Color Fundus Photography Interpretation of Ophthalmic Findings
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
This article provides a comprehensive, practical and basic overview of how to describe fundus photo findings and accurately report observations to create a list of differential diagnoses. However, fundus photos should be interpreted with other clinical data, including patient history and comprehensive eye exam findings, to ensure an accurate diagnosis. A standard fundus camera provides high-resolution images of the fundus, similar to what practitioners observe during a fundus exam[1]. These images replicate the true and natural colors of the fundus without alterations. However, there are devices equipped with various imaging modalities and filters that change the normal colors of the fundus to enhance the detection and diagnosis of specific pathologies.
While different practitioners and cameras may have varying preferences, these cameras typically capture images with a field of view ranging from 30 to 50 degrees centered on the fovea. In recent years, wide-field and ultra-wide-field imaging technologies allow for visualization of a broader retinal area, extending into the periphery.
How to tell right from left eye in a fundus photo?
The optic nerve and macula are your key identifiers when distinguishing between the left and right eye in an image. In ophthalmology, "temporal" is used instead of "lateral," and "nasal" replaces "medial." Picture yourself looking directly into the eye. The optic nerve always lies on the nasal side, while the macula is always on the temporal side. Therefore, if you see the optic nerve on the right half of the image, you're looking at a right eye. Conversely, if the optic nerve appears on the left half of the image, it's the left eye.
Anatomical Structures
Fundus photos are routinely divided into four quadrants to pinpoint abnormalities. Two ways of describing locations are suggested: 1- Cardinal Directions: superior, inferior, nasal, and temporal. 2- Combined Directions: superior temporal, superior nasal, inferior temporal, and inferior nasal. We'll review each anatomical structure with their normal and pathologic findings.
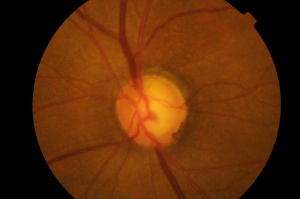
Optic Disc
The optic nerve head or disc is located nasally and visible when observed through the pupil from an angle approximately 15 degrees temporal to the optical axis (when the patient is asked to “look straight ahead”). The optic disc appears yellow-orange to pink in color, with sharp margins, particularly pronounced temporally and somewhat less so nasally. The disc is vertically oval and features a central, paler more horizontally oval pit known as the optic cup. The cup edge is best seen by the bend in small and medium-sized blood vessels as they leave or descend into the cup[2]. Size of the cup varies with the size of the disc (larger disc, larger cup), with a normal cup-to-disc ratio typically less than 0.5.
To accurately describe optic disc and cup size changes, especially in glaucomatous conditions, correct identification of scleral ring and neuroretinal rim is important. Scleral ring surrounds the outer border of the scleral ring. It is formed by the termination of the sclera as it meets the point where the optic nerve fibers enter the eye. It appears as a distinct boundary between the optic disc and the surrounding retinal tissue. Neuroretinal rim: The tissue between the border of the cup and the disc is the neuroretinal rim. This tissue consists mainly of nerve fibers with some glial cells and is usually pink. It tends to be symmetric at the superior and inferior margins of each disc. The ISNT rule is a mnemonic used to describe the normal distribution of neuroretinal rim thickness in the optic disc. In most healthy cases, the rim thickness follows this pattern: Inferior (thickest), Superior, Nasal, and Temporal (thinnest).
Optic Disc Color Changes
When light hits the fundus, it undergoes total internal reflection through the axonal fibers and reflects off the capillaries on the disc surface, creating the characteristic yellow-pink color of a healthy optic disc. Variations in the optic disc shape or color can be observed in both normal and pathologic conditions. Correct diagnosis should be made based on clinical history and eye examination.
White or pale optic disc
Typically, a white or pale color in gross pathological presentations indicates a loss of blood vessels and possible ischemia, or fibrosis and scar formation, or myelination and reactive gliosis in the nervous system.
- Ischemic (vascular) optic neuropathies: The optic disc pallor may be diffuse or segmental (sectoral). Segmental pallor occurs if part of the blood supply to the optic nerve is occluded. Anterior ischemic optic neuropathy (AION), specifically giant cell arteritis is a relatively common cause.
- Optic nerve atrophy: It is a pathological term referring to the death of the retinal ganglion cell axons that comprise the optic nerve, resulting in a pale optic nerve appearance on fundoscopy. The white appearance results from a combination of capillary loss over the optic nerve, reactive gliosis, and fibrosis[3]. Optic nerve atrophy is not a disease itself but rather a condition that can result from various underlying causes.
- Optic nerve hypoplasia (ONH): ONH is characterized by a small and gray-white optic nerve head. This can be due to conditions such as septo-optic dysplasia.
- Normal variant: Non-pathologic causes of a pale disc include, but are not limited to, pseudophakic eyes, a large physiologic cup in axial myopia, and pseudo-brightness caused by bright light during examinations.
Hyperemic optic disc
- Optic disc hyperemia: Optic disc hyperemia results from optic disc edema or inflammation, which can stem from various chronic and acute conditions. Optic disc edema refers to swelling of the nerve fiber layer at the optic nerve head due to an optic neuropathy of any etiology (inflammatory, infiltrative, compressive, etc.) whereas the term papilledema refers to optic disc edema caused by raised intracranial pressure.
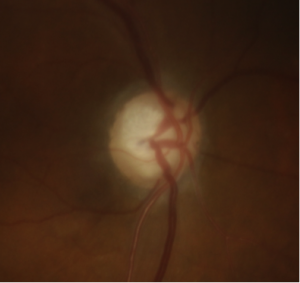
- Optic Disc Neovascularization: Abnormal, fragile blood vessels can grow on the surface of the optic disc, distinct from the normal vasculature. This can occur due to diabetes, retinal vein occlusions, and other conditions. More mature appearing collateral vessels on the optic nerve can sometimes be confused for the fine neovascular vessels.
Pigmented optic disc
- Optic Disc Melanocytoma: They appear as black lesions of varying sizes and shapes on the optic disc, typically situated eccentrically.
- Optic Disc Melanoma: Uveal melanoma can invade the optic nerve, presenting as a pigmented lesion overlying or abutting the optic disc.
Optic Disc Shape Abnormalities
Optic disc edema
Optic disc edema is the swelling of the optic nerve head, typically characterized by blurred optic disc margins and hyperemic optic disc. Papilledema refers specifically to optic disc edema caused by increased intracranial pressure. It is crucial to distinguish papilledema from pseudopapilledema, where the optic nerve head appears elevated without swelling of the nerve fiber layer. Frisén scale [4] is a widely recognized grading system to grade papilledema.
Peripapillary Atrophy (PPA)
Peripapillary atrophy involves the disruption of the retinal pigment epithelium (RPE) and chorioretinal thinning. Complete loss of RPE results in the absence of normal fundus hue and reveals large choroidal vessels underneath. In cases of incomplete RPE loss, there may be hyper- or hypopigmentation at the edges of RPE irregularities. PPA displays two distinct zones: the alpha zone, marked by irregular hyperpigmentation and hypopigmentation at the periphery of the parapapillary atrophy, and the beta zone, characterized by visible sclera and large choroidal vessels, located between the peripapillary scleral ring and the alpha zone [5]. PPA is non-specific findings and can occur in both benign and pathologic conditions such as high myopia and glaucomatous changes[6].
Physiologic Grey Crescent
It appears as a slate-gray crescent within the peripheral portion of the neuroretinal rim. These crescents are typically bilateral and found along the temporal or inferotemporal disc margin [7]. This pigmented crescent can hinder accurate assessment of the neuroretinal rim and lead to falsely high cup-to-disc ratio measurements [8]. It is commonly seen in patients of African descent with glaucoma [9].
Myopic Crescent
With progressive myopia, the disc becomes tilted, inserting into the eye at an angle. This tilted insertion along with the absence of choroid in this area and RPE thinning, the peripapillary scleral expansion appears as a whitish hypopigmented, sharply demarcated crescent on the temporal side of the optic disc. This feature is usually bilateral and is generally associated with PPA and high myopia.
Optic disc notching
Optic disc notching occurs when there is a localized loss of the Retinal Nerve Fiber Layer (RNFL), resulting in a focal notching or thinning of the optic disc contour. These changes are associated with glaucomatous alterations of the optic disc.
Myelinated Retinal Nerve Fiber Layer (MRNFL)
MRNFL are retinal nerve fibers located anterior to the lamina cribrosa that possess a myelin sheath, unlike typical retinal nerve fibers. They appear as white-grey, sharply demarcated patches with frayed or feathered borders, obscuring the underlying retinal vessels [10].
Optic Nerve Pit
An optic nerve head pit is a rare congenital anomaly and appears as a grey-white round or oval depression on the inferotemporal optic nerve, often with adjacent peripapillary atrophy or retinal pigment changes.
Optic disc drusen
Optic disc drusen are calcified, round, white-yellow deposits found on the nerve's surface or buried beneath it. The optic disc often appears elevated, with lumpy-bumpy irregular margins[9].
Vasculature
The central retinal artery and vein emerge from the optic disc, dividing into four branches superotemporal, inferotemporal, superonasal, and inferonasal branches. In approximately one-third of the population, a cilioretinal artery, derived from the ciliary circulation, supplies the area around the macula, between the superotemporal and inferotemporal regions of the inner retina. As they extend beyond one disc diameter, they are called arterioles and venules. Arteries appear narrower and lighter in color compared to veins.
Exudate
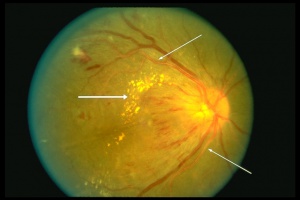
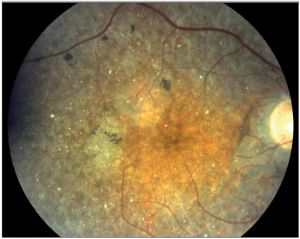
Exudate is fluid that leaks from blood vessels into surrounding tissues due to altered endothelial permeability. Exudates can be classified as hard exudates or soft exudates. Hard exudates appear as bright yellow, sharply demarcated lesions typically within or immediately beneath the retina and can be seen in any conditions with chronic vascular leakage. Soft exudates are yellow-white or grayish-white fluffy lesions with indistinct borders that result from retinal nerve fiber layer (RNFL) infarcts. Exudations may look like drusen to an inexperienced eye.
Exudative lesions in the eye can be categorized based on their vascular source: retinal exudations originating from the superficial retinal vasculature or deeper retinal and choroidal vasculature or both.
Exudates originating from retinal capillary plexus:
- Hypertensive retinopathy
- Neuroretinitis
- Retinovascular occlusion (BRVO, CRVO)
- Coat’s disease
Exudates originating from choroid:
- Choroidal neovascularization (CNV) such as CNV seen in age-related macular degeneration (AMD)
- Peripheral Exudative Hemorrhagic Chorioretinopathy
- Myopic CNV
- Traumatic CNV related to choroidal rupture
Patterns of exudate in certain retinal conditions can provide clues to the underlying pathology and aid in differential diagnosis. For example, macular star pattern, defined as a stellate pattern of hard exudates in the macula are commonly associated with hypertensive retinopathy, and neuroretinitis. Additionally, presence of exudate with drusen and sometimes hemorrhage is highly suggestive of neovascular AMD. Moreover, exudate deposition in a circinate pattern around a large vascular out pouch is suggestive or retinal macroaneurysm and lastly, in macular telangiectasia, exudates are commonly found on the macula's temporal side.
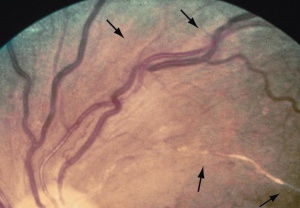
Arterio-venous nicking
It is characterized as indentation or focal attenuation (nicking) of retinal veins by adjacent stiff (arteriosclerotic) retinal arteries. The most common cause is chronic hypertension.
Microaneurysm
Microaneurysms are small local widening of retinal capillary walls which manifest as small round dots in a fundus image. They are often seen in diabetic retinopathy but can be seen in any pathologies affecting retinal microvasculature.
Intraluminal plaques
Intraluminal plaques can appear as a result of cholesterol (Hollenhorst plaque), talc, or calcium deposition at the vessels bifurcation.
Discontinuity in vessels
Vascular discontinuity in a fundus photo can occur in two main ways:
- Vessel interaction with abnormal structures: This occurs when vessels enter or exit a retinal mass or abnormal structure. Examples include vessels penetrating subretinal or intraretinal tumors such as retinoblastoma or capillary hemangioma.
- Non-perfusion conditions: In these cases, normal vessel flow is disrupted. This can be observed in conditions like branch retinal artery occlusion (BRAO) and cavernous hemangioma.
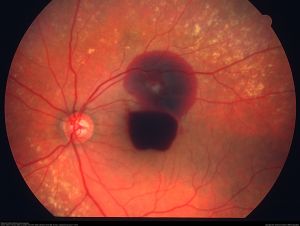
Hemorrhage
Hemorrhages can occur in different layers of the retina. A two-dimensional fundus photo often provides clues that help determine the depth of retinal lesions. Retinal hemorrhages serve as an excellent practical example for identifying lesion depth, and these principles can be helpful to determine the depth of other retinal lesions as well.
- Vitreous hemorrhage (VH): VH occurs when blood leaks into and around the vitreous humor. Since vitreous lies anterior to the retina, VH blocks the underlying retinal structures. Due to the lack of boundaries, the hemorrhage can spread throughout the vitreous humor or conform to its anatomy. Over time, chronic VH may settle inferiorly due to gravity. Initially, fresh VH appears bright red. As the hemorrhage ages, it changes to yellow, or white due to the dehemoglobinization of red blood cells.
- Pre-retinal hemorrhage: It can be categorized into two types: sub-hyaloid and sub-internal limiting membrane (ILM) hemorrhage. Sub-hyaloid hemorrhage occurs between the vitreous base and the ILM, while sub-ILM hemorrhage is located between the ILM and the retinal nerve fiber layer (RNFL). These hemorrhages often have distinctive shapes like round or scaphoid (boat-shaped), with clear boundaries. Both types can obscure underlying retinal features, posing a challenge for clinical differentiation. One distinguishing feature is their mobility: sub-hyaloid hemorrhages tend to move inferiorly with changes in head position, whereas sub-ILM hemorrhages remain stationary [11].
- Intra-retinal Hemorrhage: These hemorrhages occur within the retinal layers, and the depth and specific layer of the hemorrhage can be distinguished by their unique structural appearance.
- Retinal Nerve Fiber Layer Hemorrhage: Several types of hemorrhage can be seen at the level of RNFL.
- Flame shaped: These hemorrhages, uncommon in peripheral retina, occur due to bleeding within the superficial retina and capillary plexus and peripapillary capillary beds and they do not obscure the superficial retinal vasculature [12]. They have a thin or elongated shape (flame-shaped) due to the parallel arrangement of ganglion cell axons and the distribution of RNFL bundles in the retina. Their borders are indistinct and can appear feathered, splintered, or brush-stroke-like. Dot or blot (Intraretinal) Hemorrhage: Dot or blot hemorrhages are located within the inner nuclear or outer plexiform layers of the retina. They occur due to bleeding from the pre-venular deep capillary layer, resulting in their dark red coloration compared to the hemorrhages mentioned above. These hemorrhages have well-defined borders, and the compressive forces exerted by surrounding layers result in their characteristic dot or blot shapes.
- Roth spot: A Roth spot is a round or flame shaped hemorrhage with a white-pale center. Roth spots are the result of retinal capillary rupture and subsequent platelet-fibrin plug formation [13].
- Disc (Drance) hemorrhage: These splinter-shaped hemorrhages typically taper towards the disc and have a feathery appearance away from it, oriented perpendicular to the optic disc [14]. They are commonly found within one disc diameter, often seen in the inferotemporal and superotemporal regions, and are associated with an optic disc notch [15][16].
- Sub-retinal Hemorrhage: Sub-retinal hemorrhages occur above the RPE but below the photoreceptor layer of the retina, originating from either choroidal or retinal circulation. These hemorrhages appear deep red in color, with an amorphous shape and indistinct margins. Overlying retinal vessels are still seen traversing over subretinal hemorhages.
- SubRPE Hemorrhage: Located between the RPE and Bruch's membrane, these hemorrhages appear dark red with defined and confined borders (in contrast to subretinal hemorrhages). [See more in choroid hemorrhage below]
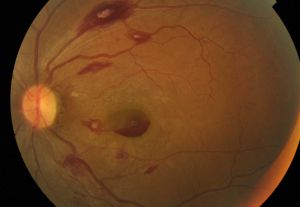
Neurosensory Retina, Macula, and Fovea
The macula is an avascular and dusky area measuring approximately 5.5 mm in diameter located two-disc diameters temporal and inferior to the optic disc. Macula generally looks darker to the surrounding retina due to taller RPE cells with higher density of pigments. Also, the choroid layer underneath macula is thicker at the center, getting thinner going to periphery. The fovea, which lies in the center of the macula, measures 1.5 mm in diameter and has the greatest density of cone photoreceptors. A small area of oxygenated carotenoids, particularly lutein and zeaxanthin, accumulate within the central fovea and contribute to its yellow color.
Crystalline Retinopathy
Crystalline deposits manifest as multiple small yellow accumulations that can occur due to a variety of etiologies and can be found in any layer of the retina. Depending on the cause, these deposits may form characteristic patterns that provide clues to the underlying pathology. Common causes include talc retinopathy, Bietti disease, Cystinosis, Tamoxifen retinopathy.
Epiretinal membrane (ERM)
ERM, also known as macular pucker, appears as clear, white, or yellow fibrovascular membrane overlying the macular area causing a loss of normal convex foveal contour and wrinkling on the retinal surface from the membrane contracture.
Retinal Pigment Epithelium (RPE)
In a fundus photograph, the RPE layer is visible at the back of the retina, situated between the retina and the choroid. Changes in the RPE can be age-related or result from pathological processes. Key structural changes include the loss of melanin granules, increased density of residual bodies, accumulation of lipofuscin, buildup of basal deposits on or within Bruch’s membrane, drusen formation, thickening of Bruch’s membrane, and microvilli atrophy [17].
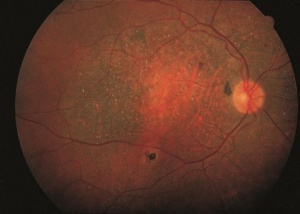
RPE Atrophy
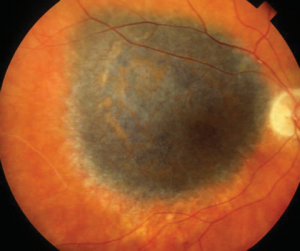
RPE atrophy, which can progress to complete RPE loss, appears as sharply demarcated edges in an oval, circular, or patchy pattern. This manifests as a loss of the normal fundus hue and increased visibility of the underlying large choroidal vessels and choroidal pigmentation. Typically, at the edge of RPE loss, areas of hyperpigmentation indicate hyperproliferation of the RPE. RPE atrophy can be observed in various conditions. In age-related macular degeneration (AMD), RPE atrophy is central to the macula, whereas in conditions like gyrate atrophy or cobblestone degeneration, it is more peripheral.
Pattern dystrophy
Pattern dystrophies are a group of disorders characterized by abnormal development and deposition of RPE pigment in the macula. Correctly identifying the patterns of each dystrophy in a fundus photo aids in accurate diagnosis. Examples include butterfly dystrophy, and adult-onset vitelliform macular dystrophy.
RPE Hyperpigmentation
RPE cells contain two different pigments, lipofuscin and melanin. RPE hyperpigmentation manifests as dark green, or black lesions compared to normal RPE cells. A classic example of RPE hypertrophy with hyperpigmentation can be seen in congenital hypertrophy of the retinal pigment epithelium (CHRPE). Another classic RPE hyperplasia associated with hyperpigmentation is Forster-Fuchs spot which is a raised, pigmented, circular scar commonly seen in pathologic myopia.
RPE Hypopigmentation
Generalized RPE hypopigmentation can be seen in individuals with lighter skin tones, which sometimes is referred to as blonde fundus. Albinoid fundus is another example and is observed in oculocutaneous albinism. Tessellated or tigroid fundus is another example of RPE depigmentation in which low pigment level in the RPE with deep pigment in choroid make the choroid vasculature more visible, and is associated with pathologic myopia.
RPE Depigmentation
RPE depigmentation can classically be seen in the chronic stage of Vogt-Koyanagi-Harada disease, leading to a pale optic nerve surrounded with peripapillary choroidal depigmentation known as a “sunset-glow fundus”.
Drusen
Drusen are round yellow lesions located between the RPE and Bruch's membrane and mostly found in the post equatorial retina. Drusen are categorized as hard (discrete with clear border), soft (amorphous and poorly demarcated), and confluent (contiguous drusen without clear boundaries).
Uvea
The color of the background retina is influenced by retinal pigmentation and the visibility of the choroidal vessels beneath it. The degree of retinal pigmentation correlates with the individual's skin and hair pigmentation. Choroidal vessels are visible beneath the RPE in a normal eye, giving the fundus photo a dark hue more easily visible in most eyes in the periphery
Chorioretinal scar
It appears as white fibrotic tissue located anywhere on the retina, depending on the underlying cause.
Choroidal folds
Choroidal folds can sometimes be seen as parallel transparent elevated lines in a fundus photo. These may indicate hypotony, posterior scleral and choroidal compression from extraocular masses, or from other causes.
Choroidal inflammation
In general, choroidal inflammation presents as yellow-white choroiditis spots with blurred margins due to surrounding edema. In Multiple Evanescent White Dot Syndrome (MEWDS) choroidal inflammation appear as multiple flat, small, white dots outside the fovea. A highly suggestive finding in MEWDS is transient foveal granularity. In birdshot chorioretinopathy, as the name implies, multiple yellow-white choroiditis spots are often initially observed inferior to the optic nerve head, displaying the characteristic "birdshot" appearance and distribution [18].
Choroidal hemangioma
Choroidal hemangiomas can present in either diffuse or circumscribed forms. A circumscribed hemangioma appears as a dome-shaped hamartoma within the choroidal vessels and can be challenging to differentiate from the surrounding normal retina. However, if the overlying RPE has degenerated or atrophied, the bright red color beneath it becomes more apparent. In diffuse hemangiomas, a homogeneous red hue beneath the retina is observed. For unilateral diffuse hemangiomas, comparing with the other eye can be helpful in diagnosis.
Choroidal nevus and melanoma
A choroidal nevus manifests as a flat or minimally elevated melanocytic lesion, typically dark gray or black. It is usually round with well-defined borders but can also appear irregular or feathery, and sometimes associated with overlying drusen. It may or may not have overlying RPE atrophy. Uveal nevus can transform to uveal melanoma, which classically presents as a unilateral, elevated, dome-shaped melanocytic lesion with irregular borders. Less commonly, melanoma can be amelanotic.
Suprachoroidal calcification
Multiple discrete white-yellow placoid lesions without a clear border, usually seen in superotemporal arcade.
Choroidal osteoma
Single yellow-white distinct lesions with overlying pigment clumps, usually seen juxtapupillary or peripupillary.
Choroidal metastases
Yellow-white and less commonly orange lesions appearing singular or multiple, unilateral or bilateral yellow lesions with plateau configuration.
Retinoblastoma
In general, retinoblastoma presents as a solitary or multifocal, well-circumscribed intraretinal mass with a white or cream-colored appearance. As the disease progresses, increased vascularization within the tumor can cause it to appear pink.
Vitreous
Normal vitreous should appear as clear, transparent media. If there’s a vitreous opacity, from causes like vitreous hemorrhage, asteroid hyalosis, or primary vitreoretinal lymphoma the vitreous can obscure the underlying structures.
Abnormal structures in the retina categorized by their color
White lesions
- Scar or fibrosis
- Necrosis
- Inflammation
- Soft exudates or Cotton wool spots
- Chorioretinal atrophy
- Myelinatied nerve fiber layer
- Calcification (calcium deposits or due to tumors with calcifications)
- Intraluminal calcium or talc plaques
Yellow Lesions
- Necrosis
- Inflammation
- Hard Exudates
- Drusen
- Crystalline deposits
- Intraluminal Cholesterol plaque
- Early necrosis following pan retinal photocoagulation therapy (PRP)
Pigmented Lesions
- RPE hyperpigmentation
- Late-stage scar of PRP
- Hemorrhage
- Choroidal nevus
- Choroidal Melanoma
- Bone spicules in retinitis pigmentosa
References
- ↑ Mishra C TK. Fundus Camera. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing 2024.
- ↑ Bourne RR, Khatib T. The optic nerve head in glaucoma. Community Eye Health 2021;34:36–9.
- ↑ Quigley HA, Anderson DR. The Histologic Basis of Optic Disk Pallor in Experimental Optic Atrophy. Am J Ophthalmol 1977;83:709–17. https://doi.org/10.1016/0002-9394(77)90138-6.
- ↑ Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982;45:13–8. https://doi.org/10.1136/jnnp.45.1.13.
- ↑ Fantes FE, Anderson DR. Clinical Histologic Correlation of Human Peripapillary Anatomy. Ophthalmology 1989;96:20–5. https://doi.org/10.1016/S0161-6420(89)32929-0.
- ↑ Manjunath V, Shah H, Fujimoto JG, Duker JS. Analysis of Peripapillary Atrophy Using Spectral Domain Optical Coherence Tomography. Ophthalmology 2011;118:531–6. https://doi.org/10.1016/j.ophtha.2010.07.013.
- ↑ Jonas JB. Optic nerve grey crescent. British Journal of Ophthalmology 2005;89:3–3. https://doi.org/10.1136/bjo.2004.051763.
- ↑ Roddy GW, Brodsky MC, Chen JJ. Optic Nerve Gray Crescent. Ophthalmology 2017;124:1022. https://doi.org/10.1016/j.ophtha.2016.12.037.
- ↑ 9.0 9.1 Shields MB. Gray Crescent in the Optic Nerve Head. Am J Ophthalmol 1980;89:238–44. https://doi.org/10.1016/0002-9394(80)90117-8.
- ↑ Shelton JB, Digre KB, Gilman J, Warner JEA, Katz BJ. Characteristics of Myelinated Retinal Nerve Fiber Layer in Ophthalmic Imaging. JAMA Ophthalmol 2013;131:107. https://doi.org/10.1001/jamaophthalmol.2013.560.
- ↑ De Maeyer K, Van Ginderdeuren R, Postelmans L, Stalmans P, Van Calster J. Sub-inner limiting membrane haemorrhage: causes and treatment with vitrectomy. British Journal of Ophthalmology 2007;91:869–72. https://doi.org/10.1136/bjo.2006.109132.
- ↑ Jia Y, Simonett JM, Wang J, Hua X, Liu L, Hwang TS, et al. Wide-Field OCT Angiography Investigation of the Relationship Between Radial Peripapillary Capillary Plexus Density and Nerve Fiber Layer Thickness. Investigative Opthalmology & Visual Science 2017;58:5188. https://doi.org/10.1167/iovs.17-22593.
- ↑ Zhang J, Chen Y, Yu Z, Liu L. Bilateral Hemorrhagic Retinopathy with Roth Spots in Pediatric-Onset Systemic Lupus Erythematosus and Associated Thrombocytopenia: A Case Report and Review of Literature. Ocul Immunol Inflamm 2018;26:1150–3. https://doi.org/10.1080/09273948.2017.1370652.
- ↑ Venkata M. Kanukollu; Syed Shoeb Ahmad. Retinal Hemorrhage n.d. https://www.ncbi.nlm.nih.gov/books/NBK560777/ (accessed July 4, 2024).
- ↑ Ozturker ZK, Munro K, Gupta N. Optic disc hemorrhages in glaucoma and common clinical features. Canadian Journal of Ophthalmology 2017;52:583–91. https://doi.org/10.1016/j.jcjo.2017.04.011.
- ↑ Yamamoto T, Iwase A, Kawase K, Sawada A, Ishida K. Optic Disc Hemorrhages Detected in a Large-Scale Eye Disease Screening Project. J Glaucoma 2004;13:356–60. https://doi.org/10.1097/01.ijg.0000137436.68060.d2.
- ↑ Bonilha V. Age and disease-related structural changes in the retinal pigment epithelium. Clinical Ophthalmology 2008:413. https://doi.org/10.2147/OPTH.S2151.
- ↑ Bergstrom R, Czyz CN. Birdshot Retinopathy. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing 2023. https://www.ncbi.nlm.nih.gov/books/NBK554416/ (accessed July 18, 2024).