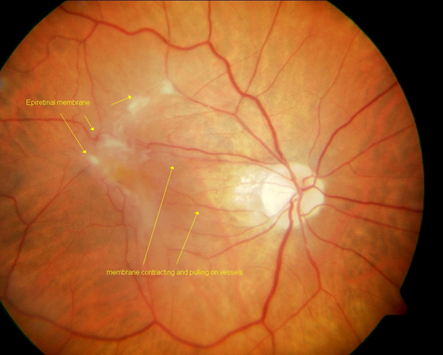
Epiretinal Membrane
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Macular Pucker ICD-9 code 362.56. Numerous terms have been used to describe this entity including: Epiretinal membrane, epimacular membrane, surface-wrinkling retinopathy, cellophane maculopathy, and preretinal macular fibrosis.
Disease
An epiretinal membrane (ERM) aka as macular pucker is a pathologic fibrocellular tissue caused by proliferation of myofibroblastic cells at the vitreo-retinal interface. It is semi-translucent and proliferates on the surface of the internal limiting membrane.
Etiology and Risk Factors
Idiopathic ERMs is the most common presentation and is noted in 95% of cases. Secondary ERMs occur in association with retinal vascular diseases including diabetic retinopathy, retinal vein occlusion, ocular inflammatory disease, trauma, intraocular surgery, intraocular tumors, and retinal tear or detachment.
Other risk factors include age, posterior vitreous detachment, and history of ERM in the fellow eye.
The mean age of ERM diagnosis is 65 years old, with most patients over 50 years of age.[1] The prevalence of ERM in the is reported to be between 7 and 11%, with as high as 17% in individuals over 80 years of age. [2] The incidence of developing an ERM in the fellow eye is 2.7% per year.
General Pathology
Retinal glial and retinal pigment epithelial cells are the major components. Fibrous astrocytes, fibrocytes, myofibrocytes, and macrophages can also be identified in pathological analysis.
Pathophysiology
It has been hypothesized that residual cortical vitreous secondary to a posterior vitreous detachment or partial separation of the posterior hyaloid can cause dehiscence of internal limiting membrane (ILM) allowing migration of microglial cells to the retinal surface in the vitreo-retinal space which differentiate into fibroblast-like cells. Hyalocytes in the vitreous when in contact with microglial cells differentiate into myofibroblasts and with microglial turned fibroblasts will form the ERM.
Inflammatory mediators also promote fibrocellular growth especially in the setting of secondary ERM formation. Retinal pigment epithelial (RPE) cells, macrophages, T- and B cells are usually observed in secondary ERMs.[3]
Primary prevention
There are no preventative measures for idiopathic ERMs. The risk of secondary ERMs can be reduced in some cases by appropriately manage the underlying cause.
Diagnosis
This is a clinical diagnosis based on history and clinical exam, including slit lamp and dilated fundus examination. In some cases, Optical Coherence Tomography (OCT) is useful in the diagnosis, quantification of retinal thickness, and management of this condition.
History
Patients with ERMs typically present over the age of 50 and both sexes are equally affected. A careful history should be obtained to investigate for any of the risk factors mentioned above.
Physical examination
Slit lamp examination with dilated fundus examination and scleral depression (to rule out peripheral breaks/lesions) are important to determine the presence and evaluate the severity of an ERM. Careful examination of the macular area is important to evaluate the ERM. However, paying attention the vitreous, retinal vasculature, and peripheral retina can provide insight as to the cause of the ERM in secondary cases.
Careful examination of the fellow eye is also recommended given that ERMs are bilateral in approximately 10-20% of patients.
Signs
A sheen or abnormal reflectivity of the macular surface is suggestive of an ERM. More advanced ERMs can become opaque.
Symptoms
Metamorphopsia, blurred vision, monocular diplopia, and micropsia can be noted with any macular pathology. The vast majority of patients with ERMs are asymptomatic.
Clinical diagnosis
Idiopathic ERMs affect the architecture of the macula. There can be blunting of the foveal contour or wrinkling on the retinal surface from membrane contracture. Most commonly it involves the foveal and parafoveal area.
Macular edema and/or pseudohole can be seen in association with an ERM. As the name implies, a pseudohole is not a full-thickness macular hole, but rather a hole or gap in the ERM that appears to be a retinal hole. The inner retina around the pseudohole is thickened. The pseudohole may not be exactly round and may have oval or irregular shape. Other associated signs include vascular tortuosity in the region of the ERM and/or intraretinal hemorrhages.
A posterior vitreous detachment is often noted which supports the pathophysiology of this entity.
Diagnostic procedures
Fluorescein angiography can be helpful in secondary cases of ERM including retinal vascular occlusions or intraocular tumors. Macular edema can be confirmed with angiography, as well.
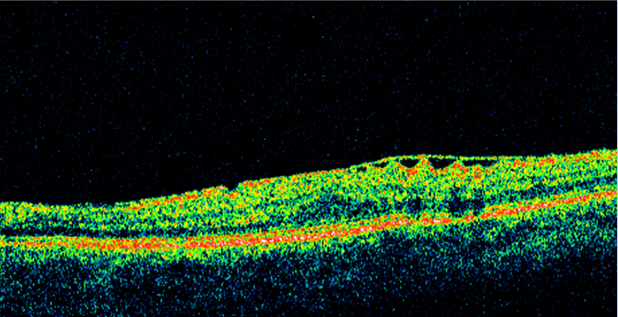
OCT has become increasingly helpful in the diagnosis and management of this disorder. ERM appears as a hyperreflective linear band on the inner retina. This high-resolution image can allow evaluation of the macula in cross section and three-dimensionally. OCT can be helpful detecting subtle ERMs as well as when associated with macular edema or other macular pathology. Some cases with ERM may have foveoschisis, a separation of the outer nuclear layer (ONL) from the outer plexiform layer (OPL). [4]
OCT can also help guide management. Some cases of ERM with vitreomacular traction are subtle clinically and better detected with OCT. One of the great advantages of the OCT is the assessment of the vitreoretinal interface. This can provide additional information regarding therapeutic options and prognosis. In surgical cases, evaluation of each scan can elucidate the best approach for removal. Spectral domain OCT can also allow evaluation of the outer retinal layers/structure which may have barring on the physiologic outcome after an ERM removal.
Laboratory test
No laboratory tests are indicated in cases of idiopathic ERMs.
Differential diagnosis
The clinical appearance of an ERM is fairly distinctive. However, macular hole, parafoveal telangiectasia, and macular edema must also be considered.
Management
General treatment
The most important issue in the management of idiopathic ERMs is the presence of visual complaints. Visual symptoms can be variable and sometimes independent of clinical severity.
Medical therapy
No medical therapy is indicated for idiopathic ERMs.
Medical follow up
See above.
Surgery
Epiretinal membrane surgery is the most common vitreoretinal surgery performed as reported by the Centers of Medicare and Medicaid Services.[5] Surgery involves pars plana vitrectomy procedure with membrane peel and removal of retinal traction. Since ILM acts as a scaffold for proliferation of myofibroblasts, it may be removed in certain cases which can minimize the recurrence of ERM. The advent of intraoperative OCT allows the surgeon to use OCT to pick the best location for beginning the ERM peeling procedure. It also allows the surgeon to check whether there is residual ERM. Theoretically, it could lead to more complete removal of the ERM. The DISCOVER study was a prospective study that evaluated the utility of intraoperative OCT for ERM peeling. The visual and anatomic results from DISCOVER were compared retrospectively to a series of ERM peeling without use of the intraoperative OCT. The study showed no significant difference in visual acuity nor retinal thickness on OCT at 12 months. However, CST was lower in the intraoperative OCT group at 6 months compared to baseline versus that of the conventional peeling group. [6]
Surgical follow up
Surgery is indicated if patient has significant visual complaints of visual decline and /or metamorphopsia.
The follow up is similar for most eyes following pars plana vitrectomy surgery. Visual acuity improvement does not occur in some patients. This is highly dependent on preoperative characteristics, duration of the ERM, as well as other factors. Most patients improve by 3-6 months postoperatively. However, some may experience improvement up to 1-2 years postoperatively.
Complications
The complications are similar to all eyes undergoing pars plana vitrectomy (including cataract in phakic, retinal break, retinal detachment, dissociated optic nerve fiber layer). In addition, macular surgery complications include intraoperative macular trauma and light toxicity.
Prognosis
The mean preoperative and postoperative visual acuity has been reported to be 20/110 and 20/55.[7] This data was a meta-analysis of three studies reporting surgical results following small incision pars plana vitrectomy.
The recurrence of ERM has been estimated to be 1%.[7]
Additional Resources
- Boyd K, Janigian RH. Macular Pucker. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/macular-pucker-list. Accessed March 18, 2019.
References
- ↑ Fraser-Bell S, Guzowski M, Rochtchina E, et al. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology 2003;110(1):34-40.
- ↑ Fung AT, Galvin J, Tran T. Epiretinal membrane: A review. Clin Exp Ophthalmol. 2021 Apr;49(3):289-308. doi: 10.1111/ceo.13914. Epub 2021 Mar 24. PMID: 33656784.
- ↑ Ueki M, Morishita S, Kohmoto R, Fukumoto M, Suzuki H, Sato T, et al.. Comparison of histopathological findings between idiopathic and secondary epiretinal membranes. Int Ophthalmol (2016) 36:713–8.
- ↑ Hubschman JP, Govetto A, Spaide RF, Schumann R, Steel D, Figueroa MS, Sebag J, Gaudric A, Staurenghi G, Haritoglou C, Kadonosono K, Thompson JT, Chang S, Bottoni F, Tadayoni R. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020;104:1741–1747.
- ↑ Gillis K. Medicare Physician Payment Schedule Services for 2001 - A Summary of Claims Data. In: Physician Marketplace Report. Chicago: American Medical Association, 2003.
- ↑ Tuifua TS, Sood AB, Abraham JR, Srivastava SK, Kaiser PK, Sharma S, Rachitskaya A, Singh RP, Reese J, Ehlers JP. Epiretinal Membrane Surgery Using Intraoperative OCT-Guided Membrane Removal in the DISCOVER Study versus Conventional Membrane Removal. Ophthalmol Retina. 2021 Dec;5(12):1254-1262. doi: 10.1016/j.oret.2021.02.013. Epub 2021 Feb 27. PMID: 33647472; PMCID: PMC8390556.
- ↑ 7.0 7.1 Gupta OP, Brown GC, Brown MM. A value-based medicine cost-utility analysis of idiopathic epiretinal membrane surgery. Am J Ophthalmol 2008;145(5):923-8.