Persistent Hyperplastic Primary Vitreous
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Nomenclature
Persistent Fetal Vasculature (PFV) is recognized by the following codes as per the International Classification of Diseases (ICD) Nomenclature:
- ICD9 743.51
- ICD10 Q14.0
Disease
Persistent Fetal Vasculature (PFV), previously known as Persistent Hyperplastic Primary Vitreous (PHPV), is a failure of the regression of a component of fetal vessels within the eye. It has been described as a “benign mimic of retinoblastoma,” being the second most common cause of infantile leukocoria [1]. Persistent fetal vasculature can be a devastating developmental anomaly that remains an important cause of amblyopia and visual disabilities in children. While PFV does not progress during a child’s lifetime, eye growth can result in tractional intraocular changes past infancy and affected children may go on to develop complications including glaucoma, cataracts, intraocular hemorrhages, retinal detachments, and/or phthisis that will further affect the child’s vision/cosmesis of the eye.
Definitions
- Persistent Hyperplastic Primary Vitreous: synonymous with Persistent Fetal Vasculature is the preferred term prior to Morton F. Goldberg’s 1997 Edward Jackson Memorial Lecture in which the term was changed to be more “encompassing, accurate, and clinically useful.” [2]
- Tunica Vasculosa Lentis: a capillary network that branches from the hyaloid artery and covers the lens surface; If this capillary network persists, the lens does not form properly; it includes anterior and posterior divisions encircling the human lens; Anteriorly, the tunica vasculosa lentis has attachments that extend to the pupillary frill of the iris and onto the anterior lens surface; Posteriorly, the tunica vasculosa lentis anastomosis with the hyaloid system and is attached to the ciliary process.
- Hyaloid System: also called primary vitreous; composed of both the hyaloid vessel that extends from the optic nerve to the posterior lens, as well as the vasculature that fills the vitreous cavity and has many attachments to the retinal surface; normally regresses by 28 to 30 weeks gestational age. It includes the vaso hyaloidea proprea, the tunica vasculosa lentis, and the pupillary membrane[3].
- Anterior PFV Syndrome: eyes with mostly tunica vasculosa changes
- Posterior PFV Syndrome: eyes with mostly hyaloid artery changes
- Mittendorf Dot: the termination of the normally regressed anterior portion of the hyaloid artery, found slightly to the nasal side of the intraocular space as the artery approaches the posterior pole of the lens; benign finding unless related to other intraocular persistent vasculature; the Brittle Star Configuration is seen when an enlarging Mittendorf Dot anastomoses with residual vessels of the posterior Tunica Vasculosa Lentis;
- Bergmeister Papilla: a remnant of the posterior portion of the hyaloid artery’s fibrous sheath at the optic disc; not known to cause any visual disorders; visualized as a linear, gray structure anterior to the optic disc.
History
Reese first described Persistent Hyperplastic Primary Vitreous (PHPV) in 1955 as a congenital malformation of the anterior portion of the primary vitreous [4]. Due to both the hyaloid vessels and the tunica vascular lentis persisting in this condition, and the fact that “primary vitreous” refers only to the hyaloid vessels, Morton F. Goldberg introduced the term “Persistent Fetal Vasculature” as the condition’s new name in the 1997 Edward Jackson Memorial Lecture stating that PHPV was a misnomer for only including those vessels that were posterior to the lens.[5]
Etiology
Despite some cases being inherited in either an autosomal dominant or recessive manner, the majority of the cases are non-heritable [6] [7]. (Some bilateral cases may be familial exudative vitreoretinopathy [FEVR], and genetic testing is considered). Although some gene involvement has been proposed, no gene has yet been reported that can account for a substantial number of the cases of PFV [8]. PFV is most frequently unilateral. When the presentation is consistent with the approximately 10% of cases that are bilateral, both the potential for an associated systemic condition or a genetic basis should be considered [9]. Associated systemic conditions include Norrie disease and multiple other conditions that are the result of a central nervous system problem, an exposure, or an infection.
The genes implicated in pathogenesis include ATOH7 (autosomal recessive PFV), and NDP (autosomal dominant PFV).
Pathophysiology
The fetal vasculature, both the tunica vasculosa and hyaloid systems, are presumed to nourish the fetus’s developing lens prior to the production of aqueous humor and formation of the anterior chamber. In normal development, fetal vasculature regresses without complications; however, in 95% of premature infants and 3% of full term infants, fetal vasculature persists and can result in a spectrum of disorders [10] [11]. Although the majority of cases are both unilateral and non-heritable, the occurrence of some familial diseases implies genetic factors [12] [13]. Nonetheless, a simple gene for PFV syndrome has not been identified. Some have postulated that if the syndromes resulting from the failure of fetal vasculature to regress were due to a somatic mutation of a crucial gene in only a subset of cells in development, this would result in the non-heritability of PFV as is seen, as well as explain the spectrum of disorders seen due to a varying proportion of cells in development lacking this crucial gene [14]. A variety of mechanisms have been postulated to affect the regression of the fetal vasculature, which begins with the smallest fetal vessels and progresses to the regression of the hyaloid artery during the third trimester. For normal vessel regression to occur, genes and growth factors must be expressed at the precise timing and at the precise level, balancing vaso-inhibitory and vaso-stimulatory factors, to allow for apoptosis and macrophage activation. [10] Some of these genes and factors that have been proposed in the mechanism of PFV are the loss of Wnt7b signals in hyalocytes, factors such as Angiopoietin 2 or p53 that may affect endothelial cell apoptosis, deregulated vascular endothelial growth factor (VEGF), and the absence of the Arf tumor suppressor; however, many studies were done in experimental models and not human. The mechanism of Persistent Fetal Vasculature continues to perplex many investigating this disorder. [14]
Diagnosis
Classification
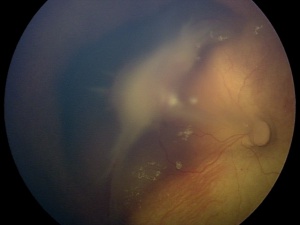
Persistent Fetal Vasculature has a spectrum of presentations, ranging from anterior findings to posterior findings. While rarely isolated, anterior PFV tends to historically be less destructive than posterior PFV, resulting in better surgical outcomes and visual results. The anterior subtype has been defined as the presence of a retrolental opacity, elongated ciliary process, or cataract [15], but can also include a membranous transformation of the anterior vitreous face causing traction on the peripheral retina, a shallow anterior chamber, poor pupil dilation, and microphthalmos. [9] The posterior subtype has been defined by a stalk of tissue extending from the optic nerve to the retrolental area causing an elevation of the vitreous membrane from the optic nerve, retinal folds, dysplasia, or detachment, and varying degrees of optic nerve dysplasia. [12]
Symptoms
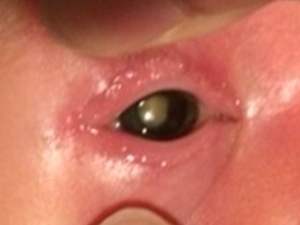
PFV typically presents with leukocoria in the smaller eye of an infant within 1 to 2 weeks of birth [16]. The classical presentation is that of leukocoria, micro-ophthalmia, and a cataract. [17]
Clinical diagnosis
Despite leukocoria being a common presentation across the entire spectrum of PFV, leukokoria results from several differing underlying features including a complete pupillary membrane, cataract formation, fibrovascular sheath attachment to the posterior lens capsule, and a cloudy cornea secondary to glaucoma [18]. Thus, infants present with a spectrum of presentations determined by the location of the persistent vasculature. Although only rarely does PFV present as purely anterior or posterior, the anterior and posterior syndromes present differently.
The characteristic finding in the anterior PFV syndrome include microphthalmia, leukocoria, a varying degree of cataract, elongated or drawn-in ciliary processes, a shallow anterior chamber, retrolental fibrovascular membranes causing traction on the peripheral retina, intralenticular hemorrhages, dilated iris vessels, glaucoma, strabismus, ectropion uvea, and coloboma iridis. The glaucoma present is usually a secondary angle-closure glaucoma associated with the swelling of the lens resulting from the tractional tearing of the posterior capsule, from bleeding, or from a contracture of the retrolental tissues. The anterior PFV may show whip-lash like movements (video) with ocular movements.
The characteristic findings in the posterior PFV syndrome include leukocoria, microphthalmia, a retinal fold possibly causing tractional retinal detachment of the posterior pole, a hypoplastic or dysplastic optic nerve, vitreous membranes and stalk, macular pigmentary disruption or hypoplastic macula, a clear lens, and strabismus. While 62% of the time PFV may present as a combination of the anterior and posterior syndromes, prognostically it is beneficial to categorize individuals into an anterior, posterior or combination of the syndromes due to patients with the purely anterior form having the most reasonable chance for a successful visual outcome following treatment of their PFV. [19] Although a posterior PFV syndrome does suggest a poorer visual outcome, other factors individually such as the severity of the anterior segment, the size of the globe, the gravity of lens involvement or even the vascular appearance does not seem to predict the amount of retinal dysplasia, which ultimately tends to determine the eye’s visual result. [10]
A smaller eye is considered typical of PFV; however, differences can be subtle or even non-existence in cases of buphthalmos associated with glaucoma.
Diagnostic procedures
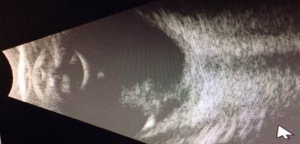
PFV is most readily diagnosed by direct visualization of any component of the persistent fetal vasculature with a careful examination. In patients with poor view of the fundus, ultrasonography can be employed. In general, ultrasound, computed tomography scanning, magnetic resonance imaging, and fluorescence angiography are all reasonable options for establishing a diagnosis. The most important differentiation when obtaining imaging is differentiating PFV from the malignant retinoblastoma (Rb). B-scan ultrasonography and computed tomographic scanning can be exceptionally helpful in this regard. Calcification seen on imaging is suggestive of malignancy due to calcification rarely being seen in PFV alone. A foci of calcification is present in 90% of retinoblastoma cases, making calcification highly suggestive of malignancy, especially if visualized in a child less than three years old. [20] B-scan ultrasonography can be used to assess for intraocular masses, fibrovascular stalk, microphthalmia and to look for calcification. In addition, Doppler associated ultrasonography can be quite useful to detect vasculature associated with PFV. [21]
A CT scan with contrast can enhance the persistent fibrovascular tissue, but it is important to remember that, due to the possibility of increasing the risk of malignancy in infants with this form of radiation, an MRI might be a superior alternative.[22] If a CT were to be used to establish a diagnosis of PFV, the CT findings could include: an absence of calcification, an increased density of the entire vitreous, tubular intravitreal density (Cloquet’s canal or nonattached retina), decubitus positioning showing a gravitational effect on fluid-fluid level due to the presence of hemorrhage in the subretinal space; a retrolental mass, micro-ophthalmia, enhancement of abnormal intravitreal tissue, and small or irregular lens.
Likewise, if an MRI were to be used, the MRI findings would include: a tubular structure, representing the hyaloid vessel; a funnel-shaped retinal detachment, with the subretinal fluid hyperintense on both T1 and T2 weighted images; fluid-fluid level due to the presence of hemorrhage in the subretinal space, a retrolental mass; micro-ophthalmia, and vitreous hemorrhage.” [15]
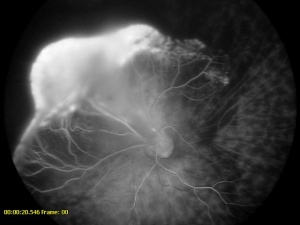
Fluorescein angiography, with either a slit-lamp camera or fundus camera, can detect abnormal vasculature in various locations and can be used to visualize the brittle-star configuration. Peripheral retinal capillary nonperfusion may also be noted in retinopathy of prematurity, familial exudative vitreoretinopathy, Norrie disease, incontinentia pigmenti, and other conditions. In leukocoria, it is prudent to perform careful retinal evaluation and fluorescein angiography in the fellow eye. The finding of non-perfused peripheral retina may point the clinician to other diagnoses, such as FEVR.
Differential diagnosis
Due to the most commonly recognized clinical manifestation of PFV being leukocoria, the differential diagnosis of PFV, especially anterior PFV, includes those conditions that may also result in leukocoria. The most concerning of these is retinoblastoma, the most common intraocular tumor of childhood, thus, retinoblastoma must be ruled-out in every case of PFV. Generally, PFV occurs in a microphthalmic eye, while retinoblastoma occurs in a normal-sized eye. Other causes of leukocoria are most commonly congenital cataract or Coats’ Disease, but the differential diagnosis should include astrocytic hamartomas (related to tuberous sclerosis), uveitis, toxocariasis, and retinopathy of prematurity. Classically, the genetic syndromes that may include PFV are Norrie disease, Trisomy 13, and the Walker-Warburg syndrome. When the eye in consideration has a total retinal detachment and retrolental fibrous tissues suggestive of posterior PFV syndrome, familial exudative vitreoretinopathy, incontinentia pigmenti, ocular toxocariasis, and retinopathy of prematurity should be especially considered. [23]
Management
Goals of Management
There is a wide diversity treatments depending on the nature of PFV. Visual outcomes also relate to the location of involvement, age of the patient, and how much pathology has occurred, especially if the retina has been involved. While the natural history of untreated PFV is likewise diverse, untreated anterior and posterior PFV can lead to recurrent intraocular hemorrhage, secondary glaucoma, and phthisis [24]. The most important factor in predicting if a patient will have successful visual rehabilitation is whether or not the posterior pole is involved, as the prognosis for pure anterior PFV is markedly better than those with posterior PFV. Other predictors of prognosis, based on a multivariate analysis, are bilaterality and microphthalmia (23). Despite a poorer visual prognosis for an eye with posterior disease, earlier detection and treatment can make it possible to obtain useful vision (24). Hunt and colleagues showed that surgery before 77 days of life was associated with useful vision. Those undergoing surgery before 77 days were approximately 13 times more likely to obtain a visual acuity of counting fingers or better than those operated on later. [12] Although advances have been made in surgical techniques, goals for management of PFV have remained the same in recent years. First, the media opacity in the affected eye should be cleared to allow visual development and to save the eye from complications of untreated PFV, such as glaucoma, intraocular hemorrhage, and phthisis. PFV eyes with myopia have less media opacity, normal corneal diameters, are detected late and have good visual prognosis.
Surgery
Treatment has changed drastically for persistent fetal vasculature since the historical treatment of a diagnosis in the first few months of an infant’s life, followed by serial exams and enucleation, often in the second or third year of life, when a complication such as secondary glaucoma appeared. Today, many eyes with PFV can retain some useful vision if diagnosed and treated within the first few months of life. Due to surgical studies of PFV being difficult to compare, particularly when assessing the visual outcomes due to visual outcomes being dependent on retinal dysplasia, the timing of the surgery, and postoperative amblyopic therapy, [10]The management of PFV often requires teams involving pediatric ophthalmology, pediatric retina and glaucoma specialists.
Eyes with PFV can have developmental abnormalities in the region of the ora serrata that predispose to intraoperative retinal tears and detachment. Surgical approaches to anterior PFV or even combined anterior/posterior PFV often requires anterior limbal lensectomy, membranectomy, and vitrectomy. A limbal approach is often safer than a pars plicata or pars plana approach since the retina/ora area may have developmental abnormalities, including noninsertion of the retina or giant dialyses. It is important to detect a dialysis or retinal detachment and ready to treat them at the time of initial surgery with endolaser or possible scleral buckle[25]. in some centers, any posterior involvement is managed by a pediatric retina trained expert.
Treatment must address the two components that affect vision, media opacity and retinal detachments and vitreoretinal traction. Media opacities can be addressed similarly to congenital cataracts and, for the most part, are handled by lens removal, refraction, and amblyopia therapy. Thus, anterior PFV is most often treated with observation, lensectomy, and glaucoma management. In both the combined anterior-posterior variety and the purely posterior form of PFV, lensectomy and vitrectomy is often required. [17] During cataract surgery, due to persistent tunica vasculosa lentis aggressive intraocular bleeding may be expected in some cases. The posterior vascular stalk in such cases may be cauterized, although this is controversial and may lead to vitreous bleeding, and Fugo Plasma blade can be used.
Medical follow up
With modern vitreoretinal techniques, aphakic rehabilitation, and aggressive amblyopic therapy, useful vision can be obtained in the majority of patients with both anterior and posterior persistent fetal vasculature. [14] There is a wide range of visual outcomes after surgery for PFV, with 10% of patients in a PEDIG registry study achieving normal vision.[26] Parental compliance is critical if visual improvement is to be seen in an individual with Persistent Fetal Vasculature. [15] Effective management of the anisometropic amblyopia requires motivated patents to ensure compliance with occlusive therapy, as well as parents willing to attend numerous visits to eye specialists.
References
- ↑ Seyedmehdi Payabvash, J. S. (2015 ). Bilateral persistent fetal vasculature due to a mutation in the Norrie disease protein gene. Neuroradiology Journal , 28, 623-627.
- ↑ Goldberg, M. F. (1997). Persistent Fetal Vasculature: An Integrated Interpretation of Sins and Symptoms Associated With Persistent Hyperplastic Primary Vitreous LIV Edward Jackson Memorial Lecture. American Journal of Ophthalmology, 124 (5), 587-626.
- ↑ Lutty GA and McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye Prog Eye Ret Res. 2018 Jan;62:58-76.
- ↑ Reese AB. Persistent hyperplastic primary vitreous. Am J Ophthalmol 1955; 40:317–31.
- ↑ Nicholas C. Farber, E. M. (2015, Feb). How to Treat Persistent Fetal Vasculature. Review of Ophthalmology.
- ↑ Ritu Galhotra, K. G. (2006). Bilateral persistent hyperplastic primary vitreous: an Egyptian family supporting a rare autosomal dominant inheritance. Journal of Genetic Counseling, 17, 441-7.
- ↑ Shagufta Khaliq, A. H. (2001). Locus for autosomal recessive nonsyndromic persistent hyperplastic primary vitreous. Investigative Ophthalmology & Visual Science, 42, 2225-8.
- ↑ Shastry, B. S. (2009). Persistent hyperplastic primary vitreous: congenital malformation of the eye. Clinical and Experimental Ophthalmology, 37, 884-890.
- ↑ Jump up to: 9.0 9.1 Nelson, L. B. (2011). Pediatric Ophthalmology: Color Atlas and Synopsis of Clinical Ophthalmology (First edition ed.). Philadelphia: Lippincott Williams & Wilkins.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 Hartnett, M. E. (2004). Pediatric Retina: Medical and Surgical Approaches (1 edition ed.). (A. C. Michael Trese MD, Ed.) Philadelphia: Lippincott Williams & Wilkins.
- ↑ Mitchell S MD Fineman, A. C. (2012). Retina: Color Atlas and Synopsis of Clinical Ophthalmology (Second edition ed.). (W. E. Institute, Ed.) Philadelphia: Lippincott Williams & Wilkins.
- ↑ Jump up to: 12.0 12.1 12.2 Hunt A, et al. Outcomes in persistent hyperplastic primary vitreous. Br J Ophthalmol 2005; 89:859-863.
- ↑ Steve Charles, A. K. (2002). Vitreous Microsurgery (Third Edition ed.). Philadelphia: Lippincott Williams & Wilkins.
- ↑ Jump up to: 14.0 14.1 14.2 Jain, T. P. (2009). Bilateral persisten hyperplastic primary vitreous. Indian Journal of Ophthalmology, 53-54.
- ↑ Jump up to: 15.0 15.1 15.2 Imran H Yusuf, C. K. ( 2015). Unilateral persistent hyperplastic primary vitreous: intensive management approach with excellent outcome beyond visual maturation. BMJ Case Rep, 1-4.
- ↑ Mira Silbert, A. S. (2000) Clinical Review: Persistent hyperplastic primary vitresous. American Academy of Optometry, 12, 131-137.
- ↑ Jump up to: 17.0 17.1 Robert A. Sisk, A. M. (2010). Visual and anatomic outcomes with or without surgery in persistent fetal vasculature. Ophthalmology, 117, 2178-83.
- ↑ Alexandrakis G, et al. (2000) Visual Acuity Outcomes with and without Surgery in Patients with Persistent Fetal Vasculature. Ophthalmology, 107:1068-1072.
- ↑ Pollard, Z. F. (1997). Persistent hyperplastic primary vitreous: diagnosis, treatment and results. Trans Am Ophthalmol Soc, 95, 487–549.
- ↑ Robert A. Mittra, L.T. (1998). Visual outcomes following lensectomy and vitrectomy for combined anterior and posterior persistent hyperplastic primary vitreous. Arch Ophthalmol, 1190-4.
- ↑ Hu A, Pei X, Ding X, Li J, Li Y, Liu F, Li Z, Zhan Z, Lu L. Combined Persistent Fetal Vasculature: A Classification Based on High-Resolution B-Mode Ultrasound and Color Doppler Imaging. Ophthalmology. 2016 Jan;123(1):19-25. doi: 10.1016/j.ophtha.2015.09.001. Epub 2015 Oct 21. PMID: 26477846.
- ↑ Deshmukh S, Magdalene D, Gupta K. Persistent fetal vasculature. TNOA J Ophthalmic Sci Res [serial online] 2018 [cited 2018 Sep 2];56:132-3. Available from: http://www.tnoajosr.com/text.asp?2018/56/2/132/238491
- ↑ Michael A. Apushkin, M. A. (2005). Retinoblastoma and simulating lesions: role of imaging. Neuroimaging Clinics of North America, 15, 49-67.
- ↑ E Shultz, B. G. (2006). Long-term visual function and relative amblyopia in posterior persistent hyperplastic primary vitreous (PHPV). Strabismus, 14, 121-125.
- ↑ Prakhunhungsit S et al. Persistent Fetal Vasculature Syndrome, in Pediatric Retina, 3rd ed. Hartnett, ME (ed-in-chief), Drack A, Capone A, Trese MT, Toth CA, Caputo G. 921-933.
- ↑ Haider KM, Repka MX, Sutherland DR, Hatt SR, Fallaha N, Kraker RT, Melia BM, Cotter SA, Holmes JM; Pediatric Eye Disease Investigator Group. Outcomes and Complications 5 Years After Surgery for Pediatric Cataract Associated With Persistent Fetal Vasculature. Am J Ophthalmol. 2024 Apr;260:30-36. doi: 10.1016/j.ajo.2023.11.002. Epub 2023 Nov 7. PMID: 37939986; PMCID: PMC11005992.