary chemotherapy options delineated above in select cases where retinoblastoma remains uncontrolled. External beam radiation is associated with orbital hypoplasia and may induce secondary cancers in the field of radiation for those with germline retinoblastoma and thus is performed less frequently than before the chemotherapy eras. In plaque radiotherapy, a custom-made plaque with radioactive seeds is surgically sutured onto the sclera over a site of intraocular retinoblastoma for a set period for proper dosing of targeted radiation to the active tumor (while attempting to spare surrounding normal retina). Radiation retinopathy and its associated sequelae (vascular non-perfusion, retinal edema, neovascularization, hemorrhage, etc.) may occur with radiation treatment.
Surgical Treatment of Intraocular Retinoblastoma
Eyes with large tumor burden (Group E eyes) and eyes that progress despite conservative treatments require enucleation. Primary enucleation may also be considered for advanced Group D or E eyes when there is grave concern for extraocular retinoblastoma spread beyond the eye without hope for useful vision; early primary enucleation in these advanced retinoblastoma cases may reduce the risk of metastatic spread that might otherwise occur during attempts at globe salvage using chemotherapy. When an eye is enucleated for retinoblastoma, the goal is to remove as much optic nerve as possible to try ensure that the cut end of the nerve is free of tumor. Enucleation may best be performed by oculoplastic surgeons who may consider lateral canthotomy and other techniques to optimize the length of optic nerve obtained on the enucleated specimen. The globe is sent for pathologic evaluation. Pathologic evaluation has clinical importance because it determines whether there are any pathologic risk factors for extraocular spread. Pathologic risk factors (PRF) that have been identified include: 1. massive choroidal invasion, 2. post-laminar invasion of the optic nerve, 3. scleral invasion, and 4. involvement of the anterior chamber. If PRFs are present, consideration should be given for adjuvant chemotherapy to decrease the risk of extraocular relapse.[28] At the time of enucleation, the largest orbital implant is placed in order to encourage normal development of the pediatric orbit. Patients who have an eye enucleated will continue to be followed to ensure there is no evidence of tumor in the other eye as well as in the orbit that surrounded the enucleated eye. They will also need updated fittings over time for possibly multiple ocular prostheses as the child and the orbit continue to grow. Revision of the enucleation site by oculoplastic surgeons using dermis fat grafts and mucous membrane grafts may sometimes be required to optimize the anatomy of the orbit and the fitting of an ocular prosthesis.
Medical Treatment of Extraocular Retinoblastoma
Historically, extraocular retinoblastoma was nearly universally fatal. If the retinoblastoma remained confined to the orbit, there was only a 10% survival rate, and cases of survival with metastatic retinoblastoma was anecdotal.[29] However, as chemotherapeutic regimens have improved, the prognosis has also improved.
For extraocular retinoblastoma limited to the orbit, clinicians are finding success with neoadjuvant chemotherapy to shrink the tumor. This is followed by surgical debulking and post-operative chemotherapy and radiation if necessary.[30][31] For systemic metastases, especially cases with CNS involvement, aggressive treatment with high dose chemotherapy (HDC) and autologous stem cell rescue (ASCR) is recommended. HDC involves the administration of high doses of chemotherapeutic agents with the aim to overcome tumor resistance and completely eradicate neoplastic cells. Unfortunately, these lethal doses are also myeloablative and concurrent ASCR must be performed to allow future reconstitution of the bone marrow. Though toxic, case series of survival after this treatment regimen are being reported.[32][33]
Currently there is a Children’s Oncology Group (COG) trial evaluating high dose systemic chemotherapy using four cycles of induction chemotherapy with vincristine, cisplatin, cyclophosphamide, and etoposide followed by one cycle of high-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for malignant brain and spinal tumors. Although promising, treatment efforts remain focused on early detection and treatment to prevent extraocular disease in the first place.
Trilateral Retinoblastoma
In additional to CNS metastases, patients with retinoblastoma can develop intracranial involvement by developing a concurrent CNS primitive neuroectodermal tumor (PNET). Trilateral retinoblastoma is the term often applied to a PNET in the setting of bilateral retinoblastoma. It is a rare neoplasm that occurs in patients with germline mutations. While this is more common in bilateral cases, some patients with unilateral retinoblastoma that carry germline mutations are also at risk for developing this tumor.[34] Thus the term trilateral retinoblastoma can create a false sense of security with unilaterally affected germline patients.
The treatment regimen for patients with PNET is similar to patients with CNS metastases. The prognosis is also dismal. As such, early detection and treatment of PNETs is recommended. Recently it was noted that there was a decrease in the incidence of PNETs as clinicians came to rely more on chemotherapy over EBRT. This is hypothesized to be secondary to either a prophylactic effect due to systemic chemotherapy or because fewer patients are receiving radiation.[35][36] Regardless, systemic chemotherapy can be considered for germline cases of retinoblastoma over local (i.e. intra-arterial, etc.) delivery.
Emerging Therapies
Tumor cells frequently develop downstream mutations following tumor initiation that allow them to escape the effects of conventional chemotherapeutic agents (i.e., tumor resistance). Additionally, non-specific chemotherapies like vincristine and carboplatin carry the risk of serious adverse effects, such as transient pancytopenia and carboplatin-induced ototoxicity.[37] Advancements in our understanding of molecular pathways involved in retinoblastoma and mechanisms underlying tumor resistance have provided opportunities to study novel targeted therapies with improved bio-availabilities and reduced chemotoxicity. New drug candidates have emerged in the last decade that have demonstrated promise in preclinical studies for retinoblastoma, including MDMX-p53 pathway antagonists (e.g., nutlin-3)[38], histone deacetylase inhibitors (e.g., trichostatin A, suberoylanilide hydroxamic acid, and MS-275)[39][40], spleen tyrosine kinase inhibitors (e.g., BAY-61-3606 and R406)[41][42], genetic and immune modulatory drugs (e.g., VCN-01 and GD2.CAR-Ts)[43][44], and CDK4/6 inhibitors (e.g., palbociclib and abemaciclib). Palbociclib and abemaciclib are of particular interest as they have recently been approved for the treatment of advanced breast carcinoma, are able to cross the blood-brain-barrier and carry reduced risk of myelosuppression.[45][46] However, prospective clinical studies are required to validate these candidate therapies prior to use in the clinical setting.
Novel drug delivery systems such as inorganic, lipid, and polymeric nanoparticles are also being investigated as a treatment option for retinoblastoma. They have shown potential for controlled, targeted, and sustained delivery of chemotherapeutic drugs, genes, and imaging agents to retinoblastoma cells. Nanoparticles can also be modified with surface ligands to specifically recognize and target tumor cells, thereby reducing the systemic toxicity of chemotherapeutic agents.[47]
Further, a recent study demonstrated the potential of circulating tumor DNA (ctDNA) in the blood of retinoblastoma patients to indicate the presence and growth of active tumors, which may have implications in assessing treatment response and risk for metastasis.[48]
Follow up
Patients who undergo any form of eye sparing treatment (external beam radiation, chemoreduction) need frequent follow-up examinations. Tumor regression is followed closely, documenting the appearance, size, location, and number of tumors during each examination. When tumor regresses after treatment, it can appear as a white, calcific mass or appear translucent and resemble fish flesh. Sometimes a flat scar or even no remnant of retinoblastoma is left behind after treatment. A typical schedule would require examinations under general anesthesia every 6-8 weeks until age three, followed by less frequent examinations if the disease becomes quiescent. Patients with hereditary retinoblastoma are at increased lifetime risk of developing secondary malignancies throughout the body. The most common secondary tumor is osteosarcoma.[49] Other tumors include PNETs, fibrosarcoma, and melanoma. Patients who have been treated with radiation are at higher risk for secondary tumors in the field of treatment. Long term follow-up of all retinoblastoma patients is mandatory with special vigilance if patients have germline mutations.
Additional Resources
- eyecancer.com
- aapos.org (FAQ section on retinoblastoma)
- Boyd K. Eye Cancer. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/eye-cancer-list. Accessed March 11, 2019.
- Boyd K, Maturi RK. Retinoblastoma. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/retinoblastoma-list. Accessed March 25, 2019.
References
- ↑ Broaddus, E., Topham, A., and Singh, A.D. 2009. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 93: 21-3.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Abramson DH, Schefler AC. Update on retinoblastoma. Retina. 2004 Dec;24:828-48.
- ↑ Jump up to: 3.0 3.1 3.2 Shields JA, Shields CL. Ocular tumors. A text and atlas. Philadelphia, PA: Saunders; 1992. p.311-12.
- ↑ Lee C, Chang JH, Lee HS, Cho Y. Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor. Genes Dev. 2002;16(24):3199–3212.
- ↑ American Academy of Pediatrics policy statement- Red Reflex Examination in Neonates, Infants, and Children. Pediatrics 2008;122: 1401-1404.
- ↑ Jump up to: 6.0 6.1 6.2 Rodriguez A, Kelley C, Patel A, Ramasubramanian A. Prenatal Diagnosis of Retinoblastomas: A Scoping Review. Int J Gen Med. 2023 Mar 27;16:1101-1110.
- ↑ Jump up to: 7.0 7.1 Lin P, O’Brien JM. Frontiers in the Management of Retinoblastoma. Am J Ophthalmol 2009; 148: 142-8.
- ↑ Sanborn GE, Augsburger JJ, Shields JA. Spontaneous regression of bilateral retinoblastoma. Br J Ophthalmol. 1982 Nov;66(11):685-90.
- ↑ Hamel P, Budning AS, Heon E, et al. Focal Therapy in the Management of Retinoblastoma: When to Start and When to Stop. J AAPOS 2000;4:334-337.
- ↑ Grossniklaus HE. Retinoblastoma. Fifty years of progress. The LXXI Edward Jackson Memorial Lecture. Am J Ophthalmol. 2014 Nov;158(5):875-91.
- ↑ Fabian ID, Stacey AW, Chowdhury T, Duncan C, Karaa EK, Scheimberg I, Reddy MA, Sagoo MS. High-Risk Histopathology Features in Primary and Secondary Enucleated International Intraocular Retinoblastoma Classification Group D Eyes. Ophthalmology. 2017 Jun;124(6):851-858.
- ↑ Berry JL, Xu L, Murphree AL, Krishnan S, Stachelek K, Zolfaghari E, McGovern K, Lee TC, Carlsson A, Kuhn P, Kim JW, Cobrinik D, Hicks J. Potential of Aqueous Humor as a Surrogate Tumor Biopsy for Retinoblastoma. JAMA Ophthalmol. 2017 Nov 1;135(11):1221-1230.
- ↑ Berry JL, Xu L, Polski A, Jubran R, Kuhn P, Kim JW, Hicks J. Aqueous Humor Is Superior to Blood as a Liquid Biopsy for Retinoblastoma. Ophthalmology. 2020 Apr;127(4):552-554.
- ↑ Eng C, Li FP, Abramson DH, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85:1121-8.
- ↑ Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23:2272-9.
- ↑ Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998;105:573-9.
- ↑ Shields JA, Shields CL, De Potter P. Photocoagulation of retinoblastoma. Int Ophthalmol Clin. 1993;33:95-9.
- ↑ Abramson DH, Frank CM, Chantada GL, de Totah AB, de Pifano IT, Ramírez GT, Gomez RT, Fandino AT, Tran HT, Madden TJ, Dunkel IJ. Intraocular carboplatin concentrations following intravenous administration for human intraocular retinoblastoma. Ophthalmic Genet. 1999 Mar;20:31-6.
- ↑ Shields CL, De Potter P, Himelstein BP, et al. Chemoreduction in the initial reduction of intraocular retinoblastoma. Arch Ophthalmol 1996;114:1330-8.
- ↑ Friedman, DL, Krailo, M, Villaluna, D, Gombos, D, Langholz, B, Jubran, R, Shields, C, Murphree, L, O'Brien, J, Kessel, S, Rodriguez‐Galindo, C, Chintagumpala, M, Meadows, AT. Systemic neoadjuvant chemotherapy for Group B intraocular retinoblastoma (ARET0331): A report from the Children's Oncology Group. Pediatr Blood Cancer. 2017; 64:e26394. DOI: 10.1002/pbc.26394
- ↑ Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A Phase I/ii study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008 Aug;115: 1398-404.
- ↑ Shields, C. L., & Shields, J. A. (2016). Intra-arterial chemotherapy for retinoblastoma. JAMA Ophthalmology, 134(10), 1201. https://doi.org/10.1001/jamaophthalmol.2016.2712
- ↑ Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Huang W, Jiang H, Xia J, Zhang S. Comparison between intravenous chemotherapy and intra-arterial chemotherapy for retinoblastoma: a meta-analysis. BMC Cancer. 2018 Apr 27;18(1):486.
- ↑ Shields CL, Bianciotto CG, Jabbour P, Griffin GC, Ramasubramanian A, Rosenwasser R, Shields JA. Intra-arterial chemotherapy for retinoblastoma: Report No 2. Treatment Complications. Arch Ophthalmol. 2011 Nov; 129: 1407-15.
- ↑ Francis, J. H., Abramson, D. H., Ji, X., Shields, C. L., Teixeira, L. F., Schefler, A. C., Cassoux, N., Hadjistilianou, D., Berry, J. L., Frenkel, S., & Munier, F. L. (2017). Risk of extraocular extension in eyes with retinoblastoma receiving intravitreous chemotherapy. JAMA Ophthalmology, 135(12), 1426–1429. https://doi.org/10.1001/jamaophthalmol.2017.4600
- ↑ Munier, F. L., Soliman, S., Moulin, A. P., Gaillard, M. C., Balmer, A., & Beck-Popovic, M. (2012). Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. British Journal of Ophthalmology, 96(8), 1084–1087. https://doi.org/10.1136/bjophthalmol-2011-301016
- ↑ Munier, F. L., Gaillard, M. C., Balmer, A., Soliman, S., Podilsky, G., Moulin, A. P., & Beck-Popovic, M. (2012). Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. British Journal of Ophthalmology, 96(8), 1078–1083. https://doi.org/10.1136/bjophthalmol-2011-301450
- ↑ International Retinoblastoma Staging Working Group. World disparities in risk definition and management of retinoblastoma: a report from the International Retinoblastoma Staging Working Group. Pediatr Blood Cancer. 2008;50:692-4.
- ↑ Rootman J, Ellsworth RM, Hofbauer J, Kitchen D. Orbital extension of retinoblastoma: a clinicopathological study. Can J Ophthalmol. 1978;13:72-80.
- ↑ Chantada G, Fandiño A, Casak S, Manzitti J, Raslawski E, Schvartzman E. Treatment of overt extraocular retinoblastoma. Med Pediatr Oncol. 2003;40:158-61.
- ↑ Kiratli H, Bilgiç S, Ozerdem U. Management of massive orbital involvement of intraocular retinoblastoma. Ophthalmology. 1998;105:322-6.
- ↑ Kremens B, Wieland R, Reinhard H, et al. High-dose chemotherapy with autologous stem cell rescue in children with retinoblastoma. Bone Marrow Transplant. 2003;31:281-4.
- ↑ Leal-Leal CA, Rivera-Luna R, Flores-Rojo M, Juárez-Echenique JC, Ordaz JC, Amador-Zarco J. Survival in extra-orbital metastatic retinoblastoma:treatment results. Clin Transl Oncol. 2006;8:39-44.
- ↑ Ibarra MS and O'Brien JM. Is Screening for Primitive Neuroectodermal Tumors in Patients With Unilateral Retinoblastoma Necessary? J AAPOS 2000;4:54-6.
- ↑ Shields CL, Meadows AT, Shields JA, Carvalho C, Smith AF. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma). Arch Ophthalmol. 2001;119:1269-72.
- ↑ Moll AC, Imhof SM, Schouten-van Meeteren AY, et al. Chemoreduction for retinoblastoma. Arch Ophthalmol. 2003;121:1513.
- ↑ Francis JH, Brodie SE, Marr B, Zabor EC, Mondesire-Crump I, Abramson DH. Efficacy and Toxicity of Intravitreous Chemotherapy for Retinoblastoma: Four-Year Experience. Ophthalmology. 2017 Apr;124(4):488-495.
- ↑ Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, Teunisse A, Lam S, Ramos Y, Mohan A, Johnson D, Wilson M, Rodriguez-Galindo C, Quarto M, Francoz S, Mendrysa SM, Guy RK, Marine JC, Jochemsen AG, Dyer MA. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006 Nov 2;444(7115):61-6.
- ↑ Zhang J, Benavente CA, McEvoy J, Flores-Otero J, Ding L, Chen X, Ulyanov A, Wu G, Wilson M, Wang J, Brennan R, Rusch M, Manning AL, Ma J, Easton J, Shurtleff S, Mullighan C, Pounds S, Mukatira S, Gupta P, Neale G, Zhao D, Lu C, Fulton RS, Fulton LL, Hong X, Dooling DJ, Ochoa K, Naeve C, Dyson NJ, Mardis ER, Bahrami A, Ellison D, Wilson RK, Downing JR, Dyer MA. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012 Jan 11;481(7381):329-34.
- ↑ Dalgard CL, Van Quill KR, O'Brien JM. Evaluation of the in vitro and in vivo antitumor activity of histone deacetylase inhibitors for the therapy of retinoblastoma. Clin Cancer Res. 2008 May 15;14(10):3113-23.
- ↑ Liu H, Zhang Y, Zhang YY, Li YP, Hua ZQ, Zhang CJ, Wu KC, Yu F, Zhang Y, Su J, Jin ZB. Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proc Natl Acad Sci U S A. 2020 Dec 29;117(52):33628-33638.
- ↑ Pritchard EM, Stewart E, Zhu F, Bradley C, Griffiths L, Yang L, Suryadevara PK, Zhang J, Freeman BB 3rd, Guy RK, Dyer MA. Pharmacokinetics and efficacy of the spleen tyrosine kinase inhibitor r406 after ocular delivery for retinoblastoma. Pharm Res. 2014 Nov;31(11):3060-72.
- ↑ Pascual-Pasto G, Bazan-Peregrino M, Olaciregui NG, Restrepo-Perdomo CA, Mato-Berciano A, Ottaviani D, Weber K, Correa G, Paco S, Vila-Ubach M, Cuadrado-Vilanova M, Castillo-Ecija H, Botteri G, Garcia-Gerique L, Moreno-Gilabert H, Gimenez-Alejandre M, Alonso-Lopez P, Farrera-Sal M, Torres-Manjon S, Ramos-Lozano D, Moreno R, Aerts I, Doz F, Cassoux N, Chapeaublanc E, Torrebadell M, Roldan M, König A, Suñol M, Claverol J, Lavarino C, Carmen de T, Fu L, Radvanyi F, Munier FL, Catalá-Mora J, Mora J, Alemany R, Cascalló M, Chantada GL, Carcaboso AM. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci Transl Med. 2019 Jan 23;11(476):eaat9321.
- ↑ Wang K, Chen Y, Ahn S, Zheng M, Landoni E, Dotti G, Savoldo B, Han Z. GD2-specific CAR T cells encapsulated in an injectable hydrogel control retinoblastoma and preserve vision. Nat Cancer. 2020 Oct;1(10):990-997.
- ↑ Goel S, DeCristo MJ, McAllister SS, Zhao JJ. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018 Nov;28(11):911-925.
- ↑ Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017 Nov;166(1):41-54.
- ↑ Onugwu AL, Ugorji OL, Ufondu CA, Ihim SA, Echezona AC, Nwagwu CS, Onugwu SO, Uzondu SW, Agbo CP, Ogbonna JD, Attama AA. Nanoparticle-based delivery systems as emerging therapy in retinoblastoma: recent advances, challenges and prospects. Nanoscale Adv. 2023 Aug 15;5(18):4628-4648.
- ↑ Silverman RF, Francis JH, Robbins MA, Dunkel IJ, Abramson DH. RB1 CIRCULATING TUMOR DNA IN THE BLOOD OF PATIENTS WITH RETINOBLASTOMA INCREASES IN UNTREATED PATIENTS. Retina. 2024 Dec 1;44(12):2123-2128. doi: 10.1097/IAE.0000000000004224. PMID: 39089006.
- ↑ MacCarthy A, Bayne AM, Draper GJ, Eatock EM, Kroll ME, Stiller CA, Vincent TJ, Hawkins MM, Jenkinson HC, Kingston JE, Neale R, Murphy MF. Non-ocular tumours followein retinoblastoma in Great Britain 1951 to 2004. BR J Ophthalmol. 2009 Sep;93: 1159-62.