All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Uveal melanoma is a malignant tumor arising from melanocytes in the uveal tract (iris, ciliary body, or choroid). It is the most common primary intraocular tumor in adults with an age-adjusted incidence of 5.1 per million.[2] There is a strong tendency for metastasis, particularly to the liver, and prognosis is poor when the tumor has disseminated. The choroid is the most common site involved and gives rise to 90% of uveal melanomas. Melanomas arising within the ciliary body comprise 7% and melanomas of the iris account for 2%.[3] This article focuses on the treatment of posterior (ciliary body and choroidal) uveal melanoma. The treatment of anterior (iris) melanoma or conjunctival melanoma is discussed elsewhere.
Risk Factors
While development of uveal melanoma is largely considered to be a sporadic event, certain risk factors including light iris color, light skin color, ability to tan, northern European ancestry, and rarely a family history of uveal melanoma may predispose individuals to the disease.[4][5] Those with pre-existing choroidal nevi are also at risk where the incidence of malignant transformation for each lesion is 1/5000 to 1/8845.[6]
Pathophysiology
Uveal melanomas develop from the unregulated clonal proliferation of uveal melanocytes. As with other malignancies, this results from a complex sequence of molecular events involving multiple driver mutations in different genes. Five genes are most commonly implicated in the tumorigenesis of uveal melanoma: GNAQ, GNA11, BAP1, SF3B1, and EIF1AX. Somatic mutations in either GNAQ or GNA11 are found in 83% to 89% of uveal melanomas and occur exclusive to one another.[7][8] Mutations involving these genes are thought to occur early in tumorigenesis as neither are significantly associated with tumor size or risk for metastatic disease.[9] Subsequent driver mutations in BAP1, SF3B1, or EIF1AX have been detected in 45%, 23% and 17% of uveal melanomas respectively and occur with almost complete exclusion to one another.[7] Driver mutations in BAP1, SF3B1, or EIF1AX are thought to represent three separate molecular pathways - each promoting tumorigenesis but conferring different levels of metastatic potential with BAP1 associated with the highest risk and EIF1AX the lowest.
Diagnosis
Symptoms
The clinical presentation of malignant uveal melanoma is characterized by nonspecific findings associated with the location of the tumor. Approximately 30% of patients are asymptomatic at presentation.[10] 38% complain of decreased vision, 9% report photopsias, 7% floaters and 6% peripheral vision loss. Only 2% of patients complain of eye pain. Very rarely an advanced case of iris melanoma may present with a secondary glaucoma due to tumor extension into the angle and pigment-laden macrophages causing blockage of the trabecular meshwork or neovascularization.[11]
Clinical Diagnosis
Choroidal Melanoma
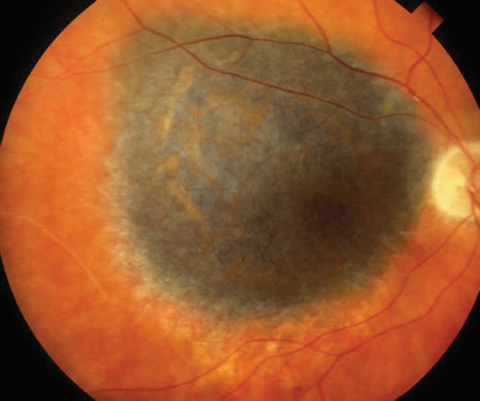
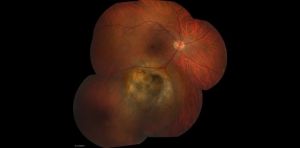
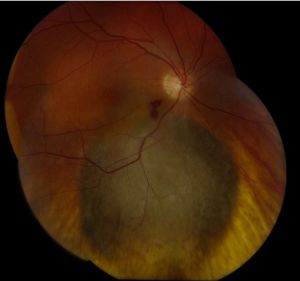
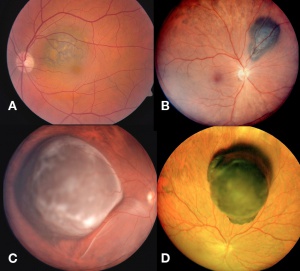
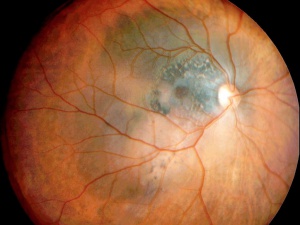
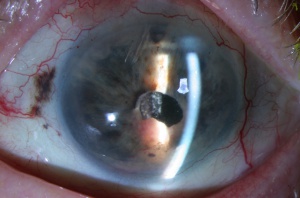
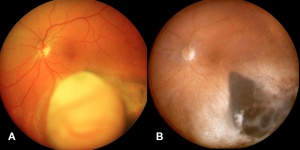
Posterior uveal melanomas typically present as a unilateral elevated domed-shaped gray-brown colored mass of the choroid with irregular margins. Less commonly, a melanoma may be amelanotic. About 20% of tumors will invade through Brüch's membrane resulting in a mushroom-shaped (or collar button) configuration. Melanomas should be differentiated from benign melanocytic lesions such as choroidal nevi or congenital hypertrophy of the retinal pigment epithelium (CHRPE) as well as choroidal hemorrhage.
Some choroidal nevi are difficult to distinguish from small choroidal melanomas. Shields et al. have identified high risk features predictive of growth in concerning choroidal nevi that can be remembered using the mnemonic TFSOM-UHHD: “To Find Small Ocular Melanoma-Using Helpful Hints Daily”:[12][13][14][15][16]
- Thickness > 2 mm
- Fluid subretinal
- Symptoms of flashes or floaters
- Orange pigment
- Margin within 3mm on the optic nerve head
- Ultrasound Hollow
- Halo absent: A halo of depigmentation surrounding a pigmented choroidal lesion is more commonly seen in nevi and indicates stability.
- Drusen absent: Drusen overlying choroidal lesions suggest chronicity.
Choroidal melanocytic tumors that display no factors have a 3% chance for growth at 5 years and most likely represent choroidal nevi. Tumors that display one factor have a 38% chance for growth, and those with two or more factors show growth in over 50% of cases at 5 years.[13]
The size of the choroidal melanomas is the most important clinical feature in determining disease prognosis. Size is classified into three categories according to a modification of the criteria of the Collaborative Ocular Melanoma Study (COMS): small, medium and large melanomas. Treatment strategies may vary depending on this classification.
| Apical height | Largest basal diameter | |
|---|---|---|
| Small | 1.0 - 2.5 mm | 5.0 - 16.0 mm |
| Medium | 2.5 - 10 mm | less than 16 mm |
| Large | more than 10 mm | more than 16 mm |
Cilary Body Melanoma
Melanomas of the ciliary body are often relatively large when they present. They have the same characteristics as choroidal melanoma but more frequently have a sentinel vessel (dilated tortuous episcleral vessel overlying the tumor). Additionally, these tumors are more likely to present with anterior displacement of the lens-iris diaphragm and a secondary angle closure glaucoma.
Diagnostic Imaging
Fundus Photography
Serial fundus photos are critical in the follow up of choroidal nevi and melanoma. The widefield retinal (WFR) imaging such as Optos creates an easily replicated map of the fundus. Certain WFR imaging systems distort the color of the tumor and fundus.
Fundus Autoflorescence
Orange lipofuscin pigmentation possesses autofluorescent properties. Liposfuscin is present in suspicious nevi and many melanomas. Lipofuscin fluorescence is brighter than that of drusen (drusen are common in choroidal nevi).
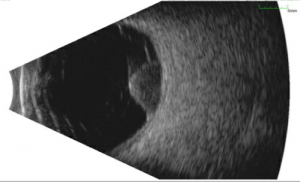
Ultrasound
This is the primary diagnostic test that confirms the diagnosis of melanoma. Ultrasound also is useful in determining the size, e.g. thickness (apical height) and basal dimension, extraocular extension (such as scleral nodules), and document growth during the follow up of a suspicious nevus/small melanoma.
- Standardized A-mode: Posterior uveal melanomas classically show medium to low internal reflectivity (88%), often in a decrescendo fashion, a.k.a, positive angle kappa, with regularity of structure. The internal blood flow (vascularity) of the tumor can be seen as a fast movement of the internal spikes. Uncommonly, a large melanoma may be more irregular, particularly if there is necrosis.
- B-mode: Shapes: Dome-shaped is most common, mushroom-shaped, i.e. collar button is the most classic shape, and an irregular shape is uncommon. Other features: acoustically hollow zone within the tumor, choroidal excavation, and subretinal fluid.
- Ultrasound biomicroscopy is utilized to better delineate ciliary body and iris melanomas.
Color Doppler ultrasound
Choroidal melanomas show pulsatile blood flow at the tumor base. This finding is not found in nevi.
Fluorescein and indocyanin green angiography (ICGA)
- Hypofluorescence: Due to blockage of the choroidal blood flow by the pigmentation inherent to the tumor.
- Hyperfluorescence: Small hyperfluorescent spots may be seen due to lipofuscin deposition at the RPE level. Pinpoint hyperfluorescence is a high risk characteristic seen in choroidal nevi and if present with other high risk characteristics, one should consider that the tumor may be undergoing malignant transformation.
- Circulation:“Double circulation” pattern consisting of an internal circulation within the lesion and the normal vascularity of the overlying retina. This characteristic is more evident in ICGA
- Neovascularization: is not typical of melanoma and its presence suggests another diagnosis should be sought.
Standard spectral domain OCT (SD-OCT)
Standard spectral domain OCT does not penetrate deeply enough for detecting the internal characteristics of choroidal neoplasms. However, it is useful in visualizing changes in the neurosensory retina and the retinal pigment epithelium (RPE). As it was mentioned earlier, posterior uveal melanomas may show serous retinal detachment in the areas adjacent to the tumor. i.e. subretinal fluid. Lipofuscin deposition is also seen at the level of the RPE.
Enhanced Depth Imaging Spectral Domain OCT (EDI-OCT)
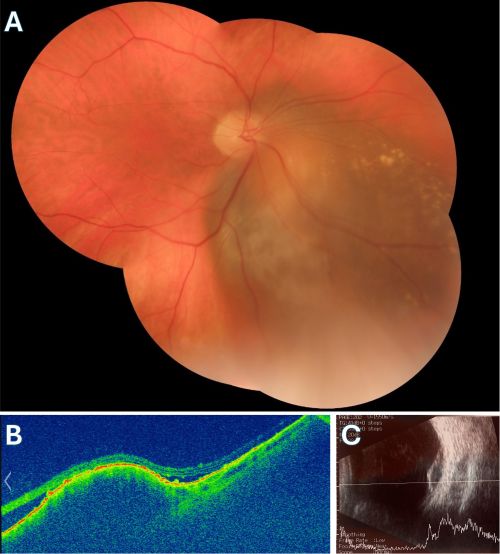
Enhanced Depth Imaging Spectral Domain OCT is a relatively new technology that is now commercially available. It is a method that allows for the evaluation of deeper structures such as the choroid and the internal portion of the sclera. In a study of 37 eyes by Shields et al.[17] it was found that the distinguishing characteristics of choroidal melanomas readily seen in EDI-OCT are:
- Optical choroidal shadowing (100%): Due to the dense pigmentation of the melanoma, there is often shadowing beneath the tumor, which can obscure deeper structures in the choroid.
- Choriocapillaris Compression and Thinning (100%): The melanoma may compress the choriocapillaris, leading to thinning over the tumor.
- Subretinal fluid (92%): Frequently associated with choroidal melanoma, the presence of subretinal fluid is an important indicator, often detected by OCT even when clinical examination might miss it.
- Bacillary Layer Detachment (BALAD): A unique feature of choroidal melanoma observed in a significant number of cases, particularly in medium-sized tumors. It is associated with more extensive subretinal fluid.
- Subretinal lipofuscin deposits (95%)
- Shaggy photoreceptors (49%): The "shaggy" appearance of photoreceptors overlying the tumor is often seen in choroidal melanoma, especially in small melanomas. This finding helps differentiate melanoma from benign nevi.
- Ellipsoid Zone (EZ) Loss/Disruption: This is one of the most common findings in choroidal melanoma, indicating damage to the photoreceptors and outer retinal layers. It is more prevalent in larger tumors.
- Subretinal Hyperreflective Material (SRHM): This material can appear as either homogeneous or heterogeneous on OCT and is a common finding. It is often associated with the presence of subretinal fluid and more advanced melanoma.
- Retinal and RPE Changes: OCT often shows retinal thinning, retinal pigment epithelium (RPE) atrophy, or thickening over the tumor. RPE hyperplasia or disruption may also be observed.
- Other retinal changes included: Loss of photoreceptors, loss of external limiting membrane, irregularity of inner plexiform layer, irregularity of ganglion cell layer, intraretinal edema.
These OCT findings are essential for assessing tumor size, monitoring changes over time, and distinguishing choroidal melanoma from benign lesions such as choroidal nevi.
Management
Depending upon several clinical factors, management options today include observation, radiation, laser, surgery, medical management, or a combination of these approaches.[18][19][20][21][22][23][24][25][26][27][28] The selected management depends on size, thickness, location, and activity of the tumor, the status of the opposite eye, and the age, general health, and psychological status of the patient. Each patient should have a detailed ophthalmic evaluation and the size and extent of the tumor carefully documented with drawings, color fundus photography, and ultrasonography. The known risk factors for growth and metastasis should be considered and therapeutic options should be discussed.[29] The potential effects of the treatment on a patient’s life prognosis, quality of life, tolerance to the proposed treatment, and expected visual outcome are important considerations.
General treatment
For centuries, immediate enucleation was believed to be the best treatment for an eye with uveal melanoma. However, despite high rates of enucleation, mortality remained high due to metastases to the liver and other sites. In the late 1970s, some authorities challenged the efficacy of enucleation for preventing metastatic disease and even proposed that enucleation may promote or accelerate metastasis.[22][23] The validity of these arguments was challenged by others, who believed that early enucleation offered the patient the best chance of cure.[24][25][30] This controversy was responsible for initiating a trend away from enucleation and increasing the use of more conservative, eye-sparing approaches.[26][27][28] In 1985, the landmark Collaborative Ocular Melanoma Study (COMS) was organized to address issues regarding the treatment of uveal melanoma.[31] The COMS answered many questions about the demographics, natural history, pathophysiology, and treatment of choroidal melanoma. More recently, key prognostic risk factors have been identified, with tumor thickness being one of the most important. As metastatic risk has been observed with increasing millimeter tumor thickness, early intervention is advised for posterior uveal melanomas.[32]
The goals of treating uveal melanoma is to achieve the following:
- Preservation of useful vision in the affected eye
- Tumor destruction
- Prevention of metastasis and recurrence
Periodic Observation
A choroidal nevus is a well-circumscribed, benign melanocytic tumor that is managed by periodic observation.[33] They typically appear as small (less than 2 mm thick, asymptomatic, and with overlying retinal pigment epithelial atrophy and drusen, signifying a chronic condition. Approximately 6% of the Caucasian population manifests a choroidal nevus.[34] About 1 in 5000 to 1 in 8800 choroidal nevi evolve into choroidal melanoma.[35][36]
Patients with suspicious nevi should be reexamined and imaged in 3 months and monitored for changes in size. If no growth is observed, then reexamination can occur every 6 months thereafter.
Documented growth of a melanocytic choroidal tumor is suggestive that the lesion is a choroidal melanoma. Growth over a short time (<2 years) is strongly suggestive of melanoma whereas growth over a long period (>10 years) is still consistent with a benign lesion.[37] Since documented growth may be associated with worse systemic prognosis, some patients with small tumors that show three or more risk factors are treated promptly, without waiting for documentation of growth.[12][13][14][15][16] Increasing tumor size both in base and thickness leads to increased risk for metastasis.[32] Based on the few patients with medium size choroidal melanoma who refuse treatment and are followed, natural history studies have found that there is greater mortality and higher risk of death.[38]
Management
Once a clinician has considerable suspicion for uveal melanoma, the decision to treat rests on several subjective and objective features. Observation of lesions is justified for small tumors where ancillary studies, not including biopsy, have failed to establish a definitive diagnosis. Additionally, smaller lesions with slow tumor doubling time may be observed if the patient is a poor surgical candidate and/or if the patient is satisfied with a conservative approach. Additionally, when the lifespan of the patient is estimated to be less than 5 years, the decision to observe may be justified in selected cases. it is important to note, that while the tumor doubling time averages 180 days, not all melanomas are slow growing and this should be taken into consideration if observation is considered. Finally, for very small tumors in the patient’s only remaining eye, observation for development of high-risk indicators is reasonable. Observation of lesions less than 3 mm in thickness should include preliminary fundus photos, fluorescein angiography, and A and B scan ultrasonography. After first diagnosis, this should be repeated at a 3-4 month follow-up. If no change in the lesions has been identified, the minimum follow-up should be fundus photography every 6-12 months for the remainder of the patient’s life.
While the above diagnostics can provide sufficient evidence for the clinical classification of uveal melanoma, biopsy of the lesion is may yield the diagnosis but is controversial due to the risk of tumor spread. Fine needle aspiration (FNA) may be performed in cases of uncertain diagnosis when clinically indicated. However, FNA is more commonly performed at the time of insertion of plaque brachytherapy.
Most ophthalmologists use clinical indicators to predict the likelihood of a borderline lesion growing and thus differentiating a benign lesion from malignant melanoma, thus guiding the workup and treatment. If multiple high-risk indicators are found, mentioned previously is found, advancing the workup is indicated and some physicians offer a biopsy.
Historical perspective
The primary goal of treatment for uveal melanomas is to prevent metastasis. It is unclear if treatment accomplishes this goal. Historically, the first treatment d for all uveal melanomas was enucleation, which provided a histological diagnosis and also guided further treatment, when necessary. Removal of the eye was the gold standard for uveal melanoma for centuries until the late 1970s due to concern that enucleation could facilitate metastasis. This inspired the use of eye-sparing strategies. In the late 1970’s advances in the field of radiation oncology opened the door to eye conserving radiotherapy for uveal melanoma. Radiotherapy treatment outcomes reported in the literature with the use of brachytherapy in the early 1980’s and charged particle radiation therapy in the early 1990’s were found to offer promising results.[39]
Brachytherapy as a treatment for uveal melanoma was led by Packer[40], who described sewing a customized plaque containing the radioisotope iodine-125, over the region affected by the tumor. With numerous ophthalmologists subsequently borrowing this mode of therapy, the techniques of brachytherapy were refined and skills were improved which suggested treatment superiority over enucleation.
Despite promising findings early in the history of brachytherapy, apprehension held by many ophthalmologists regarding the possibility for recurrence and metastasis in eye conserving therapy delayed its utilization.[41] In 2001, the Collaborative Ocular Melanoma Study (COMS), a large, multicenter, randomized control trial studying 1317 patients over 12 years concluded that there was no significant difference in mortality for iodine-125 brachytherapy compared to enucleation for choroidal melanomas.[36] The results of this COMS report, in addition to subsequent COMS publications confirming this finding, have bolstered the use of radiotherapy so that it has become a standard of treatment today for uncomplicated uveal melanomas.
Today the procedure is similar to that described by Packer, however, the specifications have been standardized. The American Brachytherapy Society currently recommends 3D tumor planning either with a multi-modality reconstruction program or with MRI to clearly demonstrate the size and location of the tumor before treatment.[42] The planning of the dosage of the radioisotope plaque is often a collaborative effort between an ophthalmologist and a radiation oncologist. The American Brachytherapy Society recommends using iodine-125 at a dosage of 0.60-1.05 Gy/h over 3 to 7 consecutive days.[42] This amount has been carefully chosen after the COMS and other researchers reported that dose rates of less than 0.60 Gy/h resulted in lower tumor control rates.[43] With a well-constructed 3D model of the tumor, the plaque is sutured to the sclera overlying the tumor by an ophthalmologist. Using this modality, great success has been reported in providing a lethal dose of radiation to a confined area with attenuated, dose-related tissue toxicity a function of distance from the site of the plaque. Currently, the most common isotope used in brachytherapy for uveal melanoma in the United States is iodine-125. This is largely a result of the well-established documentation of the isotope’s efficacy in the COMS trials. Other radioisotopes such cobalt-60 (formerly used), iridium-192, palladium-103, ruthenium-106 have also been used. The limitations of brachytherapy are generally governed by the experience of the ophthalmologist and not exceeding the tolerable dose to the sclera. Additionally, some tumors have too large a base to be covered by plaques, custom plaques are generally required for tumors with greater than an 18mm basal diameter.
Charged particle radiation therapy, notably proton beam radiotherapy is an alternative. Proton beam radiotherapy is an extraocular application of large charged particles with specified kinetic energy aimed at the exact location of interest-generally the tumor apex. This treatment precisely localizes radiation due to the quick deceleration of large mass particles and the ability of the treating ophthalmologist-oncologist to modify the kinetic energy of the beam. This treatment boasts a high degree of precision.[44] Proton beam radiotherapy has the ability to treat melanomas surrounding the optic disc.
Subsequent publications following the COMS initial reports studying the efficacy of brachytherapy or proton beam therapy in melanoma anterior to the equator reported similarly positive findings.[44] The decision to use brachytherapy vs. proton beam therapy is a function of size and location of the tumor, patient preference and availability of treatment.
Local Therapy to the Eye
Surgery
There are several methods for surgical management of posterior uveal melanoma including enucleation, exenteration, and local resection.
Enucleation
Enucleation is generally indicated for advanced melanomas that occupy most of the intraocular structures, have caused severe secondary glaucoma or total retinal detachment that is not reversible to alternative treatments, or tumors invading the optic nerve. Enucleation with a long section of the optic nerve is appropriate in such cases. Sometimes patient preference dictates this treatment as well. However, many juxtapapillary melanomas that abut the optic nerve and show no evidence of invasion can be managed by custom-designed notched radioactive plaques or proton beam therapy rather than enucleation.[45][46][47][48]
There is no role for preenucleation radiation. The large sized tumor COMS trial indicated that external beam radiotherapy prior to enucleation did not provide a survival benefit compared to enucleation alone.
The "no touch enucleation" was introduced to minimize the amount of surgical trauma and theoretically to lessen the chance of tumor dissemination at the time of surgery.[49] An essential aspect of this technique was to freeze the venous drainage from the tumor prior to cutting the optic nerve. The "no touch" technique has recently fallen into disuse at most centers because it is cumbersome and its benefits are only theoretical. However, a gentle tissue handling during a standard enucleation should be employed, without clamping the optic nerve prior to cutting it. Care should be taken not to pass needles through the sclera for traction either; rather the globe is distracted by stumps of the medial and lateral recti.
After removal of the eye, either fine needle aspiration biopsy or fixated tissue may be submitted for genomic expression profiling.
There have been advances in the types of orbital implants used following enucleation. The hydroxyapatite implant, designed to improve the ocular motility in patients undergoing enucleation, is used widely.[50][51][52] Other implants include polyethylene and polymer coated hydroxyapatite.[52][53] Furthermore, porous polyethylene implants have been used successfully.
Orbital Exenteration
The subject of orbital exenteration for uveal melanomas with extrascleral extension is controversial.[54][55] Complete orbital exenteration should not be done in cases of mild degrees of extrascleral extension. However, in the rare instance of massive orbital extension in a blind, uncomfortable eye, primary orbital exenteration is probably justified. In most instances of orbital extension for uveal melanoma, it is not necessary to sacrifice the skin of the eyelid. The eyelid-sparing exenteration provides a better cosmetic appearance.[55]
Local Resection
Local resection of melanomas involving the ciliary body and choroid can be performed using a partial lamellar sclerouvectomy technique.[56] This surgical technique is a modification of the one popularized by Foulds, and later Shields and Damato, in which the tumor is removed with the aim of leaving the retina and vitreous intact.[56][57][58][59] It is best performed in growing small size ciliary body melanomas or choroidal melanomas near the equator. It should be noted that alternative, less invasive treatments, such as plaque brachytherapy, can also be used to treat ciliary body melanomas and choroidal melanomas at the equator. There is no current evidence that local resection of posterior uveal melanoma is different from enucleation or radiotherapy with regard to patient survival. There are fewer complications and better visual results for smaller, more anteriorly located tumors than with medium or larger tumors. More complications can be expected when larger post-equatorial tumors are managed in this manner. In addition, incomplete resection of posterior melanomas is a concern.
Radiotherapy
Presently, radiotherapy is the most widely employed intervention for posterior uveal melanoma. However, between 5% and 10% of patients treated with radiotherapy ultimately require enucleation of the affected eye because of tumor recurrence or radiation complications (e.g. neovascular glaucoma, radiation retinopathy, radiation optic neuropathy).[57][60] Radiation tumor vasculopathy is also common as the irradiated tumor can become ischemic, resulting in macular edema, serous retinal detachment, retinal ischemia, and neovascular glaucoma.
At some centers, transpupillary thermotherapy, photocoagulation, and intravitreal anti-vascular endothelial growth factor or periocular triamcinolone injections are used after radiotherapy to reduce the risk of vascular complications or to actively treat radiation retinopathy and optic neuropathy.
Treatment of ciliary body melanomas with radiation may result in cataracts, punctal occlusion, keratoconjunctivitis, radiation induced anterior uveitis, or scleral necrosis (particulary if large tumors are treated).
Plaque brachytherapy
The most commonly employed form of radiotherapy is brachytherapy using a episcleral radioactive plaque that is temporarily sutured to the scleral surface.[20][45][46][47][48][56][39][61][62][63][64][65][66][67][68] Iodine-125, Ruthenium-106, and Palladium-103 plaques have largely replaced Cobalt-60 at most institutions.[39]
Following the COMS medium size tumor trial, plaque brachytherapy became the standard of care for small and medium-sized melanomas located outside the macular region and posterior to the ora serrata. Shields and associates found that plaque brachytherapy can also be custom fit to treat small, medium, and even large uveal melanoma up to approximately 12 mm in thickness (Table 1).[65] Although treating large melanoma was effective with satisfactory tumor control, complications of radiation maculopathy and papillopathy were higher and often led to poor long term vision.[65]
Innovations in radiotherapeutic planning have allowed plaque brachytherapy to be custom designed to treat uveal melanoma at any site within the eye including the macula using a round or notched plaque, juxtapapillary region using a notched plaque, ciliary body using a round or curvilinear plaque, iris using a curvilinear plaque, and extrascleral extension.[45][46][47][48][62][63][64][65]
Plaque brachytherapy maybe combined with [[Lasers (surgery).
Fine needle aspiration biopsy is usually performed prior to implantation of the radioactive plaque.
External Beam Radiotherapy
In uveal melanoma, external beam radiotherapy in the form of charged particle therapy and stereotactic radiosurgery, have been used.
Charged particle therapy
First used in 1975, charged particle therapy applies a focused beam of collimated protons or helium ions to deliver a high dose of radiation to a targeted area.[56][65][66][67][68] Damage to surrounding tissues is limited due to the Bragg peak effect, where the most destructive ionizing radiation occurs immediately before the location that the particles stop traveling. It is used in medium- to large-sized tumors or for tumors in regions inaccessible to plaque brachytherapy such as the optic disc and fovea. Proton beam therapy is a well-established treatment option but is cost prohibitive and limited to only a few dozen centers around the world.[68] Similar to plaque brachytherapy, radiation complications in the eye and adnexa can occur.[46] Tumor control with charged particle and plaque radiotherapy are similar.
Stereotactic Radiosurgery
Stereotactic radiosurgery is a non-invasive form of therapeutic radiation that is delivered over a small, well-defined 3-dimensional area. GammaKnife and CyberKnife, are examples of stereotactic radiosurgery that have been used against choroidal melanomas unsuited for plaque brachytherapy.[69][70][71] As with plaque brachytherapy, radiation injuries are common. It is noted to have significant reduction in vision in the majority of patients. [72]
Laser therapies
Laser therapies include photocoagulation, transpupillary thermotherapy, and photodynamic therapy. Sometimes lasers are used as adjuncts to brachytherapy. They are rarely used as primary intervention due to the unacceptably high rate of incomplete control, tumor recurrence and extrascleral extension.[73] Lasers are best used as treatment for radiation induced side effects such as retinal detachment, radiation retinopathy and neovascular glaucoma.[73]
Photocoagulation
Transpupillary thermotherapy has largely replaced argon laser for treating selected small choroidal melanomas, particularly those that are less than 3 mm in thickness and located more than 3 mm from the foveola.
Photocoagulation is not used in ciliary body melanomas.
Complications of photocoagulation include branch retinal vein occlusion, cystoid macular edema, epiretinal membrane formation, choroidal neovascularization, vitreous hemorrhage, retinal detachment, recurrence and extrascleral extension.
Photodynamic Therapy
Photodynamic therapy (PDT) involves intravenous administration of a photosensitizer that, when activated by a specific wavelength of light, releases reactive oxidative species within tumor vasculature and induces endothelial changes and thrombosis. PDT with verteporfin for uveal melanoma is infrequently used and very little is reported in the literature. Published studies have shown short-term tumor control while circumventing the risks of radiation retinopathy in small melanomas, but long-term tumor control rates are lower.[46][47][48] One report on 4 patients showed tumor regression for 18 months in one patient, but lack of response or continued growth in 3 patients.[74] Others have emphasized that this is best used with amelanotic choroidal melanoma.[75]
Complications
The complications associated with brachytherapy are associated with the necessity of 2 invasive operations requiring anesthesia and radiotoxic effects on healthy ocular tissue. As posterior choroidal tumors are the most common uveal melanomas, extraocular muscle repositioning is typically required. Radiation induced complications after brachytherapy are numerous and may depend on the tumor size, tumor location, dose of radiation use, and rate of use. Radiation complications are often delayed. Complications to the adnexa and anterior segment early on include diplopia and ptosis and later cataract, symptomatic dry eye, iris neovascularization, and secondary glaucoma. Posterior segment complications of brachytherapy include retinal detachment, cystoid macular edema, optic neuropathy, and hemorrhage of the vitreous, retina, choroid, or a combination of these structures. The most common posterior segment complication, however, is radiation retinopathy[76], with greater than 75% of individuals experiencing this complication following brachytherapy in some reports.[77] The mechanism of injury in radiation retinopathy is hypothesized to be secondary to free radical damage to the vascular endothelial cells leading to occlusion of the capillary beds, microaneurysm formation and retinal ischemia.[78] This can further lead to areas of retinal nonperfusion causing macular edema, neovascularization, and tractional retinal detachment. This may be managed with injections of vascular endothelial growth factor inhibitor, steroids and/or laser.
Proton beam radiotherapy has several recognized complications including cataract, chronic uveitis, hyphema, macula edema, retinal detachment, corneal melting, scleral atrophy and secondary glaucoma. Indeed the incidence of glaucoma following proton beam radiotherapy has been reported to be as high as 53%.[79] [80] Secondary glaucoma is also the leading complication necessitating subsequent enucleation.
Treatment of Metastatic Disease
Ideally, the best management of uveal melanoma would be to use methods of preventing metastasis in the early stages of the intraocular disease. Unfortunately, there is no current method of achieving this. Nearly half of all patients with uveal melanoma will develop metastatic disease. Uveal melanoma spreads exclusively hematogenously, unless it becomes large enough to infiltrate the conjunctival lymphatics. In the Collaborative Ocular Melanoma Study (COMS), the most frequent sites of metastases at time of death were the liver (93%), lung (24%), and bone (16%). More than 80% had multiple sites of metastasis.[81] The 5 and 10-year cumulative metastasis rates are 25% and 34%, respectively.[82] Prognosis is very poor after metastasis has occurred.
Locoregional Treatment of liver metastasis
The liver is the most frequent site of metastasis. About 90% of patients with large choroidal melanomas in the COMS had liver metastases at the time of death, and 57% had exclusive liver metastases.[81] Treating hepatic metastases from uveal melanoma requires a multidisciplinary approach. Treatment choice depends on the number of hepatic metastases, tumor size/volume, and time to systemic metastasis.[83]
Direct surgical procedures on the liver can be used to manage metastatic liver nodules. Other locoregional therapies involve the hepatic artery, which predominantly supplies metastatic tissue.
Surgical resection of metastatic nodules
While good outcomes have been reported in patients with uveal melanoma with metastasis to the liver,[84][85][86] surgical intervention is rarely indicated as most patients present with multiple liver metastases involving both liver lobes.[83][87]
Transcatheter intraarterial therapies
Surgical or local ablative therapies are rarely used because hepatic metastases usually involve multiple regions of the liver. Transcatheter intra-arterial therapies that target the entire liver are more commonly used. These treatments may prolong survival for a few months. However, it is unlikely that it will be curative as most patients will develop progression of liver metastases despite these treatments.
Hepatic intra-arterial chemotherapy
Liver metastases are perfused by the hepatic arteries whereas normal hepatic tissue is primarily supplied by the portal vein. Direct delivery of chemotherapy to the hepatic artery via an indwelling catheter allows maximal exposure of hepatic metastases to cytotoxic chemotherapy while minimizing systemic toxicity. Hepatic intra-arterial chemotherapy with fotemustine, melphalan, cisplatin, vinblastine, and dacarbazine on patients with metastatic uveal melanoma have been performed but no significant increase in overall survival has been observed.[88][89][90]
Transarterial chemoembolization
In hepatic transarterial chemoembolization (TACE), chemotherapy is infused through the hepatic artery followed by selective obstruction of a distal hepatic artery branch to “starve” a metastatic tumor and induce tumor necrosis. Conventional TACE involves the infusion of chemotherapy mixed with lipiodol (ethiodized oil) followed by occlusion of a prespecified hepatic artery branch that feeds the tumor with an embolic agent (e.g. polyvinyl sponge).[91] The use of conventional TACE using a wide variety of chemotherapeutic medications and protocols have been used, but the best chemotherapy choice or embolic therapy remains unknown.[83][91][92][93][94][95][96] Different embolization procedures have emerged. More recently, drug-eluting beads have been used as embolic materials that are infused with anti-cancer medications, such as doxorubicin, irinotecan, and epirubicin.[92][93][97][98][99][100][101]
Patients undergoing TACE must have disease limited to the liver or liver-dominant disease without signs of hepatic failure, such as encephalopathy, or decreased portal vein blood flow (e.g. acute or chronic portal vein thrombosis).[83][102]
Postembolization syndrome, characterized by fever, right upper quadrant abdominal pain, elevated liver enzymes, nausea, and/or vomiting, is a common complication. Other important but rare complications include cholecystitis, pulmonary embolism, hepatic abscess, bile duct injury, gastric mucosal injury, and acute pancreatitis. Hematologic or vascular complications have also been reported.
Transarterial hepatic immunoembolization
Immunoembolization involves hepatic artery embolization using granulocyte-macrophage colony-stimulating factor with lipiodol (instead of chemotherapy as in TACE) to induce tumor ischemia and stimulate antigen presenting cells to facilitate antigen uptake and enhance systemic immunity against tumor cells. One study with 34 participants with metastatic uveal melanoma showed positive outcomes, with an overall survival of 14.4 months.[103][104][105] Common complications include transaminitis, abdominal pain, and nausea/vomiting.
Selective Internal Radiation Therapy
Selective internal radiation therapy, also referred to as transarterial radioembolization (TARE), involves inserting radioactive yttrium-90 microspheres into the hepatic artery that occlude the tumor microvasculature and deliver localized radiation to hepatic tumors. Studies have shown variable results with overall survival ranging from 9 to 24 months in patients with unresectable liver metastases from uveal melanoma.[106][107][108][109][110]
Isolated hepatic perfusion
Isolated hepatic perfusion (IHP) is a complex open surgical procedure where hepatic blood flow is segregated from the systemic vasculature through cannulation and clamping of the hepatic artery and inferior vena cava and joining this circulation to an extracorporeal circuit. This distinct circulation allows administration of highly potent chemotherapies to the entire liver while bypassing systemic circulation and reducing undesirable systemic effects. Melphalan is the most commonly used chemotherapy agent.[111][112][113] However, this surgical procedure is complex and extensive, limiting its availability. IHP is also associated with considerable morbidity and mortality due to veno-occlusive disease and hepatotoxicity. In addition, because of the open surgical nature of this procedure, IHP generally is not repeated.
Percutaneous isolated hepatic perfusion (PHP) is a minimally invasive alternative to IHP. A major advantage of PHP compared to IHP is the potential for multiple perfusion treatments. As with IHP, melphalan is the most commonly investigated chemotherapy agent in metastatic uveal melanoma.[114][115] A randomized phase 3 clinical trial (NCT02678572) demonstrated superior efficacy of PHP compared with best alternative treatment with respect to disease control and overall survival.[116] PHP received FDA approval in August 2023.
Systemic therapy
Chemotherapy is reserved for metastatic uveal melanoma. However, effective chemotherapy agents used against metastatic cutaneous melanoma are generally ineffective in metastatic uveal melanoma. Various antineoplastic agents, including standard chemotherapeutic agents, targeted cancer therapy, and immunotherapy have been investigated, but no significant improvement in overall survival or progression free survival has been observed.[117][118][119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146] However, some recent studies have shown encouraging results.[147]
Conventional chemotherapy
Chemotherapeutic agents have been evaluated as monotherapy or combination therapy, including dacarbazine, temozolomide, treosulfan, and fotemustine, with response rates of <10%.[118][121][122][123][124][125]
Targeted Therapy
Uveal melanoma and cutaneous melanoma are clinically and biologically distinct. In uveal melanoma, mutations in GNAQ and GNA11 are the most common along with mutations to SF3B1, EIF1AX, and BAP1. In contrast, cutaneous melanoma most often involves mutations in BRAF, NRAS, and KIT. While effective immune checkpoint inhibitors have been identified in the treatment against metastatic cutaneous melanoma,[148] these medications have not demonstrated the same effect in metastatic uveal melanoma.[132][133][134][135][136][137][138][140][141][142][149][150][151][152][153][154][155][156][157][158][159][160] However, novel and promising treatments are under investigation.[146][161][162]
One promising treatment option is tebentafusp, a bispecific fusion protein (gp100 peptide) that is composed of a monoclonal high-affinity T cell receptor, which presents uveal melanoma antigens, fused to an anti-CD3 single-chain antibody fragment that activates cytotoxic T cells. A phase 3 trial of tebentafusp was the first to show reduced risk of death from metastatic uveal melanoma compared to investigator’s choice (dacarbazine, pembrolizumab, or ipilimumab) in treatment-naive HLAA*02:01-positive patients with metastatic uveal melanoma; FDA approval occurred in 2022.[147]
Adjuvant Therapy
At present, no effective adjuvant therapy decreases the risk of metastasis or improves overall survival.[163][164][165][166][167]
Complications
As radiotherapy is the most commonly used treatment option, the main ocular complication following radiotherapy is retinopathy with resultant decreased visual acuity.[168][169][170][171] Gunduz and associates studied retinopathy following plaque radiotherapy and noted nonproliferative retinopathy in 42% at 5 years and proliferative retinopathy in 8% at 5 years.[169] Other radiation complications included cataract (39%), papillopathy (8%), vitreous hemorrhage (7%), neovascular glaucoma (1%), and scleral necrosis (1%). The treatment of these complications involves laser photocoagulation, anti-vascular endothelial growth factor medications, and anti-inflammatory medications.[169][170]
Prognosis
The prognosis for uveal melanoma should be considered in terms of life, the globe, and visual acuity. With regards to life prognosis, uveal melanoma prognosis has been shown to be dependent on several clinical factors including tumor location in the ciliary body, large tumor size, diffuse (flat) configuration, and extraocular extension as well as histopathologic and cytogenetic factors including epithelioid cell type, increased mitotic activity, infiltrating lymphocytes, tumor vascular networks, and chromosomal mutations including monosomy 3 and 8q addition.[28][32][172][173][174] In several articles, tumor size has been identified as one of the key clinical features predictive of metastasis. One large analysis on 8033 eyes with uveal melanoma found that increasing millimeter thickness of uveal melanoma was associated with increasing risk for metastasis (Table 1).[32] These findings highlight the importance of close monitoring and early intervention.
Table 1. Probability for systemic metastasis from posterior uveal melanoma based on millimeter increments in tumor thickness at 3, 5, and 10 years after diagnosis
| Tumor thickness, mm | Probability for Systemic Metastasis, % | |||
| 3 years | 5 years | 10 years | 20 years | |
| Using 1-mm increments | ||||
| 0.0-1.0 | 2.4 | 5.7 | 5.7 | |
| 1.1-2.0 | 2.3 | 7.9 | 12.0 | |
| 2.1-3.0 | 2.0 | 4.6 | 11.8 | |
| 3.1-4.0 | 3.1 | 8.1 | 16.3 | |
| 4.1-5.0 | 7.5 | 15.2 | 26.8 | |
| 5.1-6.0 | 8.6 | 17.3 | 27.9 | |
| 6.1-7.0 | 9.5 | 15.2 | 28.8 | |
| 7.1-8.0 | 13.0 | 21.3 | 40.8 | |
| 8.1-9.0 | 17.6 | 31.1 | 50.2 | |
| 9.1-10.0 | 18.2 | 30.7 | 43.7 | |
| > 10.0 | 27.8 | 40.2 | 51.0 | |
| Using small, medium, and large COMS classifications | ||||
| Small (0.0-3.0) | 2.1 | 5.6 | 11.5 | 19.7 |
| Medium (3.1-8.0) | 7.2 | 13.9 | 25.5 | 37.3 |
| Large (> 8.0) | 22.3 | 35.0 | 49.2 | 66.9 |
Fine needle aspiration biopsy of uveal melanoma is used for prognostication and is performed immediately prior to radioactive plaque placement or in eyes undergoing brachytherapy or after enucleation. Chromosomal and genetic analysis, gene-expression-profile testing, and cytogenetic prognostic testing can aid in treatment decisions. There is an association of uveal melanoma with monosomy 3.[175] A retrospective study analyzing tumors in enucleated specimens demonstrated that no individuals with two copies of chromosomes 3 developed metastatic disease, however, 57% of those with monosomy 3 developed metastatic disease with a 3 year survival rate of 50%.[176] With this finding, direct tissue biopsy obtained via fine needle aspiration increased in popularity to offer screening for patients during therapeutic procedures for high-risk of metastasis. Patients found to have evidence for monosomy 3 were selected for adjuvant therapy or closer monitoring.
A clinically validated gene expression profile (GEP) test for uveal melanoma prognostication (DecisionDx-UM) is currently the standard of care in most ocular oncology centers in the United States (Table 2).[177] This test was validated by the Collaborative Ocular Oncology Group after it successfully classified 97.2% of cases.[178]
Table 2. Prognostication with gene expression profiling
| RNA GEP Class | Metastatic Risk | 5-year metastatic risk | Associated genetic mutation |
| Class 1A | Low | 2% | SF3B1, EIF1AX |
| Class 1B | Intermediate | 21% | SF3B1, EIF1AX |
| Class 2 | High | 72% | BAP1 |
Harbour et al described the influential role of the gene BAP1 in regulating the metastasis of uveal melanoma as it does in other cancers involving the lung, breast, and kidney.[179] The BAP1 gene codes for a deubiquitinating enzyme that binds to BRCA1 and BARD1 to create a heterodimeric tumor suppressor complex. Inactivation of this gene has been described in up to 84% of class 2 uveal melanomas.[180] As BAP1 is mapped to chromosome 3p21.31-p21.2, a loss of this region, as in monosomy 3, could predispose an individual to uveal melanoma via the classic mechanism underlying other tumor suppressor genes. While multiple genes are likely involved in the development of metastatic uveal melanoma, Onken et al has been the leader in translating this research into an applicable clinical test. [181] Onken observed that monosomy 3 had limited ability in prognosis for uveal melanoma and thereafter identified 12 genes found to be predictive of metastatic disease. Using a 15 gene microarray assay, including 3 control genes, Onken et al found this test to accurately discriminate between low-grade (class 1A and class 1B) and high-grade (class 2) uveal melanoma. Both fine needle aspirate biopsies and formalin fixed, paraffin embedded tumor tissue provide adequate supply of tumor cells while being safe and technically simple.[182] This technique, termed multi-gene expression profiling, has been compared against the other various prognostic indicators for accuracy in predicting risk of metastasis and was found to be superior to all other clinical, histopathologic and cytogenetic biomarkers.[178] [183] [184] [185] The multi-gene expression profile (GEP) assay was initially offered in 2009 and remains the only prognostic test for uveal melanoma validated in a multi-center prospective study.[178] Overexpression of the cancer-testis antigen PRAME has been identified as an additional molecular risk factor independent of GEP status. The presence of PRAME mRNA can be detected from the same fine needle aspirate biopsy obtained for GEP. A hybrid prognostic model incorporating both PRAME and GEP status demonstrated greater accuracy in predicting metastatic disease[186] and is now included as part of the interpretation of most prognostic biopsies.
Another, less commonly utilized, modality involved in the workup and prognosis of uveal melanoma is peripheral blood cytology. It has been hypothesized that most uveal melanoma has already metastasized to some extent by the time of diagnosis. Researchers looking into this found that indeed, there was evidence for cells with specific tumor markers for malignant uveal melanoma in the peripheral blood. Callejo et al[187] have demonstrated that peripheral cytology can predict, if not determine, metastasis of uveal melanoma. The strengths of this developing test are to catch metastasis as early as possible and to assist in distinguishing high-risk melanoma from lower risk lesions.
With regards to the globe, nearly 95% of patients treated with conservative measures such as radiotherapy or resection, maintain their globe on follow up. The greater the tumor thickness, the greater the risk for enucleation.[57][58][188]
With regards to visual acuity, a study on 1106 consecutive plaque-irradiated patients with uveal melanoma found poor visual acuity (20/200 or worse) in 34% at 5 years and 68% at 10 years of follow-up.[189] Factors related to poor visual acuity included increasing tumor thickness, proximity of tumor to foveola of less than 5 mm, evidence of tumor recurrence, patient age 60 years or older, subretinal fluid, cobalt isotope, tumor posterior to equator, and worse initial visual acuity.
Follow-up
Patients with uveal melanoma should have regular systemic follow up examinations by both an ocular oncologist and a medical oncologist. The ocular oncologist should monitor the uveal scar for tumor regression and complications of therapy. The medical oncologist should survey for metastatic disease. Particular evaluation of the liver, lung, and skin should be made as this malignancy most often metastasizes to these sites. Physical examination and liver function testing is recommended twice yearly, as well as annual liver magnetic resonance imaging and chest radiography for monitoring or CT chest/abdomen/pelvis or PET CT(less commonly used due to amount of radiation exposure).
Historical Perspectives
In 1809, the first known complete natural history of uveal melanoma was documented by Scottish surgeons Allan Burns and James Wardrop.[190] In 1882, Ernst Fuchs was the first to describe sarcom des uvealtractus (“uveal sarcoma,” now known as uveal melanoma) as “one of the most malignant of diseases” and recommended enucleation as the treatment of choice to prevent metastasis and death.[191][192][193]
The Zimmerman-McLean-Foster Hypothesis
Following enucleation, metastatic disease and mortality remained high. In the 1970s, a landmark paper by a group of expert ophthalmic pathologists proposed that an elevated intraocular pressure while cutting the optic nerve at the time of enucleation could facilitate the passage of tumor cells through the vortex veins into systemic circulation thereby resulting in liver metastases.[22][23][25][30] This became known as the “Zimmerman-McLean-Foster Hypothesis.” Although this hypothesis sparked controversy regarding the safety of enucleation, it also inspired new surgical techniques, such as the “no touch enucleation,” and encouraged the use of globe-salvaging therapies such as radiation and laser therapies. Further studies have refuted this hypothesis, and have attributed high frequencies of liver metastases to micrometastases that occur years prior to the initial clinical diagnosis of uveal melanoma.[194]
The Collaborative Ocular Melanoma Study
The Collaborative Ocular Melanoma Study (COMS) is a prospective multicenter trial that was funded and organized by the National Eye Institute in 1985 to evaluate the role of different interventions for patients with uveal melanoma.[195] The first patient was enrolled in 1987 and accrual was completed in 1998. To date, the COMS is the largest study performed in ocular oncology. There were three major prospective multicenter COMS trials (Table 3). The results of the COMS confirmed numerous previous publications regarding management of choroidal melanoma.
Large-size tumor trial
The “Zimmerman-McLean-Foster Hypothesis” inspired the use of additional treatments with enucleation, such as pre- or post-enucleation radiation, particularly in large choroidal melanomas. This trial sought to evaluate if pre-enucleation radiotherapy offered a survival benefit compared to enucleation alone.
Large choroidal melanomas were defined as 2.0 mm or more in apical height and greater than 16.0 mm in longest basal diameter, or more than 10.0 mm apical height regardless of basal diameter, or greater than 8.0 mm apical height regardless of basal diameter if less than 2.0 mm to the optic disc.
The randomized controlled prospective large-sized tumor trial involved 1003 treatment-naive patients with large choroidal melanomas who were treated with enucleation alone (n=506) or pretreatment external beam radiotherapy (20 Gy) followed by enucleation (n=497). In the pretreatment group, radiotherapy was delivered as five daily fractions of 4.0 Gy and eyes were enucleated within 80 hours after the final fraction. All eyes were enucleated within 4 weeks after randomization in both groups. Patients were monitored 6 and 12 months after enrollment, then annually. The primary outcome was survival rates in each treatment group. The 5-year survival rates were 57% in the enucleation alone group and 62% with pre-enucleation radiation. The authors concluded that there were no survival differences between pre-enucleation radiotherapy and enucleation alone in patients with large choroidal melanomas.[196]
The 10-year survival rates were 40% in the enucleation alone group and 45% in the pretreatment radiation group. The authors concluded that no survival differences were observed.[197]
Following these trials, pre-enucleation radiotherapy was abandoned.
Medium-size tumor trial
Following the concerns of the “Zimmermann-McLean-Foster Hypothesis,” radiotherapy was considered the best alternative to enucleation for medium-sized choroidal melanomas. This trial aimed to evaluate differences in survival rates in patients treated with iodine-125 plaque brachytherapy alone compared to treatment with enucleation alone.
Medium-sized choroidal melanomas were defined as 2.5 to 10.0 mm in apical height and no more than 16.0 mm in longest basal diameter.
The randomized controlled prospective medium-sized tumor trial involved 1317 treatment-naive patients with choroidal melanomas who were treated with enucleation (n=660) or iodine-125 plaque brachytherapy (n=657). Plaque brachytherapy treatment or enucleation was performed within 4 weeks after enrollment. The primary outcome was survival rates in each treatment group. The 5-year survival rates were 81% for the enucleation group and 82% for the iodine 125-plaque brachytherapy group (p=0.48). The authors concluded that there were no survival differences in patients treated with enucleation and patients treated with iodine-125 plaque brachytherapy.
The 12-year survival rates were 41% in the enucleation group and 43% in the iodine-125 plaque brachytherapy group.
The results of these trials encouraged the use of iodine-125 plaque brachytherapy for medium-sized choroidal melanomas.
Small-size tumor trial
The treatment of small melanomas has been controversial and many were managed with only observation. Treatment was often deferred in small melanomas because of the potential for severe visual impairment after radiation.
Small choroidal melanomas were defined as 1.0 to 3.0 mm in apical height and 5.0 to 16.0 mm in largest basal diameter.
The prospective observational small-size tumor trial involved 188 patients who were managed by observation. The primary outcome was tumor growth, which was defined as an increase to a medium-size or large-size choroidal melanoma based on COMS tumor classification criteria. In untreated small choroidal melanomas, 11% grew by 1 year, 21% by 2 years, and 31% by 5 years.
Table 3. Brief summary of three major prospective trials in the Collaborative Ocular Melanoma Study
| Trial | Design | Interventions | Findings |
| Large-size* tumor trial[196] | Randomized controlled | Enucleation alone vs external beam radiotherapy followed by enucleation | No difference between 5-year survival rates between enucleation alone (57%) and enucleation with pretreatment external beam radiotherapy (62%) in large choroidal melanomas. |
| Medium-size** tumor trial[198] | Randomized controlled | Enucleation vs iodine-125 plaque brachytherapy | No difference in 5-year survival rates between enucleation (81%) and iodine-125 plaque brachytherapy (82%) in medium-size choroidal melanomas. |
| Small-size*** tumor trial[199] | Observational | Observation | Tumor growth in untreated small choroidal melanomas were 11% by 1 year, 21% by 2 years, and 31% by 5 years. |
Additional Resources
- Shields JA, Shields CL. Intraocular Tumors: A Text and Atlas. Philadelphia: WB Saunders, 1992.
- Shields JA, Shields CL. Intraocular Tumors. An Atlas and Textbook. 2nd edition. Philadelphia, Lippincott Williams and Wilkins, 2008.
- www.fighteyecancer.com
- www.choroidalmelanoma.com
- www.melanomaeye.com
- www.malignantmelanomainfo.com
References
- ↑ American Academy of Ophthalmology. Choroidal melanoma. https://www.aao.org/image/choroidal-melanoma-4 Accessed June 28, 2019.
- ↑ Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011 Sep;118(9):1881-5.).
- ↑ Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012 Jul;32(7):1363-72.
- ↑ Ezekiel Weis, MD, MPH, Chirag P. Shah, MD, MPH, Martin Lajous, MD, Jerry A. Shields, MD, Carol L. Shields, MD. The Association Between Host Susceptibility Factors and Uveal Melanoma: A Meta-analysis. Arch Ophthalmol. 2006;124(1):54-60.
- ↑ Johanna M. Seddon, MD; Evangelos S. Gragoudas, MD; Robert J. Glynn, ScD; Kathleen M. Egan, MPH; Daniel M. Albert, MD; Peter H. Blitzer, MD. Host Factors, UV Radiation, and Risk of Uveal Melanoma. Arch Ophthalmol. 1990;108(9):1274-1280.
- ↑ Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005 Oct;112(10):1784-9
- ↑ Jump up to: 7.0 7.1 Decatur CL, Ong E, Garg N, Anbunathan H, Bowcock AM, Field MG, Harbour JW. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA Ophthalmol. 2016 Jul 1;134(7):728-33.
- ↑ Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, Sozen MM, Baimukanova G, Roy R, Heguy A, Dolgalev I, Khanin R, Busam K, Speicher MR, O'Brien J, Bastian BC. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010 Dec 2;363(23):2191-9.
- ↑ Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, Harbour JW. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008 Dec;49(12):5230-4.
- ↑ Damato EM, Damato BE. Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients. Ophthalmology. 2012 Aug;119(8):1582-9.
- ↑ Shields C, Materin M, Shields J, Gershenbaum E, Singh A, Smith A. Factors associated with elevated intraocular pressure in eyes with iris melanoma. Br J Ophthalmol 2001;85:666-669
- ↑ Jump up to: 12.0 12.1 Shields CL, Shields JA, Kiratli H, et al. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 1995;102:1351–1361.
- ↑ Jump up to: 13.0 13.1 13.2 Shields CL, Cater J, Shields JA, et al. Combination of Clinical Factors Predictive of Growth of Small Choroidal Melanocytic Tumors. Arch Ophthalmol 2000;118:360–364.
- ↑ Jump up to: 14.0 14.1 Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr Opin Ophthalmol 2002;13:135–141.
- ↑ Jump up to: 15.0 15.1 Shields CL, Kels JG, Shields JA. Melanoma of the eye: revealing hidden secrets, one at a time. Clin Dermatol 2015;33:183–196.
- ↑ Jump up to: 16.0 16.1 Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol 2009;127:981–987.
- ↑ Shields CL, Kaliki S, Rojanaporn D, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: comparison with choroidal nevus. Arch Ophthalmol. 2012 Jul;130(7):850-6
- ↑ Shields CL, Shields JA. Recent developments in the management of choroidal melanoma. Curr Opin Ophthalmol 2004;15:244–251.
- ↑ Robertson DM. Changing concepts in the management of choroidal melanoma. Am J Ophthalmol 2003;136:161–170.
- ↑ Jump up to: 20.0 20.1 Shields CL, Shields JA, Gündüz K, et al. Radiation therapy for uveal malignant melanoma. Ophthalmic Surg Lasers 1998;29:397–409.
- ↑ Shields JA, Shields CL. Surgical approach to lamellar sclerouvectomy for posterior uveal melanomas: the 1986 Schoenberg lecture. Ophthalmic Surg 1988;19:774–780.
- ↑ Jump up to: 22.0 22.1 22.2 Zimmerman LE, McLean IW. An evaluation of enucleation in the management of uveal melanomas. Int Ophthalmol Clin 1980;20:1–31.
- ↑ Jump up to: 23.0 23.1 23.2 Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br J Ophthalmol 1978;62:420–425.
- ↑ Jump up to: 24.0 24.1 Manschot WA, van Peperzeel HA. Choroidal melanoma. Enucleation or observation? A new approach. Arch Ophthalmol 1980;98:71–77.
- ↑ Jump up to: 25.0 25.1 25.2 Seigel D, Myers M, Ferris F, Steinhorn SC. Survival Rates After Enucleation of Eyes with Malignant Melanoma. Am J Ophthalmol 1979;87:761–765.
- ↑ Jump up to: 26.0 26.1 Shields JA, Shields CL, Donoso LA. Management of posterior uveal melanoma. Surv Ophthalmol 1991;36:161–195.
- ↑ Jump up to: 27.0 27.1 Shields JA, Shields CL. The management of posterior uveal melanoma. In: Smith ME, ed. Intraocular Tumors. A Text and Atlas.Vol 114. Philadelphia: Saunders; 1992:191–192.
- ↑ Jump up to: 28.0 28.1 28.2 Shields JA, Shields CL. Intraocular Tumors: An Atlas and Textbook. Lippincott Williams & Wilkins; 2015.
- ↑ Shields JA. Counseling the patient with a posterior uveal melanoma. Am J Ophthalmol 1988;106:88–91.
- ↑ Jump up to: 30.0 30.1 Singh AD, Rennie IG, Kivela T, et al. The Zimmerman-McLean-Foster hypothesis: 25 years later. Br J Ophthalmol 2004;88:962–967.
- ↑ Straatsma BR, Fine SL, Earle JD, et al. Enucleation versus plaque irradiation for choroidal melanoma. Ophthalmology 1988;95:1000–1004.
- ↑ Jump up to: 32.0 32.1 32.2 32.3 32.4 Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol 2009;127:989–998.
- ↑ Chien JL, Sioufi K, Surakiatchanukul T, et al. Choroidal nevus: a review of prevalence, features, genetics, risks, and outcomes. Curr Opin Ophthalmol 2017;28:228–237.
- ↑ Sumich P, Mitchell P, Wang JJ. Choroidal nevi in a white population: the Blue Mountains Eye Study. Arch Ophthalmol 1998;116:645–650.
- ↑ Ganley JP, Comstock GW. Benign nevi and malignant melanomas of the choroid. Am J Ophthalmol 1973;76:19–25.
- ↑ Jump up to: 36.0 36.1 Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005;112:1784–1789.
- ↑ Mashayekhi A, Siu S, Shields CL, Shields JA. Slow enlargement of choroidal nevi: a long-term follow-up study. Ophthalmology 2011;118:382–388.
- ↑ Straatsma BR, Diener-West M, Caldwell R, Engstrom RE. Mortality after deferral of treatment or no treatment for choroidal melanoma. Am J Ophthalmol 2003;136:47–54.
- ↑ Jump up to: 39.0 39.1 39.2 Shields JA, Augsburger JJ, Brady LW, Day JL. Cobalt plaque therapy of posterior uveal melanomas. Ophthalmology 1982;89:1201–1207.
- ↑ Packer S, Rotman M, Salanitro P.Iodine-125 irradiation of choroidal melanoma. Clinical experience. Ophthalmology. 1984; 91(12):1700-8.
- ↑ Gass JD. Comparison of prognosis after enucleation vs cobalt 60 irradiation of melanomas. Arch Ophthalmol. 1985 Jul;103(7):916-23.
- ↑ Jump up to: 42.0 42.1 Nag S, Quivey JM, Earle JD, et al. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 2003; 56:544.
- ↑ J.M Quivey, J Augsburger, L Snelling et al. 125I plaque therapy for uveal melanoma. Analysis of the impact of time and dose factors on local control. Cancer, 77 (1996), pp. 2356–2362
- ↑ Jump up to: 44.0 44.1 Wang Z, Nabhan M, Schild SE, et al. Charged particle radiation therapy for uveal melanoma: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2013; 86:18.
- ↑ Jump up to: 45.0 45.1 45.2 De Potter P, Shields CL, Shields JA, Cater JR. Impact of enucleation versus plaque radiotherapy in the management of juxtapapillary choroidal melanoma on patient survival. Br J Ophthalmol 1994;78:109–114.
- ↑ Jump up to: 46.0 46.1 46.2 46.3 46.4 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for choroidal melanoma encircling the optic disc (circumpapillary choroidal melanoma). Arch Ophthalmol 2007;125:1202–1209.
- ↑ Jump up to: 47.0 47.1 47.2 47.3 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma overhanging the optic disc in 141 consecutive patients. Arch Ophthalmol 2008;126:1515–1522.
- ↑ Jump up to: 48.0 48.1 48.2 48.3 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: tumor control in 650 consecutive cases. Ophthalmology 2011;118:402–407.
- ↑ Wilson RS, Fraunfelder FT. “No-touch” cryosurgical enucleation: a minimal trauma technique for eyes harboring intraocular malignancy. Ophthalmology 1978;85:1170–1175.
- ↑ Dutton JJ. Coralline hydroxyapatite as an ocular implant. Ophthalmology 1991;98:370–377.
- ↑ Shields CL, Shields JA, De Potter P. Hydroxyapatite orbital implant after enucleation. Experience with initial 100 consecutive cases. Arch Ophthalmol 1992;110:333–338.
- ↑ Jump up to: 52.0 52.1 Shields CL, Uysal Y, Marr BP, et al. Experience with the polymer-coated hydroxyapatite implant after enucleation in 126 patients. Ophthalmology 2007;114:367–373.
- ↑ Hornblass A, Biesman BS, Eviatar JA. Current techniques of enucleation: a survey of 5,439 intraorbital implants and a review of the literature. Ophthal Plast Reconstr Surg 1995;11:77–86; discussion 87–88.
- ↑ Shields JA, Shields CL, Suvarnamani C, et al. Orbital exenteration with eyelid sparing: indications, technique, and results. Ophthalmic Surg 1991;22:292–297.
- ↑ Jump up to: 55.0 55.1 Shields JA, Shields CL, Demirci H, et al. Experience with eyelid-sparing orbital exenteration: the 2000 Tullos O. Coston Lecture. Ophthal Plast Reconstr Surg 2001;17:355–361.
- ↑ Jump up to: 56.0 56.1 56.2 56.3 Seddon JM, Gragoudas ES, Albert DM, et al. Comparison of survival rates for patients with uveal melanoma after treatment with proton beam irradiation or enucleation. Am J Ophthalmol 1985;99:282–290.
- ↑ Jump up to: 57.0 57.1 57.2 Shields CL, Shields JA, Karlsson U, et al. Reasons for enucleation after plaque radiotherapy for posterior uveal melanoma. Clinical findings. Ophthalmology 1989;96:919–23; discussion 924.
- ↑ Jump up to: 58.0 58.1 Shields CL, Shields JA, Karlsson U, et al. Enucleation after Plague Radiotherapy for Posterior Uveal Melanoma: Histopathologic Findings. Ophthalmology 1990;97:1665–1670.
- ↑ Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol 2001;119:969–982.
- ↑ Shields CL, Shields JA, Karlsson U, et al. Enucleation after plaque radiotherapy for posterior uveal melanoma. Histopathologic findings. Ophthalmology 1990;97:1665–1670.
- ↑ Shields CL, Cater J, Shields JA, et al. Combined plaque radiotherapy and transpupillary thermotherapy for choroidal melanoma: tumor control and treatment complications in 270 consecutive patients. Arch Ophthalmol 2002;120:933–940.
- ↑ Jump up to: 62.0 62.1 Gündüz K, Shields CL, Shields JA, et al. Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol 1999;117:170–177.
- ↑ Jump up to: 63.0 63.1 Shields CL, Naseripour M, Shields JA, et al. Custom-designed plaque radiotherapy for nonresectable iris melanoma in 38 patients: tumor control and ocular complications. Am J Ophthalmol 2003;135:648–656.
- ↑ Jump up to: 64.0 64.1 Gündüz K, Shields CL, Shields JA, et al. Plaque radiotherapy for management of ciliary body and choroidal melanoma with extraocular extension. Am J Ophthalmol 2000;130:97–102.
- ↑ Jump up to: 65.0 65.1 65.2 65.3 65.4 Gündüz K, Shields CL, Shields JA, et al. Plaque radiotherapy for management of ciliary body and choroidal melanoma with extraocular extension. Am J Ophthalmol 2000;130:97–102.
- ↑ Jump up to: 66.0 66.1 Char DH, Kroll SM, Castro J. Ten-year follow-up of helium ion therapy for uveal melanoma. Am J Ophthalmol 1998;125:81–89.
- ↑ Jump up to: 67.0 67.1 Gragoudas ES, Goitein M, Verhey L, et al. Proton beam irradiation. An alternative to enucleation for intraocular melanomas. Ophthalmology 1980;87:571–581.
- ↑ Jump up to: 68.0 68.1 68.2 Gragoudas ES, Lane AM, Munzenrider J, et al. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans Am Ophthalmol Soc 2002;100:43–8; discussion 48–49.
- ↑ Mueller AJ, Talies S, Schaller UC, et al. Stereotactic radiosurgery of large uveal melanomas with the gamma-knife. Ophthalmology 2000;107:1381–7; discussion 1387–1388.
- ↑ Dieckmann K, Georg D, Bogner J, et al. Optimizing LINAC-based stereotactic radiotherapy of uveal melanomas: 7 years’ clinical experience. Int J Radiat Oncol Biol Phys 2006;66:S47–S52.
- ↑ Özcan G, Gündüz AK, Mirzayev İ, et al. Early Results of Stereotactic Radiosurgery in Uveal Melanoma and Risk Factors for Radiation Retinopathy. Turk J Ophthalmol 2020;50:156–162.
- ↑ van Beek JGM, van Rij CM, Baart SJ, et al. Fractionated stereotactic radiotherapy for uveal melanoma: Long-term outcome and control rates. Acta Ophthalmol. 2022;100(5):511-519. doi:10.1111/aos.15029
- ↑ Jump up to: 73.0 73.1 Maheshwari A, Finger PT. Laser treatment for choroidal melanoma: Current concepts. Surv Ophthalmol. 2023;68(2):211-224. doi:10.1016/j.survophthal.2022.05.002
- ↑ Barbazetto IA, Lee TC, Rollins IS, et al. Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol 2003;135:898–899.
- ↑ Donaldson MJ, Lim L, Harper CA, et al. Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Experiment Ophthalmol 2005;33:548–549.
- ↑ P.T Finger. Tumour location affects the incidence of cataract and retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol, 84 (2000), pp. 1068 -1070
- ↑ Murray TG, Markoe AM, Gold AS, Ehlies F, Bermudez E, Wildner A, Latiff A. Long-term follow-up comparing two treatment dosing strategies of (125) I plaque radiotherapy in the management of small/medium posterior uveal melanoma. J Ophthalmol. 2013;2013:517032.
- ↑ Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy--clinical, histopathological, ultrastructural and experimental correlations. Eye. 1991;5 ( Pt 2):239-251.
- ↑ Rundle P, Singh AD, Rennie I. Proton beam therapy for iris melanoma: a review of 15 cases. Eye (Lond). 2007 Jan;21(1):79-82.
- ↑ Tsuji H, Ishikawa H, Yanagi T, et al.: Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a Phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys 67 (3): 857-62, 2007.
- ↑ Jump up to: 81.0 81.1 Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol 2001;119:670–676.
- ↑ Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthal 2005;123:1639–1643.
- ↑ Jump up to: 83.0 83.1 83.2 83.3 Sato T. Locoregional management of hepatic metastasis from primary uveal melanoma. Semin Oncol 2010;37:127–138.
- ↑ Aoyama T, Mastrangelo MJ, Berd D, et al. Protracted survival after resection of metastatic uveal melanoma. Cancer 2000;89:1561–1568.
- ↑ Frenkel S, Nir I, Hendler K, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol 2009;93:1042–1046.
- ↑ Hand F, Doherty S, Gullo G, et al. Metastatic uveal melanoma: A valid indication for liver resection. J BUON 2020;25:1161–1165.
- ↑ Carvajal RD, Schwartz GK, Tezel T, et al. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 2017;101:38–44.
- ↑ Leyvraz S, Piperno-Neumann S, Suciu S, et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol 2014;25:742–746.
- ↑ Boone BA, Perkins S, Bandi R, et al. Hepatic artery infusion of melphalan in patients with liver metastases from ocular melanoma. J Surg Oncol 2018;117:940–946.
- ↑ Melichar B, Voboril Z, Lojík M, Krajina A. Liver metastases from uveal melanoma: clinical experience of hepatic arterial infusion of cisplatin, vinblastine and dacarbazine. Hepatogastroenterology 2009;56:1157–1162.
- ↑ Jump up to: 91.0 91.1 Huppert PE, Fierlbeck G, Pereira P, et al. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol 2010;74:e38–44.
- ↑ Jump up to: 92.0 92.1 Carrasco CH, Wallace S, Charnsangavej C, et al. Treatment of hepatic metastases in ocular melanoma. Embolization of the hepatic artery with polyvinyl sponge and cisplatin. JAMA 1986;255:3152–3154.
- ↑ Jump up to: 93.0 93.1 Patel K, Sullivan K, Berd D, et al. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a phase II study. Melanoma Res 2005;15:297–304.
- ↑ Vogl T, Eichler K, Zangos S, et al. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol 2007;133:177–184.
- ↑ Valpione S, Aliberti C, Parrozzani R, et al. A retrospective analysis of 141 patients with liver metastases from uveal melanoma: a two-cohort study comparing transarterial chemoembolization with CPT-11 charged microbeads and historical treatments. Melanoma Res 2015;25:164–168.
- ↑ Shibayama Y, Namikawa K, Sone M, et al. Efficacy and toxicity of transarterial chemoembolization therapy using cisplatin and gelatin sponge in patients with liver metastases from uveal melanoma in an Asian population. Int J Clin Oncol 2017;22:577–584.
- ↑ Agarwala SS, Panikkar R, Kirkwood JM. Phase I/II randomized trial of intrahepatic arterial infusion chemotherapy with cisplatin and chemoembolization with cisplatin and polyvinyl sponge in patients with ocular melanoma metastatic to the liver. Melanoma Res 2004;14:217–222.
- ↑ Poon RTP, Tso WK, Pang RWC, et al. A Phase I/II Trial of Chemoembolization for Hepatocellular Carcinoma Using a Novel Intra-Arterial Drug-Eluting Bead. Clin Gastroenterol Hepatol 2007;5:1100–1108.
- ↑ Fiorentini G, Aliberti C, Del Conte A, et al. Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo 2009;23:131–137.
- ↑ Tan AL, Eschelman DJ, Gonsalves CF, et al. Treatment of bulky uveal melanoma (UN) hepatic metastases with doxorubicin eluting beads (DEBDOX) followed by 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) TACE: an initial experience. J Vasc Interv Radiol 2014;25:S45.
- ↑ Nam HC, Jang B, Song MJ. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma. World J Gastroenterol 2016;22:8853.
- ↑ Piscaglia F, Ogasawara S. Patient Selection for Transarterial Chemoembolization in Hepatocellular Carcinoma: Importance of Benefit/Risk Assessment. Liver Cancer 2018;7:104–119.
- ↑ Valsecchi ME, Terai M, Eschelman DJ, et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol 2015;26:523–32.e2.
- ↑ Yamamoto A, Chervoneva I, Sullivan KL, et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology 2009;252:290–298.
- ↑ Sato T, Eschelman DJ, Gonsalves CF, et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol 2008;26:5436–5442.
- ↑ Eldredge-Hindy H, Ohri N, Anne PR, et al. Yttrium-90 Microsphere Brachytherapy for Liver Metastases From Uveal Melanoma: Clinical Outcomes and the Predictive Value of Fluorodeoxyglucose Positron Emission Tomography. Am J Clin Oncol 2016;39:189–195.
- ↑ Gonsalves CF, Eschelman DJ, Adamo RD, et al. Radioembolization for treatment of uveal melanoma hepatic metastasis: Results of a phase II, single institution, prospective trial. J Clin Orthod 2018;36:9535–9535.
- ↑ Tulokas S, Mäenpää H, Peltola E, et al. Selective internal radiation therapy (SIRT) as treatment for hepatic metastases of uveal melanoma: a Finnish nation-wide retrospective experience. Acta Oncol 2018;57:1373–1380.
- ↑ Gonsalves CF, Eschelman DJ, Adamo RD, et al. A Prospective Phase II Trial of Radioembolization for Treatment of Uveal Melanoma Hepatic Metastasis. Radiology 2019;293:223–231.
- ↑ Ponti A, Denys A, Digklia A, et al. First-Line Selective Internal Radiation Therapy in Patients with Uveal Melanoma Metastatic to the Liver. J Nucl Med 2020;61:350–356.
- ↑ Alexander HR, Libutti SK, Bartlett DL, et al. A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res 2000;6:3062–3070.
- ↑ Olofsson R, Ny L, Eilard MS, et al. Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases (the SCANDIUM trial): study protocol for a randomized controlled trial. Trials 2014;15:317.
- ↑ Ben-Shabat I, Belgrano V, Hansson C, Olofsson Bagge R. The effect of perfusate buffering on toxicity and response in isolated hepatic perfusion for uveal melanoma liver metastases. Int J Hyperthermia 2017;33:483–488.
- ↑ Karydis I, Gangi A, Wheater MJ, et al. Percutaneous hepatic perfusion with melphalan in uveal melanoma: A safe and effective treatment modality in an orphan disease. J Surg Oncol 2018;117:1170–1178.
- ↑ Meijer TS, Burgmans MC, de Leede EM, et al. Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Ocular Melanoma Metastases Confined to the Liver: A Prospective Phase II Study. Ann Surg Oncol 2021;28:1130–1141.
- ↑ Zager JS, Orloff MM, Ferrucci PF, et al. FOCUS phase 3 trial results: Percutaneous hepatic perfusion (PHP) with melphalan for patients with ocular melanoma liver metastases (PHP-OCM-301/301A). Journal of Clinical Oncology 2022 40:16
- ↑ Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev 2012;38:549–553.
- ↑ Jump up to: 118.0 118.1 Kivelä T, Suciu S, Hansson J, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer 2003;39:1115–1120.
- ↑ Albert DM, Ryan LM, Borden EC. Metastatic ocular and cutaneous melanoma: a comparison of patient characteristics and prognosis. Arch Ophthalmol 1996;114:107–108.
- ↑ Pons F, Plana M, Caminal JM, et al. Metastatic uveal melanoma: is there a role for conventional chemotherapy? - A single center study based on 58 patients. Melanoma Res 2011;21:217–222.
- ↑ Jump up to: 121.0 121.1 Bedikian AY, Papadopoulos N, Plager C, et al. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res 2003;13:303–306.
- ↑ Jump up to: 122.0 122.1 Piperno-Neumann S, Diallo A, Etienne-Grimaldi M-C, et al. Phase II Trial of Bevacizumab in Combination With Temozolomide as First-Line Treatment in Patients With Metastatic Uveal Melanoma. Oncologist 2016;21:281–282.
- ↑ Jump up to: 123.0 123.1 Schmittel A, Schuster R, Bechrakis NE, et al. A two-cohort phase II clinical trial of gemcitabine plus treosulfan in patients with metastatic uveal melanoma. Melanoma Res 2005;15:447–451.
- ↑ Jump up to: 124.0 124.1 O’Neill PA, Butt M, Eswar CV, et al. A prospective single arm phase II study of dacarbazine and treosulfan as first-line therapy in metastatic uveal melanoma. Melanoma Res 2006;16:245–248.
- ↑ Jump up to: 125.0 125.1 Spagnolo F, Grosso M, Picasso V, et al. Treatment of metastatic uveal melanoma with intravenous fotemustine. Melanoma Res 2013;23:196–198.
- ↑ Triozzi PL, Singh AD. Adjuvant Therapy of Uveal Melanoma: Current Status. Ocul Oncol Pathol 2014;1:54–62.
- ↑ Piperno-Neumann S, Rodrigues MJ, Servois V, et al. A randomized multicenter phase 3 trial of adjuvant fotemustine versus surveillance in high risk uveal melanoma (UM) patients (FOTEADJ). J Clin Orthod 2017;35:9502–9502.
- ↑ Richtig E, Langmann G, Schlemmer G, et al. [Safety and efficacy of interferon alfa-2b in the adjuvant treatment of uveal melanoma]. Ophthalmologe 2006;103:506–511.
- ↑ Wu X, Zhou J, Rogers AM, et al. c-Met, epidermal growth factor receptor, and insulin-like growth factor-1 receptor are important for growth in uveal melanoma and independently contribute to migration and metastatic potential. Melanoma Res 2012;22:123–132.
- ↑ Valsecchi ME, Orloff M, Sato R, et al. Adjuvant Sunitinib in High-Risk Patients with Uveal Melanoma: Comparison with Institutional Controls. Ophthalmology 2018;125:210–217.
- ↑ Ren X, Hu B, Colletti L. Stem cell factor and its receptor, c-kit, are important for hepatocyte proliferation in wild-type and tumor necrosis factor receptor-1 knockout mice after 70% hepatectomy. Surgery 2008;143:790–802.
- ↑ Jump up to: 132.0 132.1 Sacco JJ, Nathan PD, Danson S, et al. Sunitinib versus dacarbazine as first-line treatment in patients with metastatic uveal melanoma. J Clin Orthod 2013;31:9031–9031.
- ↑ Jump up to: 133.0 133.1 Daud A, Kluger HM, Kurzrock R, et al. Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. Br J Cancer 2017;116:432–440.
- ↑ Jump up to: 134.0 134.1 Scheulen ME, Kaempgen E, Keilholz U, et al. STREAM: A randomized discontinuation, blinded, placebo-controlled phase II study of sorafenib (S) treatment of chemonaïve patients (pts) with metastatic uveal melanoma (MUM). J Clin Orthod 2017;35:9511–9511.
- ↑ Jump up to: 135.0 135.1 Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014;311:2397–2405.
- ↑ Jump up to: 136.0 136.1 Carvajal RD, Piperno-Neumann S, Kapiteijn E, et al. Selumetinib in Combination With Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J Clin Oncol 2018;36:1232–1239.
- ↑ Jump up to: 137.0 137.1 Nathan PD, Marshall E, Smith CT, et al. A Cancer Research UK two-stage multicenter phase II study of imatinib in the treatment of patients with c-kit positive metastatic uveal melanoma (ITEM). J Clin Orthod 2012;30:8523–8523.
- ↑ Jump up to: 138.0 138.1 Piulats Rodriguez JM, Ochoa de Olza M, Codes M, et al. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): The GEM-1 trial. J Clin Orthod 2014;32:9033–9033.
- ↑ Najjar YG, Navrazhina K, Ding F, et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study. J Immunother Cancer 2020;8:e000331.
- ↑ Jump up to: 140.0 140.1 Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naïve patients with metastatic uveal melanoma. PLoS One 2015;10:e0118564.
- ↑ Jump up to: 141.0 141.1 Joshua AM, Monzon JG, Mihalcioiu C, et al. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res 2015;25:342–347.
- ↑ Jump up to: 142.0 142.1 Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122:3344–3353.
- ↑ Sato T, Nathan PD, Hernandez-Aya LF, et al. Intra-patient escalation dosing strategy with IMCgp100 results in mitigation of T-cell based toxicity and preliminary efficacy in advanced uveal melanoma. J Clin Orthod 2017;35:9531–9531.
- ↑ Chandran SS, Somerville RPT, Yang JC, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol 2017;18:792–802.
- ↑ Hasanov M, Rioth MJ, Kendra K, et al. A Phase II Study of Glembatumumab Vedotin for Metastatic Uveal Melanoma. Cancers 2020;12:2270.
- ↑ Jump up to: 146.0 146.1 Buder K, Gesierich A, Gelbrich G, Goebeler M. Systemic treatment of metastatic uveal melanoma: review of literature and future perspectives. Cancer Med 2013;2:674–686.
- ↑ Jump up to: 147.0 147.1 Nathan P, Hassel JC, Rutkowski P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med 2021;385:1196–1206.
- ↑ Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–2526.
- ↑ Surriga O, Rajasekhar VK, Ambrosini G, et al. Crizotinib, a c-Met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol Cancer Ther 2013;12:2817–2826.
- ↑ Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:782–789.
- ↑ Shoushtari AN, Khan S, Komatsubara K, et al. A Phase Ib Study of Sotrastaurin, a PKC Inhibitor, and Alpelisib, a PI3Kα Inhibitor, in Patients with Metastatic Uveal Melanoma. Cancers 2021;13:5504.
- ↑ Shah S, Luke JJ, Jacene HA, et al. Results from phase II trial of HSP90 inhibitor, STA-9090 (ganetespib), in metastatic uveal melanoma. Melanoma Res 2018;28:605–610.
- ↑ Zeldis JB, Heller C, Seidel G, et al. A randomized phase II trial comparing two doses of lenalidomide for the treatment of stage IV ocular melanoma. J Clin Oncol 2009;27:e20012–e20012.
- ↑ Moser JC, Pulido JS, Dronca RS, et al. The Mayo Clinic experience with the use of kinase inhibitors, ipilimumab, bevacizumab, and local therapies in the treatment of metastatic uveal melanoma. Melanoma Res 2015;25:59–63.
- ↑ Francis JH, Kim J, Lin A, et al. Growth of Uveal Melanoma following Intravitreal Bevacizumab. Ocul Oncol Pathol 2017;3:117–121.
- ↑ Hussain RN, Heimann H, Damato B. Neoadjuvant intravitreal ranibizumab treatment in high-risk ocular melanoma patients: a two-stage single-centre phase II single-arm study. Melanoma Res 2020;30:102–106.
- ↑ Tarhini AA, Frankel P, Margolin KA, et al. Aflibercept (VEGF Trap) in inoperable stage III or stage iv melanoma of cutaneous or uveal origin. Clin Cancer Res 2011;17:6574–6581.
- ↑ Mouriaux F, Servois V, Parienti JJ, et al. Sorafenib in metastatic uveal melanoma: efficacy, toxicity and health-related quality of life in a multicentre phase II study. Br J Cancer 2016;115:20–24.
- ↑ Namikawa K, Takahashi A, Mori T, et al. Nivolumab for patients with metastatic uveal melanoma previously untreated with ipilimumab: a single-institution retrospective study. Melanoma Res 2020;30:76–84.
- ↑ Rossi E, Pagliara MM, Orteschi D, et al. Pembrolizumab as first-line treatment for metastatic uveal melanoma. Cancer Immunol Immunother 2019;68:1179–1185.
- ↑ Mattei J, Ballhausen A, Bassett R, et al. A phase II study of the insulin-like growth factor type I receptor inhibitor IMC-A12 in patients with metastatic uveal melanoma. Melanoma Res 2020;30:574–579.
- ↑ Gezgin G, Luk SJ, Cao J, et al. PRAME as a Potential Target for Immunotherapy in Metastatic Uveal Melanoma. JAMA Ophthalmol 2017;135:541–549.
- ↑ McLean IW, Berd D, Mastrangelo MJ, et al. A randomized study of methanol-extraction residue of bacille Calmette-Guerin as postsurgical adjuvant therapy of uveal melanoma. Am J Ophthalmol 1990;110:522–526.
- ↑ Voelter V, Schalenbourg A, Pampallona S, et al. Adjuvant intra-arterial hepatic fotemustine for high-risk uveal melanoma patients. Melanoma Res 2008;18:220–224.
- ↑ Lane AM, Egan KM, Harmon D, et al. Adjuvant interferon therapy for patients with uveal melanoma at high risk of metastasis. Ophthalmology 2009;116:2206–2212.
- ↑ Binkley E, Triozzi PL, Rybicki L, et al. A prospective trial of adjuvant therapy for high-risk uveal melanoma: assessing 5-year survival outcomes. Br J Ophthalmol 2020;104:524–528.
- ↑ Khan S, Lutzky J, Shoushtari AN, et al. Adjuvant crizotinib in high-risk uveal melanoma following definitive therapy. J Clin Orthod 2020;38:10075–10075.
- ↑ Gündüz K, Shields CL, Shields JA, et al. Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am J Ophthalmol 1999;127:579–589.
- ↑ Jump up to: 169.0 169.1 169.2 Gündüz K. Radiation Retinopathy Following Plaque Radiotherapy for Posterior Uveal Melanoma. Arch Ophthalmol 1999;117:609–614.
- ↑ Jump up to: 170.0 170.1 Wen JC, McCannel TA. Treatment of radiation retinopathy following plaque brachytherapy for choroidal melanoma. Curr Opin Ophthalmol 2009;20:200–204.
- ↑ Horgan N, Shields CL, Mashayekhi A, Shields JA. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol 2010;21:233–238.
- ↑ Shields JA, Shields CL. Prognostic factors for uveal melanoma. In: Gospodarowicz MK, O’Sullivan B, Sobin LH, eds. Prognostic Factors in Cancer, 3rd Edition. Wiley-Liss, Inc; 2006:269–272.
- ↑ Paul EV, Leslie Parnell B, Fraker M. PROGNOSIS OF MALIGNANT MELANOMAS OF THE CHOROID AND CILIARY BODY. Int Ophthalmol Clin 1962;2:387–402.
- ↑ Diener-West M, Hawkins BS, Markowitz JA, Schachat AP. A Review of Mortality From Choroidal Melanoma. Arch Ophthal 1992;110:245–250.
- ↑ F. Tschentscher, J. Husing, T. Holter et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res, 63 (2003), 2578–2584.
- ↑ S E Coupland, S L Lake, M Zeschnigk and B E Damato. Molecular pathology of uveal melanoma. Eye. 2013; 27, 230-242.
- ↑ Jump up to: 177.0 177.1 Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol 2014;25:234–239.
- ↑ Jump up to: 178.0 178.1 178.2 Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012; 119(8): 1596–1603.
- ↑ Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010; 330:1410.
- ↑ Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998; 16(9): 1097–1112.
- ↑ Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64(20):7205–7209.
- ↑ Onken MD, Worley LA, Tuscan MD, Harbour JW. An Accurate, Clinically Feasible Multi-Gene Expression Assay for Predicting Metastasis in Uveal Melanoma. J Mol Diagn. 2010 July; 12(4): 461–468
- ↑ Gill HS, Char DH. Uveal melanoma prognostication: from lesion size and cell type to molecular class. Can J Ophthalmol. 2012 Jun;47(3):246-53.
- ↑ Onken MD, Worley LA, Dávila RM, Char DH, Harbour JW. Prognostic Testing in Uveal Melanoma by Transcriptomic Profiling of Fine Needle Biopsy Specimens. J Mol Diagn. 2006 Nov; 8(5): 567–573.
- ↑ Petrausch U, Martus P, Tönnies H, Bechrakis NE, Lenze D, Wansel S, Hummel M, Bornfeld N, Thiel E, Foerster MH, Keilholz U. Significance of gene expression analysis in uveal melanoma in comparison to standard risk factors for risk assessment of subsequent metastases. Eye (Lond). 2008 Aug;22(8):997-1007.
- ↑ Cai L, Paez-Escamilla M, Walter SD, Tarlan B, Decatur CL, Perez BM, Harbour JW. Gene Expression Profiling and PRAME Status Versus Tumor-Node-Metastasis Staging for Prognostication in Uveal Melanoma. Am J Ophthalmol. 2018 Nov;195:154-160. doi: 10.1016/j.ajo.2018.07.045. Epub 2018 Aug 6. PMID: 30092184
- ↑ Callejo SA, Antecka AE, Blanco PL, Edelstein C, Burnier MN. Identification of circulating malignant cells and its correlation with prognostic factors and treatment in uveal melanoma: A prospective longitudinal study. Eye (2007) 21, 752–759.
- ↑ Jampol LM, Moy CS, Murray TG, et al. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma: IV. Local Treatment Failure and Enucleation in the First 5 Years after Brachytherapy. COMS Report No. 19. Ophthalmology 2002;127:S148–S157.
- ↑ Shields CL, Shields JA, Cater J, et al. Plaque Radiotherapy for Uveal Melanoma. Long-term Visual Outcome in 1106 Consecutive Patients. Arch Ophthal 2000;118:1219–1228.
- ↑ Kivelä TT. The first description of the complete natural history of uveal melanoma by two Scottish surgeons, Allan Burns and James Wardrop. Acta Ophthalmol 2018;96:203–214.
- ↑ Fuchs E. Das Sarcom des Uvealtractus. W. Braumuller; 1882.
- ↑ Shields JA, Shields CL. Management of posterior uveal melanoma: past, present, and future: the 2014 Charles L. Schepens lecture. Ophthalmology 2015;122:414–428.
- ↑ Zozolou M, Tsoucalas G, Karamanou M, et al. The distinguished Austrian ophthalmologist Ernst Fuchs (1851-1930) and the “sarcom des uvealtractus.” J BUON 2018;23:1563–1568.
- ↑ Eskelin S, Pyrhönen S, Summanen P, et al. Tumor doubling times in metastatic malignant melanoma of the uvea: Tumor progression before and after treatment. Ophthalmology 2000;107:1443–1449.
- ↑ Honavar SG. Is Collaborative Ocular Melanoma Study (COMS) still relevant? Indian J Ophthalmol 2018;66:1385–1387.
- ↑ Jump up to: 196.0 196.1 The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma II: initial mortality findings. COMS report no. 10. Am J Ophthalmol 1998;125:779–796.
- ↑ Hawkins BS, Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol 2004;138:936–951.
- ↑ Melia BM, Abramson DH, Albert DM, et al. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology 2001;108:348–366.
- ↑ Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 1997;115:1537–1544.