Managing Choroidal Effusions after Glaucoma Filtration Surgery
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
A choroidal detachment (CD) is defined by the abnormal presence of fluid or blood in the suprachoroidal space, which is the potential space between the choroid and the sclera. It may occur in two types—serous and hemorrhagic. Serous choroidal detachments, also known as choroidal effusions, are a frequent complication of glaucoma surgery.[1][2]
The terms choroidal effusion, ciliochoroidal effusion, choroidal detachment, and ciliochoroidal detachment describe similar entities and are sometimes used interchangeably in the literature. However, choroidal detachment is a broader term than choroidal effusion. The terms choroidal effusion, ciliochoroidal effusion, and serous choroidal detachment describe the same entity.
ICD-10:
- H31.4 Choroidal detachment
- H31.8 Other specified disorders of choroid
- H59.8 Other intraoperative and postprocedural complications and disorders of eye and adnexa, not elsewhere classified
Pathophysiology
Glaucoma filtering procedures may result in hypotony, particularly in the setting of over-filtration or a bleb leak. When hypotony occurs after glaucoma surgery, it results in pressure-driven osmotic shifts of serous fluid from the choroidal capillaries into the suprachoroidal space by causing decreased vascular permeability. Once choroidal effusions develop, they may further exacerbate hypotony by reducing aqueous humor production.[3]
In a healthy eye, several factors—intraocular pressure (IOP), osmotic diffusion, vortex vein drainage, and uveoscleral outflow—create an equilibrium between protein and fluid levels in the suprachoroidal layer. Disturbances in the hydrostatic and oncotic pressure gradients result in serum or blood accumulation due to the high permeability of the choriocapillaris. This fluid accumulation leads to thickening of the choroid and the formation of a fluid-filled suprachoroidal layer. Low IOP or a disruption to uveoscleral outflow may promote fluid accumulation within the suprachoroidal space.[4][5]
Serous
Serous choroidal effusions occur due to the transudation of serum into the suprachoroidal space. According to the Starling equation, the movement of fluid between the plasma and interstitium is determined by the relative hydrostatic and oncotic pressures of these compartments.[6] Starling’s equation states that Q = Kf (Pcap − Pint) – σ (πcap − πint), where Q is the flow rate; Kf is the filtration coefficient of plasma; Pcap and Pint represent the hydrostatic pressures within the capillaries and interstitium, respectively; σ is the reflection coefficient of the capillary endothelium to molecules within plasma; and πcap and πint represent the oncotic pressures within the capillaries and interstitium, respectively. The filtration coefficient Kf varies depending on the surface available for filtration and the permeability of the capillary wall and is typically expressed in mL/min/mmHg.[7]
In applying the Starling equation to the pathophysiology of fluid accumulation in the suprachoroidal space, we can consider the suprachoroidal layer to represent the interstitial space, with intraocular pressure (IOP) providing the interstitial hydrostatic pressure. Thus, a decrease in IOP (low Pint)—such as that occurring after incisional glaucoma surgery—would allow fluid to accumulate in the interstitial space, given a higher capillary pressure relative to interstitial pressure. Other factors which can contribute to the formation of choroidal effusions include inflammation-induced increases in choroidal capillary permeability (high Kf) and high hydrostatic pressure in the choroidal vascular plexus secondary to hypertension (high Pcap). As choroidal fluid accumulates in the suprachoroidal space, hypotony may be exacerbated by decreased aqueous humor production in the setting of impaired ciliary body function. When proteinaceous serum accumulates in the suprachoroidal space (high πint), the equilibrium between the oncotic pressure in the interstitium and plasma limits uveal resorption, particularly if the effusion is large or long-standing. These non-resolving choroidal effusions may result in serous retinal detachment due to failure of the retinal pigment epithelium pump mechanism. [8][9]
Hemorrhagic
In a hemorrhagic choroidal detachment, blood rapidly accumulates in the suprachoroidal space due to the rupture of branches of the short or long posterior ciliary arteries. The pathogenesis of intraoperatively suprachoroidal hemorrhages likely involves arterial rupture secondary to precipitous drops in IOP, which may occur during incisional ocular surgery, blunt trauma, or even spontaneously.[10] Post-operative choroidal hemorrhage is typically related to prolonged hypotony and inflammation.[11] While hemorrhagic choroidal detachments may develop intraoperatively or postoperatively, the clinical severity differs between these two situations. Intraoperative hemorrhagic CD represents an ocular emergency due to a high propensity for expulsion of the intraocular contents through the surgical wound; post-operative hemorrhagic CD typically develop more gradually and are not associated with expulsion of intraocular contents. The use of an antimetabolite medication (mitomycin-C or 5-fluorouracil) in the setting of filtering glaucoma surgeries may contribute to the formation of post-operative hemorrhagic choroidal detachments, possibly related to significant IOP reductions when these medications are applied.[12]
Etiology
Serous
- Post-operative hypotony is the most common causative factor. This most frequently occurs after glaucoma filtering surgeries, such as trabeculectomy surgery or the implantation of a glaucoma drainage device (a.k.a glaucoma seton or tube shunt). However, post-operative hypotony can also occur in the setting of other intraocular surgeries.[13]
- Glaucoma surgery is the most common cause of choroidal detachments, which may occur in 3-34% of trabeculectomies and 3%-35% of glaucoma implant procedures.[14][15][16][17] While the reported incidence of choroidal effusions varies in the literature, the multi-centered Tube versus Trabeculectomy Study found 19% of trabeculectomies and 16% of glaucoma drainage implants developed post-operative choroidal effusion.[18]
- The rate of postoperative choroidal effusions may be lower in non-penetrating glaucoma surgeries such as deep sclerectomy, canaloplasty, and the broad array of newer micro-invasive glaucoma surgeries (MIGS). These procedures are less effective in lowering IOP with an accompanying decrease in the risk of post-operative hypotony.[19]
- In a large retrospective study of a nationally pooled database in the U.S., the reported incidence of hemorrhagic effusion following trabeculectomy was 0.6%-1.4%. The study reported a significantly higher incidence of hemorrhagic effusion following tube shunt surgeries, 1.2-2.7%, possibly because patients who require drainage devices have higher preoperative IOPs, resulting in steeper IOP reductions postoperatively.[20] A study in Australia found similar results.[21] MIGS were developed in part to decrease the risk of suprachoroidal hemorrhage given the less invasive technique and potentially more reliable decreases in IOP achieved with these procedures; however, the risk of choroidal detachment is still present.[22][23]
- Other causes of choroidal detachment include infection (e.g., herpesviruses, human immunodeficiency virus), inflammation (e.g., posterior scleritis, drug-induced cyclitis, Vogt-Koyanagi-Harada syndrome), malignancy (e.g., primary intraocular lymphoma or metastatic carcinoma), or episcleral venous congestion (e.g., Sturge-Weber syndrome, dural arteriovenous fistula).[24][25][26][27][28]
- The use of certain medications has been reported in association with serous choroidal detachments, and these are listed below in the Risk Factors section.
- Uveal effusion syndrome is a rare cause of idiopathic choroidal effusions that may involve impaired posterior segment drainage and congenital anomaly of the sclera resulting in scleral thickening.[29]
Hemorrhagic
- Hemorrhagic choroidal effusions frequently occur in the setting of severe globe trauma.[30][31]
- Rarely, choroidal hemorrhage occurs intraoperatively. Sudden globe decompression during surgery predisposes to hemorrhagic choroidal detachment, particularly if the eye is affected by glaucoma and surgery is initiated when the IOP is still elevated.[32]
- Choroidal hemorrhage may develop post-operatively, typically in the setting of prolonged hypotony and inflammation.[33]
Risk Factors
Surgeries
- Glaucoma filtration surgery (e.g., trabeculectomy or XEN gel stent procedure)[34][35][36]
- Tube shunt procedures (especially those using a valved device)[37][38]
- Laser peripheral iridotomy[39]
- Retinal surgery (pars plana vitrectomy or scleral buckling procedure)[40][41]
- Any glaucoma surgery with intraoperative or postoperative hypotony[42]
Ocular medications
- Antimetabolites (mitomycin C or 5-fluorouracil) used in conjunction with trabeculectomy surgery are a significant risk factor for clinically significant choroidal effusions, which typically develop in the setting of prolonged hypotony.[43][44]
- The use of topical aqueous suppressants (e.g., timolol and dorzolamide) after trabeculectomy or glaucoma drainage device implantation may place patients at increased risk for developing hypotony and choroidal effusions postoperatively.[45][46]
- Topical prostaglandin analogs (e.g., latanoprost, travoprost, and bimatoprost) have been associated with late choroidal effusions following Cataract extraction or glaucoma filtering surgery.[47]
- Intravitreal injection of ocriplasmin has also been reported as a cause of choroidal effusion.[48]
Systemic medications
- Oral and topical carbonic anhydrase inhibitors[49][50][51][52][53][54][55][56]
- Anticoagulants[57][58]
- Topiramate[59][60][61][62][63]
- Tamsulosin[64][65]
- Sulfonamides (e.g., chlorthalidone, sulfamethoxazole-trimethoprim, indapamide)[66][67][68][69]
- Antidepressants (e.g., escitalopram, venlafaxine, bupropion)[70][71][72]
- Angiotensin receptor blockers (e.g., losartan)[73]
- Chemotherapeutics (e.g., docetaxol and gemcitabine)[74]
- Pergolide (a dopamine agonist used to treat Parkinson’s disease)[75]
- Drugs of abuse (e.g., 3,4-methylenedioxymethamphetamine a.k.a. “ecstasy” or MDMA)[76]
Pre-existing conditions
- Nanophthalmos, Sturge-Weber syndrome, hypermetropia, systemic hypertension, atherosclerosis, diabetes, prior cataract surgery, glaucoma, diffuse choroidal hemangioma, older age, history of choroidal detachment in the other eye, pseudoexfoliative glaucoma[77][78][79]
- Lower postoperative IOP, full-thickness filtration surgery, ocular inflammation, aqueous suppressant therapy[80]
Ocular trauma
- Severe ocular trauma is a frequent cause of hemorrhagic CD. In retrospective studies about traumatic ruptured globe injuries, hemorrhagic CD was found in 11-39% of cases.[81][82][83][84]
The risk for hemorrhagic choroidal detachments is also increased in patients with systemic hypertension, intraoperative tachycardia, arteriosclerosis, high myopia, increased axial length, aphakia, and glaucoma. Further, elevated IOP pre-operatively or acute intraoperative effusion places a patient at higher risk for developing choroidal hemorrhage.[85][86][87]
Primary Prevention
Acute glaucoma surgery-related choroidal effusions may be prevented by minimizing hypotony, bleeding, and inflammation intra-operatively and post-operatively.
Pre-operative care: Pre-operative osmotic agents or carbonic anhydrase inhibitors can be used to decrease the IOP to a safe level before surgery. The risk of choroidal effusion attributable to anticoagulant use is unclear. However, aspirin or anticoagulants may be discontinued in preparation for glaucoma surgery, if this is medically reasonable. Patients with a serous choroidal effusion should be counseled to avoid intense exercise and heavy lifting to reduce the risk of suprachoroidal hemorrhage postoperatively.
Operative technique: Several important measures can be taken to minimize hypotony, bleeding, and inflammation intraoperatively and during the early postoperative period. When performing a trabeculectomy, careful surgical technique is important to ensure consistent results. Scleral cautery can be performed to prevent bleeding during flap creation, with the scleral flap constructed at 50-75% scleral depth. To prevent early hypotony, multiple sutures can be placed in the scleral flap, and suture release should be delayed by at least one week. A stable anterior chamber can be achieved with a cohesive ophthalmic viscosurgical device or anterior chamber maintainer. Antimetabolites should be used carefully, as their use can increase the risk of CD.
For non-valved glaucoma drainage devices, the tube should be ligated with dissolvable polyglactin suture at the time of surgery, or two-stage surgery should be performed with the tube remaining in the subconjunctival space outside the eye and being placed into the anterior chamber at a later time. These techniques allow time for the formation of a fibrous capsule around the plate, avoiding excessive filtration in the early post-operative period and thereby minimizing hypotony and its related sequelae. The risk of hemorrhagic CD can be diminished by placing the sclerotomy anteriorly to avoid the ciliary body. At the conclusion of a surgery which is expected to lower IOP immediately, the conjunctiva requires watertight closure, in order to prevent hypotony due to a wound leak. Releasable sutures and restrictive devices may be used to reduce hypotony, but these tools do not prevent choroidal detachment completely.
High-risk patients: Prophylactic sclerotomies may be performed as a preventive measure in patients with nanophthalmos, Sturge-Weber syndrome, or previous postoperative choroidal effusions. This is discussed further in the Treatment section below.
Post-operative care: Topical and systemic aqueous suppressants should be discontinued post-operatively, and early laser suture lysis should be avoided.
Diagnosis
Presentation
Serous
Serous choroidal detachments typically develop in the setting of post-operative hypotony, most frequently 2-5 days after glaucoma filtering surgery. Small, peripheral effusions are frequently asymptomatic, with unchanged visual acuity and minimal to no shallowing of the anterior chamber. However, large choroidal effusions may cause peripheral visual field constriction and can even result in a significant decline in central visual acuity due to direct obstruction of the visual axis. Further, anterior displacement of the lens-iris diaphragm can cause a myopic shift and result in secondary angle closure. Appositional choroidal detachments, which extend from the optic nerve to the lens, are more likely to obscure central vision and cause secondary angle closure.[92] In the early post-operative period after glaucoma filtration surgery, older patients are more likely to develop choroidal effusions, while younger patients are more likely to develop hypotony maculopathy. However, hypotony maculopathy may also develop in the setting of longstanding choroidal effusions, resulting in decreased central visual acuity. When hypotony maculopathy persists, permanent vision loss may result.[93]
Hemorrhagic
The intra-operative development of hemorrhagic choroidal detachment is visualized by the surgeon as an enlarging dark mass obscuring the fundus reflex. The patient often complains of severe pain due to stretching of the ciliary nerves, which can be accompanied by nausea and vomiting. This intra-operative emergency is characterized by the development of positive pressure and a tendency for extrusion of the intraocular contents. In the post-operative period, hemorrhagic choroidal detachments are characterized by sudden, excruciating throbbing pain with an immediate loss of vision. This pattern of symptoms in the setting of recent glaucoma filtering surgery is nearly pathognomonic.[94][95]
Signs
- The anterior chamber (AC) can be of normal depth, shallow, or flat. When present, anterior chamber shallowing is typically diffuse, as pressure from choroidal swelling is indirectly transmitted to the posterior surface of the lens via the vitreous body.
- Intraocular pressure can be normal, low, or elevated in the setting of choroidal detachment. Typically, low IOP accompanies serous choroidal detachments, and high IOP accompanies hemorrhagic detachments.
- Fundus examination enables direct visualization of choroidal detachments, which are often located peripherally with a multi-lobed appearance. Up to four smooth lobes may be visualized, extending to the vortex veins. The fluid-filled lobes of serous detachments transilluminate, while hemorrhagic detachments do not transilluminate. Choroidal detachments can be distinguished from retinal detachments based on their more anterior location, extension to the ora serrata, and unique morphology resulting from the choroid’s strong attachments at the sites of the vortex veins.
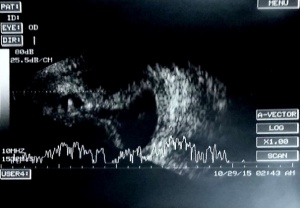
- B-scan ultrasonography can distinguish serous vs. hemorrhagic CD. Serous detachments appear as rounded, peripheral lobes filled with echo-lucent fluid. In contrast, hemorrhagic detachments appear echo-dense.
- Intraoperative hemorrhages can be complicated by loss of eye contents, resulting in vitreous, retina, and/or lens remnants incarcerated in the surgical incision and/or visible in the anterior chamber.
- A serous, or exudative, retinal detachment can be superimposed upon a choroidal detachment and is characterized by shifting subretinal fluid.
- The retinal vasculature is usually normal in appearance, though hypotony maculopathy may present with macular striae and distortion of the retinal vessels.
- The ora serrata may be visible without indentation.
Diagnostic Procedures
- B-scan echography helps to differentiate choroidal effusions from retinal detachments and serous from hemorrhagic effusions, as described in the section titled Signs above. In summary, choroidal detachments can be distinguished from retinal detachments based on their anterior location, extension to the ora serrata, and multiple round lobes demarcated by choroidal attachments to vortex veins. Further, hemorrhagic CD appear as echo-dense lobes on B-scan, while serous CD appear echo-lucent.[100] B-scan ultrasonography offers a method for detecting a small accumulation of fluid in the supra-choroidal space not readily apparent on clinical examination. However, these small choroidal effusions are not typically visually or clinically significant and frequently resolve on their own.[101]
- Ultrasound biomicroscopy (UBM) can be used to visualize anterior rotation of the ciliary body in the setting of a choroidal detachment. UBM can serve a helpful diagnostic role in difficult cases, as it can distinguish secondary angle closure due to choroidal effusions or a choroidal tumor from a pupillary block angle-closure mechanism.[102]
- While not routinely used in the management of CD, wide field fundus photography and swept source optical coherence tomography can also be used to detect and monitor choroidal detachments, with improved detection of peripheral CD.[103][104]
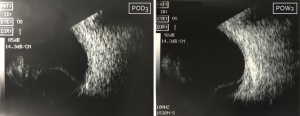
 Appositional hemorrhagic choroidal detachments in two patients. In both patients, these images were captured at a follow-up visit after surgical repair of a traumatic globe injury. Left: B-scan US performed on an elderly female with hemorrhagic choroidal detachment. Right: B-scan US performed on a young male with hemorrhagic choroidal detachment.
Appositional hemorrhagic choroidal detachments in two patients. In both patients, these images were captured at a follow-up visit after surgical repair of a traumatic globe injury. Left: B-scan US performed on an elderly female with hemorrhagic choroidal detachment. Right: B-scan US performed on a young male with hemorrhagic choroidal detachment.
Differential Diagnosis
- Retinal detachment
- Choroiditis
- Central serous chorioretinopathy
- Choroidal melanoma
- Uveal effusion syndrome
- Idiopathic ciliochoroidal effusion
- Pseudophakic or aphakic pupillary block
- Malignant glaucoma
Management
Most serous choroidal effusions are self-limited, resolving with observation as the IOP gradually increases postoperatively.[108] If treatment is warranted, conservative management may be associated with better visual acuity outcomes compared to surgical intervention. A randomized prospective study showed that postoperative trabeculectomy patients diagnosed with flat anterior chambers with iridocorneal touch who were treated with surgical drainage had ≥ 2-line reductions in visual acuity compared to patients who were treated with atropine, phenylephrine, and/or oral acetazolamide alone.[109]
Medical
The initial treatment for persistent post-operative choroidal effusions is typically topical steroids and longer-acting topical cycloplegics (e.g., atropine or cyclopentolate). Topical cycloplegics deepen the anterior chamber by rotating the ciliary body posteriorly; cycloplegics are beneficial in patients with moderate choroidal effusions to prevent apposition of cornea with the iris or lens. Topical steroids are used in an effort to increase IOP and control any inflammation which may be contributing to CD. In addition to intense treatment of ocular inflammation with topical steroids, any medications promoting ocular inflammation should be discontinued. In severe cases that are refractory to topical medications, systemic steroids may be used.[110][111][112]
Hypotensive medications (e.g., topical prostaglandin analogs and aqueous suppressants) should be discontinued in the setting of postoperative hypotony.[113] In contrast, the precipitous rise in IOP seen in cases of acute suprachoroidal hemorrhage may require treatment with topical aqueous suppressants, including beta-blockers, alpha-agonists, and carbonic anhydrase inhibitors. Additionally, oral carbonic anhydrase inhibitors may be required. The pain associated with hemorrhagic CD may be managed with topical cycloplegics and oral acetaminophen (Tylenol), though aspirin and NSAIDs should be avoided due to an increased risk of bleeding.[114]
Surgical
Serous
Office-based procedures are frequently beneficial in cases of persistent hypotony with choroidal effusions. If a wound leak is identified on Seidel testing, a bandage contact lens or suture(s) placed with a tapered (blood vessel) needle can be used to close leaking areas, if the anterior chamber is still formed and deep. Similarly, for an over-filtrating trabeculectomy, transconjunctival sutures may be placed using a tapering needle to secure the scleral flap.[115] Reforming the anterior chamber by inserting a cohesive or ultracohesive viscoelastic material (e.g., Healon 5 or Healon GV, respectively) through the already-formed intraoperative paracentesis track is a highly effective method of transiently increasing IOP, promoting fluid resorption, and decreasing corneal endothelial cell death resulting from a flat anterior chamber.[116] In some instances, serial viscoelastic injections may be necessary to maintain anterior chamber depth and IOP. Injections are performed under topical anesthesia, and IOP should be measured afterward to monitor for ocular hypertension due to over-installation of viscoelastic.[117]
Surgical drainage of choroidal detachments is indicated in the following cases:[118][119]
- Flat anterior chamber
- Persistent corneal edema with a shallow anterior chamber
- Significant eye pain
- Elevated IOP refractory to medical management
- Longstanding choroidal effusion
- Appositional choroidal effusion and/or apposition of the central retina
- Hemorrhagic choroidal detachment
- Decreased vision
Drainage procedures involve deepening the anterior chamber while choroidal fluid egresses out of a full-thickness scleral incision behind the limbus. First, a tangential conjunctival incision is made 3-6 mm from the limbus in the quadrant where the effusion is most substantial; a 2-3 mm radial sclerectomy is then placed 3-4 mm posterior to the limbus. Choroidal fluid egresses spontaneously and/or with assistance by holding the incision open with forceps or gently depressing the adjacent sclera with a cyclodialysis spatula. Finally, the anterior chamber is deepened by injecting saline solution via a paracentesis incision. This procedure has a reported 77% success rate by 12 months follow-up, defined as complete resolution of the choroidal detachment, normalization of anterior chamber depth, and resolution of hypotony. Most patients had improved visual acuity as well.[120]
Cataract extraction is part of treating CD. When a visually significant cataract is present in combination with CD, then combined cataract extraction and CD drainage should be performed. Cataract extraction may be considered for non-resolving choroidal detachment, as this may in some cases result in resolution. In a study of persistent choroidal detachments after glaucoma filtering surgery by Berke et al. in 1987, they state that “46 choroidal detachments in 14 eyes were recorded and required drainage of suprachoroidal fluid on 34 occasions.” In this study, all eyes developed visually significant cataracts, and complete resolution of the recurrent or chronic choroidal detachment occurred following cataract extraction in six of fourteen eyes.[121]
Hemorrhagic
In general, hemorrhagic choroidal detachments are treated with surgical drainage. However, surgical treatment is controversial for hemorrhagic choroidal detachments developing post-operatively, as there is limited data on whether surgical intervention is superior to conservative management, especially if the hemorrhage is small.[122][123] Further, the precise timing of surgical drainage for post-operative hemorrhagic CD is still debated. In the setting of hemorrhagic CD due to ocular trauma, surgical drainage is delayed by 10-14 days after the inciting event to allow time for clot lysis, which makes for easier and more complete drainage. While a similar strategy can be followed for postoperative hemorrhagic CD, it can be difficult to ascertain the exact timing of onset, and the clinical course is typically more indolent. Thus, the timing varies for surgical drainage of hemorrhagic CD developing in the post-operative period after glaucoma surgery. If the choroidal detachment recurs after surgical drainage and persistent over-filtration is suspected, the glaucoma surgery should be revised or closed.[124] Vitreoretinal surgery (e.g., PPV) may be indicated in addition to drainage in cases of hemorrhagic choroidal detachment with concurrent retinal detachment, vitreoretinal traction, or vitreous hemorrhage.
Summary
In summary, medical treatment of choroidal detachments often begins conservatively with observation, followed by topical steroids and cycloplegics. Topical and systemic aqueous suppressant therapy should be discontinued in the setting of hypotony. Chronic and recurrent choroidal detachments should include intense treatment of ocular inflammation and discontinuation of medications that can incite ocular inflammation. In the short term, serial intracameral injections of an ophthalmic viscosurgical material may be considered to reform the anterior chamber and raise IOP. Surgical drainage of choroidal detachments should be performed for serous CD meeting certain criteria, as listed above, and for the majority of hemorrhagic CD. When a visually significant cataract is present, cataract extraction combined with a choroidal tap should be performed.[125]
Prognosis
Serous
Serous choroidal effusions are usually benign and do not significantly reduce visual acuity. A large, persistent effusion, however, may cause significant morbidity, particularly when it is associated with hypotony maculopathy or serous retinal detachment.[126]
Hemorrhagic
Suprachoroidal hemorrhage is a potentially vision-threatening event even with appropriate intervention. The prognosis is worse when the effusion involves all four quadrants and/or results in retinal detachment. In a retrospective case series of 48 patients, 23% had a final visual acuity of no light perception following surgical drainage for suprachoroidal hemorrhage.[127]
Follow Up
Post-operative visits for glaucoma surgical patients should include an assessment of visual acuity, anterior chamber depth, and IOP, as well as careful evaluation for a choroidal detachment in patients with post-operative hypotony or a previously detected CD. In an undilated patient, the direct ophthalmoscope can be utilized to screen for large choroidal detachments, which appear as a rounded peripheral shadow within the red reflex. Dilated fundoscopy and B scan ultrasonography are helpful in assessing CD, including an evaluation of their size and location(s); both are effective strategies for following CD for resolution over time.[128]
In patients who have undergone glaucoma filtering surgery, each visit should also include a careful evaluation of bleb morphology and performance of a Seidel test, utilizing fluorescein staining to evaluate for a leak. If a bleb leak is detected, this merits repair, given the increased risk for bleb-related endophthalmitis as well as hypotony-related sequelae such as CD.[129]
Complications
Poor vision can result from chronic hypotony maculopathy. Further, serous or hemorrhagic choroidal detachments can result in cataract formation, intractable secondary glaucoma, chronic retinal detachment, or phthisis.[130][131]
References
- ↑ Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Current Opinion in Ophthalmology. 2015;26(2):134-42.
- ↑ Roa T, De La Rosa S, Netland P. Five Pointers on Choroidal Effusion and Suprachoroidal Hemorrhage. Glaucoma Today. 2019 Jul/Aug. Accessed 9/2020.<https://glaucomatoday.com/articles/2019-july-aug/five-pointers-on-choroidal-effusion-and-suprachoroidal-hemorrhage>.
- ↑ Reddy A, Salim S. Choroidal Effusions. EyeNet Magazine. 2012 Nov. Edited by Fekrat S and Scott I. Accessed 9/2020. <https://www.aao.org/eyenet/article/choroidal-effusions>.
- ↑ Bellows AR, Chylack LT, Jr., Hutchinson BT. Choroidal detachment. Clinical manifestation, therapy and mechanism of formation. Ophthalmology. 1981;88(11):1107-1115.
- ↑ Diep MQ, Madigan MC. Choroidal detachments: what do optometrists need to know? Clinical & Experimental Optometry. 2019;102(2):116-125.
- ↑ Capper SA, Leopold IH. Mechanism of serous choroidal detachment; a review and experimental study. Arch Ophthalmol. 1956;55:101-13.
- ↑ Levy MM. Pulmonary Capillary Pressure: Clinical Implications. Critical Care Clinics. 1996;12(4):819-839.
- ↑ Pederson JE, Gaasterland DE, MacLellan HM. Experimental ciliochoroidal detachment. Effect on intraocular pressure and aqueous humor flow. Arch Ophthalmol. 1979;97:536-41.
- ↑ Bellows AR, Chylack LT, Jr., Hutchinson BT. Choroidal detachment. Clinical manifestation, therapy and mechanism of formation. Ophthalmology. 1981;88(11):1107-1115.
- ↑ Manschot WA. The pathology of expulsive hemorrhage. Am J Ophthalmol. 1955;40(1):15–24.
- ↑ Ruderman JM, Harbin TS, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol. 1986 Feb;104(2):201–205.
- ↑ Tuli SS, WuDunn D, Ciulla TA, Cantor LB. Delayed suprachoroidal hemorrhage after glaucoma filtration procedures. Ophthalmology. 2001;108(10):1808-1811.
- ↑ Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Current Opinion in Ophthalmology. 2015;26(2):134-42.
- ↑ Learned D, Eliott D. Management of Delayed Suprachoroidal Hemorrhage after Glaucoma Surgery. Seminars in Ophthalmology. 2018;33(1):59-63.
- ↑ Haga A, Inatani M, Shobayashi K, Kojima S, Inoue T, Tanihara H. Risk factors for choroidal detachment after trabeculectomy with mitomycin C. Clin Ophthalmol. 2013;7:1417-1421.
- ↑ Maheshwari D, Kanduri S, Kadar MA, Ramakrishnan R, Pillai MR. Midterm outcome of mitomycin C augmented trabeculectomy in open angle glaucoma versus angle closure glaucoma. Indian J Ophthalmol. 2019;67(7):1080-1084.
- ↑ Shin DY, Jung KI, Park HYL, Park CK. Risk Factors for Choroidal Detachment After Ahmed Valve Implantation in Glaucoma Patients. American J Ophthalmol. 2020;211:105-113.
- ↑ Gedde SJ, Herndon LW, Brandt JD, et al, Tube Versus Trabeculectomy Study Group. Surgical complications in the Tube Versus Trabeculec- tomy Study during the first year of follow-up. Am J Ophthalmol 2007;143:23–31.
- ↑ Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmology. 2013;131(12):1573-1582.
- ↑ Vaziri K, Schwartz SG, Kishor KS, et al. Incidence of postoperative suprachoroidal hemorrhage after glaucoma filtration surgeries in the United States. Clin Ophthalmol. 2015;9:579-584.
- ↑ Jeganathan VS, Ghosh S, Ruddle JB, Gupta V, Coote MA, Crowston JG. Risk factors for delayed suprachoroidal haemorrhage following glaucoma surgery. British J Ophthalmol. 2008;92(10):1393-1396.
- ↑ Liu JC, Green W, Sheybani A, Lind JT. Intraoperative suprachoroidal hemorrhage during Xen gel stent implantation. Am J Ophthalmol Case Rep. 2020;17:100600.
- ↑ Prokosch-Willing V., Vossmerbaeumer U., Hoffmann E., Pfeiffer N. Suprachoroidal bleeding after XEN gel implantation. J Glaucoma. 2017 Dec;26(12):e261–e263.
- ↑ Fineman MS, Emerick G, Dudley D, Katz LJ, Maguire JI. Bilateral choroidal effusions and angle-closure glaucoma associated with human immunodeficiency virus infection. Retina. 1997; 17: 455-457.
- ↑ Quinlan MP, Hitchings RA. Angle-closure glaucoma secondary to posterior scleritis. Br J Ophthalmol. 1978; 62: 330-335.
- ↑ Kawano Y, Tawara A, Nishioka Y, Suyama Y, Sakamoto H, Inomata H. Ultrasound biomicroscopic analysis of transient shallow anterior chamber in Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 1996; 121: 720-723.
- ↑ Diep MQ, Madigan MC. Choroidal detachments: what do optometrists need to know? Clinical & Experimental Optometry. 2019;102(2):116-125.
- ↑ Reddy A, Salim S. Choroidal Effusions. EyeNet Magazine. 2012 Nov. Edited by Fekrat S and Scott I. Accessed 9/2020. <https://www.aao.org/eyenet/article/choroidal-effusions>.
- ↑ Shah PR, Yohendran J, Hunyor AP, Grigg JR, McCluskey PJ. Uveal Effusion: Clinical Features, Management, and Visual Outcomes in a Retrospective Case Series. J Glaucoma. 2016;25(4):e329-335.
- ↑ Almendárez JE, Vargas DM, González C, Takane M, Koga W. Ultrasound findings in ocular trauma. Arch Soc Esp Oftalmol. 2015 Dec;90(12):572-7.
- ↑ Andreoli MT, Yiu G, Hart L, Andreoli CM. B-scan ultrasonography following open globe repair. Eye (Lond). 2014 Apr;28(4):381-5.
- ↑ Speaker MG, Guerriero PN, Met JA, Coad CT, Berger A, Marmor M. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98(2):202-10.
- ↑ Ruderman JM, Harbin TS, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol. 1986 Feb;104(2):201–205.
- ↑ Ruderman JM, Harbin TS, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol. 1986 Feb;104(2):201–205.
- ↑ Liu JC, Green W, Sheybani A, Lind JT. Intraoperative suprachoroidal hemorrhage during Xen gel stent implantation. Am J Ophthalmol Case Rep. 2020;17:100600.
- ↑ Prokosch-Willing V., Vossmerbaeumer U., Hoffmann E., Pfeiffer N. Suprachoroidal bleeding after XEN gel implantation. J Glaucoma. 2017 Dec;26(12):e261–e263.
- ↑ Vaziri K, Schwartz SG, Kishor KS, et al. Incidence of postoperative suprachoroidal hemorrhage after glaucoma filtration surgeries in the United States. Clin Ophthalmol. 2015;9:579-584.
- ↑ Jeganathan VS, Ghosh S, Ruddle JB, Gupta V, Coote MA, Crowston JG. Risk factors for delayed suprachoroidal haemorrhage following glaucoma surgery. British J Ophthalmol. 2008;92(10):1393-1396.
- ↑ Sakai H, Ishikawa H, Shinzato M, Nakamura Y, Sakai M, Sawaguchi S. Prevalence of ciliochoroidal effusion after prophylactic laser iridotomy. Am J Ophthal. 2003;136(3):537-8.
- ↑ Chandra A, Xing W, Kadhim MR, Williamson TH. Suprachoroidal hemorrhage in pars plana vitrectomy: Risk factors and outcomes over 10 years. Ophthalmology. 2014;121(1):311-7.
- ↑ Pavlin CJ, Rutnin SS, Devenyi R, Wand M, Foster FS. Supraciliary Effusions and Ciliary Body Thickening after Scleral Buckling Procedures. Ophthalmology. 1997;104(3):433-438.
- ↑ Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmology. 2013;131(12):1573-1582.
- ↑ Haga A, Inatani M, Shobayashi K, Kojima S, Inoue T, Tanihara H. Risk factors for choroidal detachment after trabeculectomy with mitomycin C. Clin Ophthalmol. 2013;7:1417-1421.
- ↑ Maheshwari D, Kanduri S, Kadar MA, Ramakrishnan R, Pillai MR. Midterm outcome of mitomycin C augmented trabeculectomy in open angle glaucoma versus angle closure glaucoma. Indian J Ophthalmol. 2019;67(7):1080-1084.
- ↑ Goldberg S, Gallily R, Bishara S, Blumenthal EZ. Dorzolamide-induced choroidal detachment in a surgically untreated eye. Am J Ophthalmol. 2004;138(2):285-286.
- ↑ Callahan C, Ayyala RS. Hypotony and choroidal effusion induced by topical timolol and dorzolamide in patients with previous glaucoma drainage device implantation. Ophthalmic Surg Lasers Imaging. 2003;34(6):467-9.
- ↑ Nakakura S, Noguchi A, Tabuchi H, Kiuchi Y. Bimatoprost-induced late-onset choroidal detachment after trabeculectomy: A case report and review of the literature. Medicine. 2017;96(5):e5927.
- ↑ McClintock M, MacCumber M. Lowered intraocular pressure in a glaucoma patient after intravitreal injection of ocriplasmin. Clin Ophthalmol 2015; 23: 1995–1998.
- ↑ de Rojas V, Gonzalez‐Lopez F, Baviera J. Acetazolamide‐induced bilateral choroidal effusion following insertion of a phakic implantable collamer lens. J Refract Surg 2013;29:570–572.
- ↑ Fan JT, Johnson DH, Burk RR, Transient myopia, angleclosure glaucoma and choroidal detachment after oral acetazolamide. Am J Ophthalmol. 1993;115:813-814.
- ↑ Llovet‐Rausell A, Ruiz Tolosa F, Kudsieh B. Severe ocular side effects with acetazolamide: a case report. Arch Soc Esp Oftalmol 2016;91:543–546.
- ↑ Malagola R, Giannotti R, Pattavina L et al. Acute cilio‐choroidal effusion due to acetazolamide: unusual posterior involvement. Drug Des Devel Ther 2013;7:33–36.
- ↑ Malagola R, Giannotti R, Pattavina L, Arrico L. Acute cilio-choroidal effusion due to acetazolamide: unusual posterior involvement (OCT aspects). Eye (London). 2013;27(6):781-782.
- ↑ Mancino R, Varesi C, Cerulli A et al. Acute bilateral angle‐closure glaucoma and choroidal effusion associated with acetazolamide administration after cataract surgery. J Cataract Refract Surg 2011;37:415–417.
- ↑ Kwon S, Park D, Shin J. Bilateral transient myopia, angle‐closure glaucoma, and choroidal detachment induced by methazolamide. Jpn J Ophthalmol 2012;56:515–517.
- ↑ Shaheen, A., Schultis, S., Magraner, M., Correa, Z. M., Yannuzzi, N. A., & Greenfield, D. S. (2023). Acute serous and hemorrhagic choroidal effusion associated with topical dorzolamide therapy. American Journal of Ophthalmology Case Reports, 31, 101866. https://doi.org/10.1016/j.ajoc.2023.101866
- ↑ Law S, Song B, Yu F et al. Hemorrhagic complications from glaucoma surgery in patients on anticoagulation therapy or antiplatelet therapy. Am J Ophthalmol 2008;145:736–746.
- ↑ Nguyen H, Nork T. Massive spontaneous suprachoroidal hemorrhage in a young woman with cystic fibrosis and diabetes mellitus on anticoagulants. Retin Cases Brief Rep 2012;6:216–218.
- ↑ Abtahi M, Abtahi S, Fazel F et al. Topiramate and the vision: a systematic review. Clin Ophthalmol 2012;6:117–131.
- ↑ Banta JT, Hoffman K, Budenz DL, Ceballos E, Greenfield DS. Presumed topiramate-induced bilateral acute angle-closure glaucoma. Am J Ophthalmol. 2001;132(1):112-4.
- ↑ Craig JE, Ong TJ, Louis DL, Wells JM. Mechanism of topiramate-induced acute-onset myopia and angle closure glaucoma. Am J Ophthalmol. 2004;137(1):193-195.
- ↑ Dehghani A, Abtahi M, Abtahi S et al. Massive bilateral choroidal detachment induced by administration of topiramate. Case Rep Ophthalmol 2011;2:251–255.
- ↑ Quagliato L, Barella K, Abreu Neto J et al. Topiramate‐associated acute, bilateral, angle‐closure glaucoma: case report. Arg Bras Oftalmol 2013;76:48–49.
- ↑ Kerimoglu H, Zengin N, Ozturk B et al. Unilateral chemosis, acute onset myopia and choroidal detachment following the use of tamsulosin. Acta Ophthalmol 2010;88:e20–e1.
- ↑ Lubbers S, Japing W. Unilateral choroidal detachment following the use of tamsulosin. Can J Ophthalmol 2017;52:e75–e7.
- ↑ Durai I, Dhavalikar MM, Anand CP, Ganesh V, Krishnadas R. Case Reports in Ophthalmological Medicine. 2016;e1-e5.
- ↑ Postel EA, Assalian A, Epstein DL. Drug-induced transient myopia and angle-closure glaucoma associated with supraciliary choroidal effusion. Am J Ophthalmol. 1996;122(1):110-112.
- ↑ Vegh M, Hari‐Kovacs A, Rez K et al. Indapamide‐induced transient myopia with supraciliary effusion: case report. BMC Ophthalmol 2013;13:58.
- ↑ Phylactou M, Matarazzo F, Jones E. Indapamide-induced bilateral choroidal effusion in pseudophakic patient. Case Reports. 2018;2018:bcr-2018-225920.
- ↑ de Guzman M, Thiagalingam S, Ong P et al. Bilateral acute angle closure caused by supraciliary effusions associated with venlafaxine intake. Med J Aus. 2005;182:121–123.
- ↑ Zelefsky JR, Fine HF, Rubinstein VJ, Hsu IS, Finger PT. Escitalopram-Induced Uveal Effusions and Bilateral Angle Closure Glaucoma. Am J Ophthalmol. 2006;141(6):1144-1147.
- ↑ Takusagawa H, Hunter R, Jue A et al. Bilateral uveal effusion and angle‐closure glaucoma associated with bupropion use. Arch Ophthalmol 2012;130:120–122.
- ↑ Lipa RK, Sánchez ME, Ordovas CA, Aragües AR, Borque CG. Circumscribed Ciliochoroidal Effusion Presenting as an Acute Angle Closure Attack. J Ophthalmic Vis Res. 2017;12(1):117-119.
- ↑ Valeshabad A, Mieler W, Setlur V et al. Posterior segment toxicity following gemcitabine and docetaxel chemotherapy. Optom Vis Sci 2015;92:e110–e3.
- ↑ Andreatta W, Shah P. Reversible hypotony and choroidal effusion following the use of pergolide for Parkinson's disease. Semin Ophthalmol 2013;30:423–425.
- ↑ Kumar RS, Grigg JR, Farinelli AC. Ecstasy induced acute bilateral angle closure and transient myopia. British Journal of Ophthalmology. 2007;91(5):693-5.
- ↑ Berke SJ, Bellows AR, Shingleton BJ, Richter CU, Hutchinson BT. Chronic and recurrent choroidal detachment after glaucoma filtering surgery. Ophthalmology. 1987;94(2):154-62.
- ↑ Wu W, Dawson DG, Sugar A, et al. Cataract surgery in patients with nanophthalmos: results and complications. J Cataract Refract Surg. 2004;30(3):584-590.
- ↑ Ying, Stephaniea; Sidoti, Paul A.a,b; Panarelli, Joseph F.c. Risk factors and management of choroidal effusions. Current Opinion in Ophthalmology 34(2):p 162-167, March 2023. | DOI: 10.1097/ICU.0000000000000929
- ↑ Berke SJ, Bellows AR, Shingleton BJ, Richter CU, Hutchinson BT. Chronic and recurrent choroidal detachment after glaucoma filtering surgery. Ophthalmology. 1987;94(2):154-62.
- ↑ Andreoli MT, Yiu G, Hart L, Andreoli CM. B-scan ultrasonography following open globe repair. Eye (Lond). 2014 Apr;28(4):381-5.
- ↑ Almendárez JE, Vargas DM, González C, Takane M, Koga W. Ultrasound findings in ocular trauma. Arch Soc Esp Oftalmol. 2015 Dec;90(12):572-7.
- ↑ Read S, Cavuoto K. Traumatic open globe injury in young pediatric patients: characterization of a novel prognostic score. J AAPOS 2016; 20: 141–144.
- ↑ Sheng J, Bauza A, Langer P et al. A 10‐year review of open‐globe trauma in elderly patients at an urban hospital. Retina 2015; 35: 105–110.
- ↑ Ruderman JM, Harbin TS, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol. 1986 Feb;104(2):201–205.
- ↑ Speaker MG, Guerriero PN, Met JA, Coad CT, Berger A, Marmor M. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98(2):202-10.
- ↑ Maumenee AE, Schwartz MF. Acute intraoperative choroidal effusion. Am J Ophthalmol. 1985;100(1):147-54.
- ↑ Lin S. Building a safer trabeculectomy. Br J Ophthalmol. 2006. 90:4-5.
- ↑ Stalmans I, Gillis A, Lafaut AS, Zeyen T. Safe trabeculectomy technique: long term outcome. Br J Ophthalmol. 2006;90(1):44-7.
- ↑ Wells AP, Bunce C, Khaw PT. Flap and suture manipulation after trabeculectomy with adjustable sutures: titration of flow and intraocular pressure in guarded filtration surgery. J Glaucoma. 2004 Oct;13(5):400-6.
- ↑ Fellenbaum PS, Almeida AR, Minckler DS, Sidoti PA, Baerveldt G, Heuer DK. Krupin disk implantation for complicated glaucomas. Ophthalmology. 1994;101(7):1178-1182.
- ↑ Bowling B, Chen SD, Salmon JF. Outcomes of referrals by community optometrists to a hospital glaucoma service. Br J Ophthalmol. 2005 Sep;89(9):1102-1104
- ↑ Fannin L, Schiffman J, Budenz D. Risk factors for hypotony maculopathy. Ophthalmology 2003;110:1185–1191
- ↑ Ruderman JM, Harbin TS Jr, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol. 1986;104:201–205
- ↑ Learned D, Eliott D. Management of delayed suprachoroidal hemorrhage after glaucoma surgery. Semin Ophthalmol. 2018;33:59–63
- ↑ Chu T, Green R. Suprachoroidal hemorrhage. Surv Ophthalmol 1999;43:471–486
- ↑ Diep MQ, Madigan MC. Choroidal detachments: what do optometrists need to know? Clin Exp Optom. 2019 Mar;102(2):116-125
- ↑ Fannin L, Schiffman J, Budenz D. Risk factors for hypotony maculopathy. Ophthalmology 2003; 110: 1185–1191)
- ↑ Healey P, Herndon L, Smiddy W. Management of suprachoroidal hemorrhage. J Glaucoma 2007;16:577–579
- ↑ Chu T, Green R. Suprachoroidal hemorrhage. Surv Ophthalmol 1999;43:471–486
- ↑ Reddy A, Salim S. Diagnosis and management of choroidal effusions. EyeNet Magazine 2012; November:47–49
- ↑ Sharan S, Grigg J, Higgins R. Nanophthalmos: ultrasound biomicroscopy and Pentacam assessment of angle structures before and after cataract surgery. J Cataract Refract Surg 2006;32:1052–1055
- ↑ Michalewska Z, Michalewski J, Nawrocka Z et al. Suprachoroidal layer and suprachoroidal space delineating the outer margin of the choroid in swept‐source optical coherence tomography. Retina 2015;35:244–249
- ↑ Adhi M, Liu J, Qavi A et al. Choroidal analysis in healthy eyes using swept source optical coherence tomography compared to spectral domain optical coherence tomography. Am J Ophthalmol 2014;157:1272–1281
- ↑ Shah P, Yohendran J, Hunyor A et al. Uveal effusion: Clinical features, management and visual outcomes in a retrospective case series. J Glaucoma 2016;25:e329–e35
- ↑ Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Curr Opin Ophthalmol 2015;26:134–142
- ↑ Kahook M, and Noecker, R. Why Do Choroidals Form, and How Do You Treat Them? https://glaucomatoday.com/articles/2007-sept-oct/GT0907_07-php?c4src=issue:feed. Published 2007. Accessed January 26, 2021
- ↑ Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Curr Opin Ophthalmol. 2015;26(2):134-142.
- ↑ de Barros DS, Navarro JB, Mantravadi AV, et al. The early flat anterior chamber after trabeculectomy: a randomized, prospective study of 3 methods of management. J Glaucoma. 2009;18(1):13-20.
- ↑ Ku W, Lin Y, Chuang L et al. Choroidal detachment after filtration surgery. Chang Gung Med J 2005; 28: 151–158
- ↑ Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Curr Opin Ophthalmol 2015; 26: 134–142
- ↑ Krishnan M, Baskaran R. Management of postoperative choroidal detachment. Indian J Ophthalmol 1985; 33: 217–220
- ↑ Nakakura S, Noguchi A, Tabuchi H et al. Bimatoprost‐induced late‐onset choroidal detachment after trabeculectomy. Medicine 2017; 96:1–4
- ↑ Chu T, Green R. Suprachoroidal hemorrhage. Surv Ophthalmol 1999; 43:471–486
- ↑ Eha J, Hoffmann EM, Pfeiffer N. Long-term results after transconjunctival resuturing of the scleral flap in hypotony following trabeculectomy. Am J Ophthalmol. 2013;155(5):864-869.
- ↑ de Barros DS, Navarro JB, Mantravadi AV, et al. The early flat anterior chamber after trabeculectomy: a randomized, prospective study of 3 methods of management. J Glaucoma. 2009;18(1):13-20.
- ↑ Kahook M, and Noecker, R. Why Do Choroidals Form, and How Do You Treat Them? https://glaucomatoday.com/articles/2007-sept-oct/GT0907_07-php?c4src=issue:feed. Published 2007. Accessed October 17, 2020.
- ↑ Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Curr Opin Ophthalmol. 2015;26(2):134-142.
- ↑ Bellows AR, Chylack LT, Jr., Hutchinson BT. Choroidal detachment. Clinical manifestation, therapy and mechanism of formation. Ophthalmology. 1981;88(11):1107-1115.
- ↑ WuDunn D, Ryser D, Cantor LB. Surgical drainage of choroidal effusions following glaucoma surgery. J Glaucoma. 2005;14(2):103-8.
- ↑ Berke SJ, Bellows AR, Shingleton BJ, Richter CU, Hutchinson BT. Chronic and recurrent choroidal detachment after glaucoma filtering surgery. Ophthalmology. 1987;94(2):154-62
- ↑ Vaziri K, Schwartz SG, Kishor KS, et al. Incidence of postoperative suprachoroidal hemorrhage after glaucoma filtration surgeries in the United States. Clin Ophthalmol. 2015;9:579-584.
- ↑ Jeganathan VS, Ghosh S, Ruddle JB, Gupta V, Coote MA, Crowston JG. Risk factors for delayed suprachoroidal haemorrhage following glaucoma surgery. British J Ophthalmol. 2008;92(10):1393-1396.
- ↑ Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Curr Opin Ophthalmol. 2015;26(2):134-142.
- ↑ Berke SJ, Bellows AR, Shingleton BJ, Richter CU, Hutchinson BT. Chronic and recurrent choroidal detachment after glaucoma filtering surgery. Ophthalmology. 1987;94(2):154-62
- ↑ Altan T, Temel A, Bavbek T, Kazokoglu H. Hypotonic maculopathy after trabeculectomy with postoperative use of 5-fluorouracil. Ophthalmologica. 1994;208(6):318-320.
- ↑ Wirostko WJ, Han DP, Mieler WF, Pulido JS, Connor TB, Jr., Kuhn E. Suprachoroidal hemorrhage: outcome of surgical management according to hemorrhage severity. Ophthalmology. 1998;105(12):2271-2275.
- ↑ Diep MQ, Madigan MC. Choroidal detachments: what do optometrists need to know? Clin Exp Optom. 2019 Mar;102(2):116-125
- ↑ Monteiro de Barros DS, Navarro JB, Mantravadi AV, et al. The early flat anterior chamber after trabeculectomy: a randomized, prospective study of 3 methods of management. J Glaucoma 2009;18:13-20
- ↑ Berke SJ, Bellows AR, Shingleton BJ, Richter CU, Hutchinson BT. Chronic and recurrent choroidal detachment after glaucoma filtering surgery. Ophthalmology. 1987;94(2):154-62
- ↑ Wirostko W.J., Han D.P., Mieler W.F., Pulido J.S., Connor T.B., Jr., Kuhn E. Suprachoroidal hemorrhage: outcome of surgical management according to hemorrhage severity. Ophthalmology. 1998 Dec;105(12):2271–2275.