Congenital Hypertrophy of the Retinal Pigment Epithelium
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) is a generally asymptomatic congenital hamartoma of the retina. Typical (solitary and grouped) and atypical variant forms are described. Atypical CHRPE is associated with familial adenomatous polyposis (FAP).
Disease Entity
ICD-10: Q14.1 - congenital malformation of the retina.
Disease
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) is a typically benign, asymptomatic, pigmented fundus lesion. It is a congenital hamartoma of the retinal pigment epithelium (RPE) and occurs in three variant forms: solitary (unifocal), grouped (multifocal) and atypical. Atypical CHRPE is associated with familial adenomatous polyposis (FAP), an autosomal dominant cancer syndrome, characterized by numerous adenomatous polyps of the colon and rectum. Left untreated, virtually all FAP patients develop colorectal carcinoma/s by middle age. FAP sub-types, including Gardner syndrome (FAP plus skeletal hamartomas and various soft tissue tumors) and Turcot syndrome (FAP plus various brain tumors) are also associated with atypical CHRPE.
Epidemiology
The prevalence of CHRPE in the general optometric population has been estimated to be 1.2%.[1]
Risk Factors
Atypical CHRPE is the earliest and most common extra-colonic manifestation of FAP, present in up to 90% of patients.[2][3]
General pathology
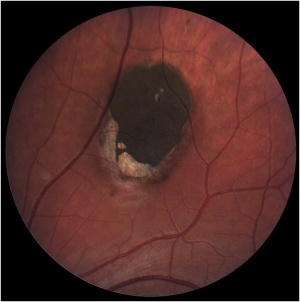
Mutations in the adenomatous polyposis coli (APC) gene are responsible for FAP. The gene encodes a tumor suppressor protein and is located on the long arm of chromosome 5 (5q21-q22). The severity of disease and presence of extracolonic features are associated with the location of the APC mutation.[5] The phenotypic expression of CHRPE in FAP is regularly present with mutations between codons 446-1338 of the APC gene, but absent with mutations between codons 1445-1578.[6]
Histopathology
Most solitary and grouped CHRPE lesions are characterized by a monocellular layer of hypertrophied RPE cells, densely packed with large, round macromelanosomes.[7][8] The underlying Bruch’s membrane may be thickened and the overlying photoreceptor layer degenerates with increasing age.[7][9] The choroid, choriocapillaris and inner retinal layers are unaffected.[10] Glial cells replace the RPE and photoreceptor layer in areas of depigmented lacunae.[7]
In comparison, atypical CHRPE lesions associated with FAP show RPE hypertrophy and hyperplasia, retinal invasion and retinal vascular changes.[10] These lesions may be multi-layered or involve the full thickness of the retina.[11][12]
Diagnosis
CHRPE is usually an incidental finding made on routine ophthalmological examination. The identification of multiple or bilateral lesions should alert the clinician to the possibility of underlying FAP.
History
Almost exclusively asymptomatic. A subset of patients may be known with FAP.
Physical examination
Lesions are usually detected on dilated examination of the peripheral retina.
Signs
Solitary (Unifocal)
Solitary CHRPE is typically a single, flat, round, hyperpigmented retinal lesion. Color may vary from light gray to brown to black, with smooth or scalloped margins. There is typically a sharp demarcation between CHRPE and adjacent normal RPE, with a normal appearance of the overlying retina and vasculature. Lesions are generally located equatorially, with a predominance in the superotemporal quadrant, but may be located throughout the fundus.[10] Macular involvement is rare. Size varies from 100 μm to several disc diameters.[10] The lesion may be surrounded by a marginal depigmented halo or contain multiple hypopigmented lacunae. These hypopigmented areas show a tendency to enlarge slowly over time.[7][14]
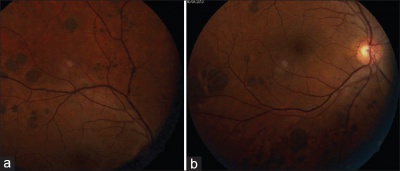
Grouped (Multifocal)
Multiple lesions arranged in a cluster constitute grouped CHRPE. Each cluster may include up to 30 lesions , which may vary from 100-300 μm in size, and are usually confined to one sector or quadrant of the fundus.[10] Lesions tend to increase in size towards the fundus periphery; lack haloes and lacunae; and have been termed “bear tracks” due to their resemblance to animal footprints.[10]
Atypical
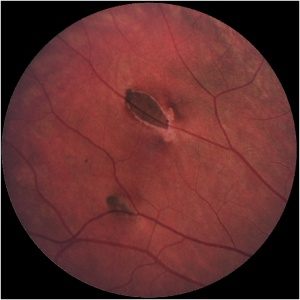
CHRPE lesions associated with FAP are typically smaller in diameter (50-100 μm) than solitary lesions.[10] Clinically they appear as multiple oval, spindle, comma or fishtail-shaped lesions haphazardly distributed across the fundus. Retinal invasion and proliferation of RPE, capillaries and glial cells are typical.[10] Larger lesions may contain depigmented lacunae and may be surrounded by depigmented haloes, mottled RPE and small, pigmented satellite lesions.[10][15] Bilateral lesions occur in 78% of patients.[15] If these typical lesions are seen in a family member with FAP pedigree, it is almost certainly associated with malignant adenomatous polyposis. [16]
Diagnostic procedures
The diagnosis of CHRPE is usually made clinically and no diagnostic procedures are generally necessary. Color fundus photography is useful for documentation and follow up of lesions and wide-field scanning-laser ophthalmoscopy has been recommended as a screening tool.[17] Ancillary testing may be beneficial in uncertain cases.
- Lesions typically demonstrate hypoautofluorescence on fundus autofluorescence (FAF) due to their high melanin content. Non-pigmented haloes or lacunae may show autofluorescence.[18]
- No leakage is demonstrated on fluorescein angiography (FA) or indocyanine green angiography (ICGA). Lesions typically block underlying choroidal fluorescence, except in areas of depigmented lacunae or haloes.[10]
- Optical coherence tomography findings include retinal thinning and photoreceptor loss over lesions, with absence of RPE and increased transmission of light in areas of lacunae.[19]
- Use of Optical coherence tomography angiography (OCTA) in evaluation of CHRPE has limitations due to thick RPE and increased cumulative melanin granules. However this modality is better that FA or ICG in visualizing choroidal vasculature.[20]
- Electroretinogram (ERG), electrooculogram (EOG), A-scan and B-scan ultrasonography are non-contributory.[10]
Differential diagnosis
- Choroidal melanoma
- Choroidal nevus
- Melanocytoma
- Focal pigmentation (caused by injury, inflammation, drug toxicity)
- True hyperplasia of the RPE
- Black sunburst lesion in sickle cell retinopathy
- Congenital grouped albinotic retinal pigment epithelial spots (CGARPES) ("polar bear tracks") may resemble grouped CHRPE but are characterized by multiple grouped, white, variably-sized, albinotic spots of the RPE.[21] The nature of these lesions has not been investigated histologically.
Management
Screening
CHRPE is a non-invasive, rapid, early phenotypical screening marker of FAP. Clinical recognition further allows increased gene analysis efficiency, even though the absence of CHRPE alone cannot exclude FAP.[22]
Medical therapy
No active intervention is generally indicated or required. Proton beam therapy has been described for rare complicated cases.[23]
Complications
CHRPE lesions have been documented to enlarge in 46-83% of cases over at least three years of follow up.[10] Foveal extension may result in impaired visual acuity.[24] Rarely, nodular pigmented adenocarcinomas may arise from within areas of CHRPE.[25][26] Untreated nodular lesions have been documented to progress to pedunculated tumors with associated serous retinal detachment.[26] Premacular gliosis and cystoid macular edema is frequently seen when peripheral pigmented or non-pigmented RPE tumors arise within CHRPE. [27]CHRPE may occasionally be complicated by choroidal neovascularization.[28]
Additional Resources
References
- ↑ Coleman P, Barnard NA. Congenital hypertrophy of the retinal pigment epithelium: prevalence and ocular features in the optometric population. Ophthalmic Physiol Opt. 2007;27:547–555.
- ↑ Wallis YL, Macdonald F, Hulten M, et al: Genotype-phenotype correlation between position of constitutional APC gene mutation and CHRPE expressed in FAP. Hum Gene. 1994;94:543-548.
- ↑ Traboulsi ET, Apostoides J, Giardiello FM, et al. Pigmented ocular fundus lesions and APC mutations in familial adenomatous polyposis. Ophth Genet. 1996;17:167-174.
- ↑ 4.0 4.1 Deibert B, Ferris L, Sanchez N, Weishaar P. The link between colon cancer and congenital hypertrophy of the retinal pigment epithelium (CHRPE). Am J Ophthalmol Case Rep. 2019 Sep;15:100524.
- ↑ Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61:153-161.
- ↑ Caspari R, Olschwang S, Friedl W, et al. Familial adenomatous polyposis: desmoid tumours and lack of ophthalmic lesions (CHRPE) associated with APC mutations beyond codon 1444. Hum Mol Genet. 1995;4:337-340.
- ↑ 7.0 7.1 7.2 7.3 Buettner H. Congenital hypertrophy of the retinal pigment epithelium. Am J Ophthalmol. 1975;79:177–189.
- ↑ Lloyd WC III, Eagle RC Jr, Shields JA, et al. Congenital hypertrophy of the retinal pigment epithelium: electron microscopic and morphometric observations. Ophthalmology. 1990;97:1052–1060.
- ↑ Kasner L, Traboulsi EI, Delacruz Z, et al. A histopathologic study of the pigmented fundus lesions in familial adenomatous polyposis. Retina. 1992;12:35-42.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 Meyer CH, Gerding H. Congenital Hypertrophy of the Retinal Pigment Epithelium. In: Ryan SJ (ed.). Retina. 5th edition. Elsevier; 2013. 2209-2213.
- ↑ Buettner H. Congenital hypertrophy of the retinal pigment epithelium in familial polyposis coli. Int Ophthalmol. 1987;10:109–110.
- ↑ Traboulsi EI, Murphy SF, de la Cruz ZC, et al. A clinicopathologic study of the eyes in familial adenomatous polyposis with extracolonic manifestations (Gardner's syndrome). Am J Ophthalmol. 1990;110:550–561.
- ↑ Mishra A, Aggarwal S, Shah S, Negi P. An interesting case of "bear track dystrophy". Egypt Retina J. 2014;2:114-117.
- ↑ Shields CL, Mashayekhi A, Ho T, et al. Solitary congenital hypertrophy of the retinal pigment epithelium. Clinical features and frequency of enlargement in 330 patients. Ophthalmology. 2003;110:1968–1976.
- ↑ 15.0 15.1 Traboulsi EI, Krush AJ, Gardner EJ, et al. Prevalence and importance of pigmented ocular fundus lesions in Gardner’s syndrome. N Engl J Med. 1987;316:661–667.
- ↑ Rushwurm I, Zehetmayer M, Dejaco C, Wolf B, Karner-Hanusch J Ophthalmic and genetic screening in pedigrees with familial adenomatous polyposis. Am J Ophthalmol 1998; 125: 680-686
- ↑ Meyer CH, Holz FG. Documentation of congenital hypertrophy of the retinal pigment epithelium with wide-field funduscopy. Semin Ophthalmol. 2009;24:251-3.
- ↑ Shields CL, Pirondini C, Bianciotto C, et al. Autofluorescence of congenital hypertrophy of the retinal pigment epithelium. Retina. 2007;27:1097-1111.
- ↑ Shields CL, Materin MA, Walker C, et al. Photoreceptor loss overlying congenital hypertrophy of the retinal pigment epithelium by optical coherence tomography. Ophthalmology. 2006;113:661-665.
- ↑ Shanmugam PM, Konana VK, Ramanjulu R, Mishra KCD, Sagar P, Simakurthy S. Ocular coherence tomography angiography features of congenital hypertrophy of retinal pigment epithelium. Indian J Ophthalmol. 2019;67(4):563-566.
- ↑ Arana LA, Sato M, Arana J. Familial Congenital Grouped Albinotic Retinal Pigment Epithelial Spots. Arch Ophthalmol. 2010;128(10):1362–1364.
- ↑ Bonnet LA, Conway RM, Lim LA. Congenital Hypertrophy of the Retinal Pigment Epithelium (CHRPE) as a Screening Marker for Familial Adenomatous Polyposis (FAP): Systematic Literature Review and Screening Recommendations. Clin Ophthalmol. 2022 Mar 15;16:765-774. doi: 10.2147/OPTH.S354761. PMID: 35321042; PMCID: PMC8934868.
- ↑ Moulin AP, Zografos L, Schalenbourg A. RPE adenocarcinoma arising from a congenital hypertrophy of the RPE (CHRPE) treated with proton therapy. Klin Monatsbl Augenheilkd. 2014;231:411-413.
- ↑ Van der Toren K, Luyten GP. Progression of papillomacular congenital hypertrophy of the retinal pigment epithelium associated with impaired visual function. Arch Ophthalmol. 1998;116:256–257.
- ↑ Shields JA, Eagle Jr RC, Shields CL, et al. Malignant transformation of congenital hypertrophy of the retinal pigment epithelium. Ophthalmology. 2009;116:2213–2216.
- ↑ 26.0 26.1 Trichopoulos N, Augsburger JJ, Schneider S. Adenocarcinoma arising from congenital hypertrophy of the retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 2006;244:125–128.
- ↑ Shields JA, Shields CL, Eagle RC, Singh AD. Adenocarcinoma Arising From Congenital Hypertrophy of Retinal Pigment Epithelium. Arch Ophthalmol. 2001;119(4):597–602. doi:10.1001/archopht.119.4.597
- ↑ Bellamy JP, Cohen SY, Congenital Hypertrophy of the Retinal Pigment Epithelium Complicated by Choroidal Neovascularization. Ophthalmol Retina; 2022 Jun;6(6):511. doi: 10.1016/j.oret.2022.02.005.

