Primary Vitreoretinal Lymphoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Primary vitreoretinal lymphoma (PVRL) is the name given to primary central nervous system lymphoma (PCNSL) when its initial or primary manifestation occurs in the retina or vitreous. In this article the more general term, PCNSL will be used as the article summarizes material from both the ophthalmic and CNS literature. PCNSL is generally a high grade diffuse large B cell lymphoma. The principle treatment involves systemic chemotherapy.
Disease Entity
Primary Vitreoretinal Lymphoma
Lymphoma is a malignant neoplasia of white blood cells of lymphocytic origin. Lymphomas are diagnosed according to the World Health Organization (WHO) Classification of Haematolymphoid Tumors which outlines the criteria for each subtype of lymphoma based on histologic, cytogenetic and immunophenotypic characteristics.[1] Primary vitreoretinal lymphoma (PVRL) is classified with primary CNS lymphoma which is a subtype of diffuse large B-cell lymphoma. By contrast, uveal lymphoma and ocular adnexal lymphoma are most commonly of the extranodal marginal zone subtype and are less aggressive.
Secondary vitreoretinal lymphoma differs from PVRL in that it occurs within the context of a systemic lymphoma which involves other parts of the body (such as the brain or testis) before intraocular lesions develop. Up to 5% of systemic lymphomas will manifest secondary infiltration of the retina and vitreous.[2]
Primary Uveal Lymphoma
This rare form of lymphoma affects the uvea and is distinctly different from PCNSL. Primary uveal lymphoma is generally a low grade lymphoma of B cell origin. In primary uveal lymphoma, the uvea is the primary site of involvement, i.e. there is no systemic lymphoma at the time of diagnosis. This type of intraocular lymphoma is not covered in this article.
Etiology
Vitreoretinal lymphomas represent < 1% of all intraocular tumors[3], 4-6% of all intracranial tumors and 1-2% of all extra nodal non-Hodgkin’s lymphomas.[4] Involvement of the CNS is common, developing in 35-90% along the course of the disease. Women are more commonly affected than men[3] and patients generally present in 4th to 6th decade,[4] although a case as young as 15 years has been reported.[5][6] Eighty to ninety percent patients will have bilateral disease, although initial presentation may be unilateral or asymmetric.[4]
Risk Factors
There are two major risk factors for PCNSL, these are age and compromised immune status. In the non-immunocompromised group of patients, PCNSL is more common in older patients, generally with onset in the 5th and 6th decades of life. The onset of PCNSL in immunocompromised patients occurs at a younger age, typically in the 3rd and 4th decades. An increased risk of PCNSL is known to be present for several types of immunocompromise, including HIV infection and post transplant immunocompromise.
Diagnosis
Signs
On examination , anterior segment may exhibit anterior chamber cells as well as keratic precipitates in both the primary presentation as well as in recurrences.[7][8] Confocal microscopy of such "keratic precipitates" has demonstrated features that recapitulate atypical large lymphocytes with large nuclei and minimal cytoplasm.[9] These cells can even layer and create a hypopyon; in these situations, the eye is generally more quiet than it would be with a hypopyon from an infectious etiology.
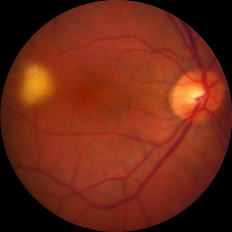
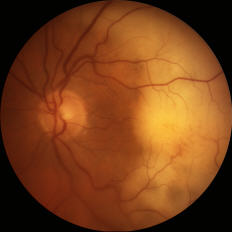
The disease may present with new vitreous opacities, subretinal lesions, or both. Subretinal lesions may begin as small, yellow to white mounds, which enlarge and expand and further coalesce to produce large yellow subretinal masses with brown pigmentation in the center known as leopard skin pigmentation. These lesions may become atrophic and shrink with treatment and the passage of time.
The lesions may involve optic disc producing optic nerve head swelling. Vasculitis with retinal hemorrhages can also be seen.[10][11] Sheathing of the vessels may be seen which could be reactive or due to lymphoma cell infiltration.
Figure: Small (left) and large (right) subretinal infiltrates in patients with primary vitreoretinal lymphoma
Clinical Diagnosis
Vitreoretinal lymphoma can be challenging to diagnose as its clinical presentation closely resembles many uveitic conditions. Diseases that should be considered on the differential diagnosis include chronic endophthalmitis, syphilis, tuberculosis, Behcet disease, birdshot chorioretinopathy, secondary intraocular lymphoma, primary uveal lymphoma, and birdshot chorioretinopathy. Patients given a presumptive diagnosis of uveitis will often receive corticosteroid treatment initially. Vitreous and subretinal lesions will typically regress after starting steroids but then recur or progress following the initial response. A diagnostic and therapeutic vitrectomy may result without a diagnosis of a lymphomatous process, particularly when a patient is still using topical or systemic corticosteroids. A study from the National Eye Institute found that patients underwent a mean of 2.1 procedures prior to receiving a diagnosis of vitreoretinal lymphoma. Furthermore, they found an average of 13.9 months from onset of symptoms to a confirmed histopathological diagnosis.[12] PVRL should thus be suspected in any patient with posterior or intermediate uveitis without a complete or sustained response to systemic corticosteroids.
Diagnostic Vitrectomy
For diagnosis, the gold standard is cytopathologic inspection of ocular fluid or chorioretinal biopsies. Small gauge vitrectomy may help with the yield and it is important to obtain an undiluted specimen (0.5 - 1 ml) at a low cut rate.
The sample may then be evaluated for:
- Cytology
- Immunohistochemistry
- Directed polymerase chain reaction (PCR) for gene rearrangements in immunoglobulin heavy chain genes or (if T-cell lymphoma is suspected) T-cell receptor genes[13]
- Directed PCR for mutation in the MYD88 gene involving codon L265P[14]
- Cytokine measurement
Cytology
Cytological examination is the gold standard for diagnosis[15] which shows large atypical lymphoid cells with pleomorphic nuclei, scant basophilic cytoplasm and prominent nucleoli. However, Kimura et al showed that cytology was sufficient in only 48% of cases.[16] The reason for such low yields includes the fact that lymphomatous cells may necrose and be misinterpreted.
Immunophenotyping
Flow cytometry is a powerful technique to characterize cells removed from the vitreous on the basis of the presence of identifiable surface proteins. For example, the cells can be identified as B or T cells, and the number with kappa or lambda light chains can be identified. This information complements the findings on cytology, and can help confirm that there is a subpopulation of atypical cells that exhibit “clonality”, i.e. all share the same surface light chain.
Cytokine Ratios
Assay of the amounts of interleukin 6 (IL6) and interleukin 10 (IL10) have been used to distinguish benign intraocular inflammatory conditions from PVRL. In benign infiltrates the IL10:IL6 ratio is typically less than 1, whereas in PVRL it is typically greater than 1. There is some overlap between the different types of infiltrates, and some variation in ratios obtained in different laboratories; however, this ratio may be useful in initial diagnosis and in follow up for recurrences.
Genetic Testing
Polymerase chain reaction (PCR) analysis should be performed for any vitreous specimen with suspected PVRL to determine mutation status of the myeloid differentiation primary response 88 (MYD88) gene. Proline for leucine substitution at codon 265 results in constitutive activation of this oncogene and genetic testing for this mutation may detect PVRL with very high sensitivity. Up to 87% of PVRL specimens are positive for the L265P mutation in MYD88.[17]
An interleukin (IL)-10:IL-6 ratio > 1, positive L265P mutation of the MYD88 gene and monoclonality confirmed by flow cytometry are all positive diagnostic criteria for PVRL.[18]
Neuroimaging
MRI should be performed for all patients with suspected PVRL as there is a high likelihood of CNS involvement either at the time of the initial recognition of ocular involvement, or subsequently.[19] In addition, patients should also have cerebrospinal fluid cytology as leptomeningeal involvement may be present even in the absence of a detectable CNS parenchymal lesion. As other forms of systemic diffuse large B cell lymphoma may affect the eyes and CNS secondarily, physical examination, bone marrow examination and imaging may be indicated in some patients.
Management
Systemic Therapy
As mentioned above, retinal or vitreous involvement alone or in combination with brain or leptomeningeal lymphoma is classified as primary central nervous system lymphoma (PCNSL), a subtype of Diffuse Large B cell Lymphoma (DLBCL). This is a high grade lymphoma that is treated with systemic combination chemotherapy. Treatment of the eye involvement should be done in conjunction with a neuro-oncologist or a medical oncologist. Prior to initiating therapy, patients should undergo a staging evaluation to document the extent of disease at presentation. This should include lumbar puncture and neuro-imaging.
There are a variety of chemotherapy regimens for DLBCL, but the vast majority of these include high dose methotrexate in combination with other agents. Increasingly, rituximab, a chimeric antibody that recognizes and induces apoptosis in CD20 positive cells (B-cells), is used in the chemotherapy regimen for patients with DLBCL. CNS involvement develops in 80-90% of patients that initially present with PVRL, so systemic therapy should be strongly considered even if initial disease manifestation is limited to the eye.
Ocular Therapy
As mentioned above, ocular therapy should be considered in the context of what type of systemic therapy is being delivered to the patient. If the patient is receiving high dose systemic chemotherapy the eyes may be observed for response. Many systemically administered agents reach therapeutic levels in the ocular fluids, so if intraocular lesions respond to systemic therapy, no additional treatment will be required.
There is a high percentage of patients for whom the medical or neuro-oncologist will elect not to treat with systemic chemotherapy. In many elderly patients with no brain or leptomeningeal involvement the side effects of therapy may be considered to outweigh the benefits of treatment in the absence of the life-threatening manifestations of the disease. In this setting the treating ophthalmologist must decide whether to treat the eyes alone. This decision should rest on the patient’s visual symptoms, and the threat the ocular involvement poses for permanent vision loss. If the vitreous infiltrate is sufficiently dense to lower vision, vitrectomy will generally return vision to a near normal level. Intraocular chemotherapy is also effective in clearing the vitreous of cells, resulting in visual improvement. If there are extensive subretinal infiltrates, particularly if they threaten the fovea, the use of intravitreal chemotherapy is generally effective in eliminating the infiltrates. Also, in settings of ischemic retinal or optic nerve disease, the use of intravitreal chemotherapy is effective in removing the cells from the vitreous and retina.
Methotrexate or rituximab are most commonly utilized for this indication. These two agents appear to be well tolerated with minimal drug related toxicity. The protocol for intravitreal chemotherapy requires an induction phase of twice-weekly injections for 1 month followed by a consolidation phase of weekly injections for 2 months and finally a maintenance phase of monthly injections for 9 months. Intravitreal methotrexate delivered according to this protocol has been shown to induce complete regression of intraocular lesions in up to 98.5% of eyes with PVRL.[20] Intravitreal rituximab only achieves this in 65%.[21] It is important to emphasize that treatment of intraocular disease is strictly palliative and there is little to no evidence to suggest that achieving local control of PVRL can improve overall survival prognosis as the majority of patients will still develop CNS relapse.[22]
Radiation Therapy
External beam radiation (XRT) was once considered the standard of care for treating PVRL. Due to the risk of radiation retinopathy and optic neuropathy, intravitreal chemotherapy with methotrexate or rituximab has largely replaced radiation as first-line treatment for intraocular lesions. XRT may still be offered as a second-line alternative to patients who cannot tolerate intravitreal injections. Whole brain radiation in conjunction with chemotherapy in older patients is now recognized to be associated with a high incidence of severe dementia, and offers little improvement in survival compared to chemotherapy alone.
Prognosis
Gene expression studies indicate that PCNSL is a distinct type of DLBCL, differing in some aspects of gene expression from the better known germinal center cell and activated B-cell subtypes. The 5 year disease free survival rates are very different in these two forms of DLBCL: 70% survival for germinal center cell type, and 30% for the activated B-cell type. Gene expression studies and survival rates for PCNSL are much more like the rates seen in the activated B-cell type, indicating that PCNSL has a poorer prognosis and that it is very likely that there will be recurrence of disease at some time following initial treatment.
With systemic chemotherapy alone or in combination with intraocular therapy, there is a high risk of recurrent disease, so patients need to be followed indefinitely for signs of recurrence. The 5 year survival data for primary CNS lymphoma is now over 3 years in multiple clinical series in which the principle therapy is systemic chemotherapy. This is a dramatic improvement over earlier series in which survival was on the order of 1 year with radiation.
References
1. Wilson DJ, Braziel R, Rosenbaum J. Intraocular lymphoma: An immunopathologic study of vitreous biopsies. Arch Ophthalmol 110:1455-1458, 1992.
2. Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol 115: 1157-60, 1997
3. Fishburne BC, Wilson DJ, Rosenbaum JT, Neuwelt EA. Intravitreal methotrexate as an adjunctive treatment of intraocular lymphoma. Arch Ophthalmol 115:1152-1156, 1997.
4. Smith JR, Rosenbaum JT, Wilson DJ, Doolittle ND, Siegal T, Neuwelt EA, Pe’er J. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology 109:1709-1716, 2002.
5. Abrey LE, DeAngelis LM, and Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol 16:859-63, 1998.
6. Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma.
7. Berenbom A, Davila RM, Lin HS, et al. Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye: 21: 1198-201, 2007.
8. Freeman LN, Schachat AP, Knox DL, et al. Clinical features, laboratory investigations, and survival in ocular reticulum cell sarcoma. Ophthalmology 94: 1631-9, 1986.
9. Chan CC, Gonzales JA: Primary Intraocular Lymphoma. Singapore: World Scientific Publishing Co; 2007.
- ↑ Alaggio R, Amador C, Anagnostopoulos I et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022 Jul;36(7):1720-1748. doi: 10.1038/s41375-022-01620-2. Epub 2022 Jun 22. Erratum in: Leukemia. 2023 Sep;37(9):1944-1951
- ↑ Salomão DR, Pulido JS, Johnston PB, Canal-Fontcuberta I, Feldman AL. Vitreoretinal presentation of secondary large B-cell lymphoma in patients with systemic lymphoma. JAMA Ophthalmol. 2013 Sep;131(9):1151-8
- ↑ Jump up to: 3.0 3.1 Bardenstein DS. Intraocular lymphoma. Cancer Control. 1998;5:317–325.
- ↑ Jump up to: 4.0 4.1 4.2 Freeman LN, Schachat AP, Knox DL, et al. Clinical features laboratory investigations, and survival in ocular reticulum sarcoma. Ophthalmology 1987;94: 1631-1639.
- ↑ Cohen IJ, Vogel R, Matz S, et al. Successful non-neurotoxic therapy (without radiation) of a multifocal primary brain lymphoma with a methotrexate, vincristine, and BCNU protocol (DEMOB). Cancer. 1986;57:6–11
- ↑ Wilkins CS, Goduni L, Dedania VS, Modi YS, Johnson B, Mehta N, Weng CY. Diagnostic and Therapeutic Challenge. Retina. 2021 Jul 1;41(7):1570-1576. doi: 10.1097/IAE.0000000000002820. PMID: 32332425.
- ↑ Biswas J, Majumdar PD .Uveitis: An Update .Goto H.Intraocular lymphoma.2016. 93-100
- ↑ Hoffman PM, McKelvie P, Hall AJ, Stawell RJ, Santamaria JD. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye 2003;17:513-521
- ↑ Zhang P, Tian J, Gao L. Intraocular lymphoma masquerading as recurent iridocyclitis: findings based on in vivo confocal microscopy. Ocul Immunol Inflamm 2018;26(3):362-364
- ↑ Akpek EK, Ahmed I, Hochberg FH, Soheilian M, Dryja TP, Jakobiec FA, Foster CS. Intraocular-central nervous system lymphoma: clinical features, diagnosis and outcomes. Ophthalmol 1999;106(9):1805-1810
- ↑ Katoch D, Bansal R, Nijhawan R, Gupta A. Primary intraocular central nervous system lymphoma masquerading as diffuse retinal vasculitis. BMJ Case Rep 2013;1-4
- ↑ Dalal M, Casady M, Moriarty E, Faia L, Nussenblatt R, Chan CC, Sen HN. Diagnostic procedures in vitreoretinal lymphoma. Ocul Immunol Inflamm. 2014 Aug;22(4):270-6.
- ↑ Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc 2003;101:269-286
- ↑ Pulido JS, Salomão DR, Frederick LA, Viswanatha DS. MyD-88 L265P mutations are present in some cases of vitreoretinal lymphoma. Retina 2015;35(4):624-627
- ↑ Chan CC, Sen HN. Current concepts in diagnosing and managing primary vitreoretinal (intraocular) lymphoma. Discov Med 2013;15:93‐100.
- ↑ Kimura K, Usui Y, Goto H, et al. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol 2012; 56: 383-389.
- ↑ Raja, Harish MD; Salomão, Diva R. MD; Viswanatha, David S. MD; Pulido, Jose S. MD, MS, MPH, MBA. PREVALENCE OF MYD88 L265P MUTATION IN HISTOLOGICALLY PROVEN, DIFFUSE LARGE B-CELL VITREORETINAL LYMPHOMA. Retina 36(3):p 624-628, March 2016.
- ↑ Carbonell, D., Mahajan, S., Chee, S. P., Sobolewska, B., Agrawal, R., … Bülow, T. (2021). Consensus Recommendations for the Diagnosis of Vitreoretinal Lymphoma. Ocular Immunology and Inflammation, 29(3), 507–520.
- ↑ Pulido JS, Johnston PB, Nowakowski GS, Castellino A, Raja H. The diagnosis and treatment of primary vitreoretinal lymphoma: a review. Int J Retina Vitreous. 2018 May 7;4:18. doi: 10.1186/s40942-018-0120-4. Erratum in: Int J Retina Vitreous. 2018 Jun 4;4:22
- ↑ Habot-Wilner Z, Frenkel S, Pe'er J. Efficacy and safety of intravitreal methotrexate for vitreo-retinal lymphoma - 20 years of experience. Br J Haematol. 2021 Jul;194(1):92-100.
- ↑ Larkin KL, Saboo US, Comer GM, Forooghian F, Mackensen F, Merrill P, Sen HN, Singh A, Essex RW, Lake S, Lim LL, Vasconcelos-Santos DV, Foster CS, Wilson DJ, Smith JR. Use of intravitreal rituximab for treatment of vitreoretinal lymphoma. Br J Ophthalmol. 2014 Jan;98(1):99-103.
- ↑ Jahnke K, Korfel A, Komm J, Bechrakis NE, Stein H, Thiel E, Coupland SE. Intraocular lymphoma 2000-2005: results of a retrospective multicentre trial. Graefes Arch Clin Exp Ophthalmol. 2006 Jun;244(6):663-9.