Pars Plana Vitrectomy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Overview
Pars plana vitrectomy (PPV) is a commonly employed technique in vitreoretinal surgery that enables access to the posterior segment for treating conditions such as retinal detachments, vitreous hemorrhage, endophthalmitis, and macular holes in a controlled, closed system. The procedure derives its name from the fact that vitreous is removed (i.e., vitreous + ectomy = removal of vitreous) and the instruments are introduced into the eye through the pars plana.
History
David Kasner first described vitrectomy, or removal of the vitreous body, using an open-sky technique in 1969.[1] Two years later, Robert Machemer created the first closed-system vitrectomy setup—which enabled intraocular pressure (IOP) control—using 17-gauge instruments with a pars plana approach, the beginning of what became known as pars plana vitrectomy.[2][3] In 1974, Conor O’Malley and Ralph Heintz developed the modern-day 3-port vitrectomy system—with dedicated ports for vitreous removal using the vitrectomy cutter, infusion of fluid to maintain IOP, and illumination of the posterior segment—using 20-gauge instruments.[4] Subsequent innovations include the development of a trocar-cannula system for insertion of instruments by Robert Machemer and Dyson Hickingbotham, 23-gauge instrumentation by Gholam Peyman and Claus Eckardt, 25-gauge instrumentation by Gildo Fujii and Eugene de Juan, and 27-gauge instrumentation by Yusuke Oshima and colleagues more recently in 2010.[5][6][7][8][9][10][11][12][13]
Indications
Indications for pars plana vitrectomy include removal of vitreous opacities, relieving vitreoretinal traction, restoring the normal anatomical relationship of the retina and retinal pigment epithelium (RPE), and accessing the subretinal space.
Specific conditions include the following:
- Macular hole
- Epiretinal membrane
- Vitreomacular traction
- Vitreous hemorrhage
- Tractional retinal detachment
- Rhegmatogenous retinal detachment
- Refractory macular edema
- Vitreous biopsy
- Endophthalmitis
- Dislocated intraocular lens
- Retained lens material
- Intraocular foreign bodies
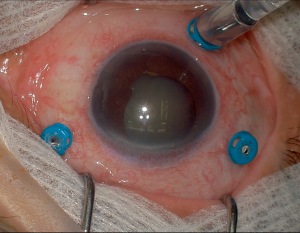
Basic Setup
The basic components of a vitrectomy setup include the following elements:
- Vitrectomy machine (e.g., Alcon Constellation, DORC EVA, Bausch + Lomb Stellaris PC)
- Surgical microscope and wide-angle viewing system (e.g., Zeiss RESIGHT, Oculus BIOM, AVI)
- Infusion cannula: to maintain IOP set by the vitrectomy machine
- Endoillumination light source: for visualization of the posterior segment including vitreous and retina
- Vitrectomy cutter (or vitrector): for vitreous removal, aspiration, and peeling and cutting membranes, among other functions
Gauges
The gauge refers to the size of the instruments with higher numbers corresponding to smaller instruments (20-gauge = 0.9-mm diameter, 23-gauge = 0.6-mm diameter, 25-gauge = 0.5-mm diameter, 27-gauge = 0.4-mm diameter). When vitrectomy was first introduced, 20-gauge instrumentation was the most commonly used.[4] Sclerotomies created with 20-gauge instruments would need to be sutured. Although it is seldom used nowadays, there are still indications for which 20-gauge is necessary, including removal of retained lens material using the fragmatome and removal of intraocular foreign bodies (IOFB) using IOFB forceps. In addition, patients with 20-gauge, sutured wounds may have a decreased rate of endophthalmitis compared to patients undergoing smaller-gauge vitrectomy surgery, a finding possibly related to hypotony and wound leakage more commonly seen in sutureless surgery. Previous studies have found endophthalmitis rates 12 to 28 times higher with 25-gauge vitrectomy compared to 20-gauge vitrectomy, although endophthalmitis rates were low in both groups.[14][15] However, subsequent studies have found similar rates of endophthalmitis with 20-gauge and 25-gauge vitrectomy.[16][17]
On the other hand, there are numerous advantages of small-gauge vitrectomy, including the following:
- Ability to use trocar-cannula entry systems which reduce retinal and vitreous incarceration, neovascularization at sclerotomy sites, and iatrogenic breaks and dialyses at the vitreous base[18]
- Increased patient comfort[19][20]
- Decreased corneal astigmatism[21][22]
- Decreased operative times[19][20]
- Decreased conjunctival scaring[23]
Moreover, the previously held concern that small-gauge vitrectomy systems were only suitable for cases not requiring extensive vitrectomy or membrane dissection—such as epiretinal membrane or macular hole cases—due to lack of instrumentation is no longer true given advances in technology.[18]
Viewing Systems
Visualization of the posterior segment is critical to performing pars plana vitrectomy. The advent of wide-angle viewing systems in the 1980s has revolutionized vitreoretinal surgery by enabling a panoramic view of the posterior segment, thereby enabling safer and more efficient surgery.[24][25][26]
There are 2 main categories of surgical viewing systems: noncontact systems and contact lens systems. Both require the use of an operating microscope. An example of a contact lens system is the Advanced Visual Instruments or AVI system which offers 2 different handheld lenses: one with a 68-degree view to the equator and one with a 130-degree view to the ora serrata.[27] The AVI system requires coupling of the lens to the cornea with viscoelastic and an assistant to hold the lens in place while the eye is maintained in primary position. It is usually used with Leica scopes.
Two popular noncontact viewing systems are the Zeiss RESIGHT and Oculus BIOM wide-angle viewing systems.[28] The Zeiss lens requires use of a Zeiss microscope, whereas the Oculus lens is typically used with Leica scopes but can be adapted to fit Zeiss microscopes as well. Both lens systems do not require coupling to the corneal surface, but viscoelastic is placed on the corneal surface to help maintain a clear view. In addition, no assistant is needed to hold the lens in place as the lenses attach directly to the operating microscope, and they allow for up to 130 degrees of visualization.
For macular surgery, special contact lenses such as the DORC flat vitrectomy lens enable higher resolution and magnification viewing of the posterior pole for more delicate maneuvers such as epiretinal membrane (ERM) and internal limiting membrane (ILM) peeling.[28] However, noncontact macular lenses also exist such as with the Zeiss RESIGHT system.
Instruments
Below is a list of commonly used instruments in pars plana vitrectomy:[29]
- Trocar-cannula system: for placing ports
- Available in 20-, 23-, 25-, and 27-gauge
- Endoilluminators: available in focal and wide-angle illumination
- Uses varying light sources including Xenon and LED
- Chandelier lighting systems: placed using trocar-cannula systems that enable bimanual surgery without having to devote one hand to holding a traditional endoilluminator
- Vitreous cutter: now available with cut speeds up to and exceeding 10,000 cuts per minute
- Forceps: for grasping membranes including ERM, ILM, and proliferative membranes from proliferative vitreoretinopathy (PVR) and diabetes
- e.g., ILM forceps, Grieshaber Maxgrip serrated forceps
- Scissors: for cutting retinal bands and tractional membranes
- Available in horizontal, vertical, and angled designs
- Membrane scrapers: for lifting an edge of a membrane
- e.g., Finesse Flex loop, Tano scraper, pick
- Extrusion cannulas: used to drain subretinal fluid, perform fluid-air exchange, and disperse fluids resting on the retinal surface
- e.g., soft-tip cannula, backflush cannula
- Diathermy: for providing hemostasis, marking the border of retinal tears, and creating drainage retinotomies
- Endolaser: to perform laser photocoagulation intraoperatively
- Available in straight and curved designs
- Fragmatome: for pars plana lensectomy
Dyes
Adequate visualization of tissues and tissue planes is a prerequisite for safe and efficient surgery. Chromovitrectomy—the use of vital dyes during vitreoretinal surgery—aids with such visualization. An ideal dye should be safe to use intraocularly, be easy to apply and remove, and provide adequate staining of the desired tissue.[30] The following 4 dyes are the most commonly used in vitreoretinal surgery:
- Triamcinolone acetonide: available in a non–preservative-free formulation called Kenalog and preservative-free formulation called Triesence, the latter of which is approved by the Food and Drug Administration (FDA) for intraocular use. Triamcinolone is particularly useful for staining the vitreous gel.[31][32]
- Trypan blue: a hydrophilic dye also used for staining the anterior capsule during phacoemulsification surgery. It is used in vitreoretinal surgery to stain the ERM and ILM and is FDA approved for use in ERM removal cases.[33]
- Brilliant blue: commonly used worldwide and approved by the FDA in 2019, it is used primarily for staining the ILM with minimal toxicity.[34]
- Indocyanine green: traditionally used for angiography, indocyanine green also effectively stains the ILM. However, at higher concentrations, it is toxic to the retina and RPE.[35][36]
Surgical Principles
The following section provides an overview of 3 common surgeries that utilize pars plana vitrectomy: retinal detachment repair, membrane peeling, and crystalline lens removal.
Retinal Detachment
The principles of retinal detachment repair via pars plana vitrectomy are to remove the vitreous gel and any vitreoretinal traction, locate and laser any retinal tears, and insert an intraocular tamponade.[28]
The basic steps are as follows:
- Insert trocars in the pars plana (3-4 mm from the limbus depending on the lens status) typically using a beveled incision technique.
- Perform a core vitrectomy to remove the central vitreous gel. Use of triamcinolone can aid with vitreous removal.
- Induce a posterior vitreous detachment if a natural one has not already occurred. This is often done using the vitrectomy cutter on the aspiration-only setting (i.e., the cutter function is turned off).
- Perform a peripheral vitrectomy and release traction over the detached retina, at the retinal tears, and at any areas of lattice degeneration. Again, triamcinolone can be helpful during this step.
- Flatten the retina by draining subretinal fluid from a preexisting break or a newly created drainage retinotomy while performing a fluid-air exchange, typically using a soft-tip cannula. Alternatively, heavy liquids such as perfluorocarbons can be injected posteriorly to push subretinal fluid anteriorly out through a preexisting break.
- Once the retina is flattened, endolaser is then performed around the retinal breaks.
- Insert an intraocular tamponade. SF6 (lasting 2-3 weeks) and C3F8 (lasting 6-8 weeks) gas are most commonly used, although there are indications for silicone oil (lasting permanently until it is taken out) if longer tamponade is needed or in patients who are monocular, must fly, or cannot position.
Membrane Peel
To treat conditions such as epiretinal membranes, macular holes, vitreomacular traction, tractional retinal detachments, and proliferative vitreoretinopathy, membrane peeling may be necessary. As with retinal detachment surgery, the initial steps of inserting trocars, performing a core vitrectomy, and inducing a posterior vitreous detachment if not already present are similar. The degree of peripheral vitrectomy performed may depend on the clinical scenario.
Next, attention is turned to the membrane itself that requires peeling. A different set of techniques is used here compared to retinal detachment surgery. First, a higher magnification and resolution lens is used, which may be the 68-degree AVI lens, the green macular lens with the Zeiss RESIGHT, or the DORC flat vitrectomy lens.[27][28] Next, a vital dye can be instilled in the posterior segment to improve tissue visualization. With any membrane peel, an initial flap must be created if not naturally present, followed by peeling of the membrane off the retinal surface. The initial flap can be created with Maxgrip or ILM forceps, a microvitreoretinal (MVR) blade, a Tano scraper, a Finesse Flex loop, or a pick.[28]
Lens Removal
Removal of the crystalline lens during pars plana vitrectomy may be needed in several situations. If visualization of the posterior segment is poor due to media opacity from a cataract, either phacoemulsification or pars plana lensectomy can be performed. If there is retained nucleus or retained cortical fragments causing significant inflammation and IOP rise, pars plana lensectomy must be performed.[37] There may also be a benefit to removing both the crystalline lens and lens capsule in PVR cases to remove a scaffold for future membrane growth.[38]
For pars plana lensectomy, soft lenses can be removed using the vitrectomy cutter, but denser lenses may require use of the fragmatome. The fragmatome operates similarly to a phacoemulsification probe although use of the conventional fragmatome requires enlarging sclerotomies, often using an MVR blade. If the lens is sitting in the vitreous cavity, a thorough vitrectomy should be performed first to remove vitreous attachments to any lens fragments. If indicated, lens capsule can subsequently be removed using different types of intraocular forceps.
Complications
The following are complications that may be seen intraoperatively or postoperatively with pars plana vitrectomy:[39][40][41]
- Cataract
- Glaucoma
- Endophthalmitis
- Retinal tear
- Retinal detachment
- Hypotony
- Suprachoroidal effusion
- Suprachoroidal hemorrhage
- Vitreous hemorrhage
- Cystoid macular edema
- Optic neuropathy
- Phototoxicity
- Retained perfluorocarbon liquid–induced toxicity
References
- ↑ Kasner D. Vitrectomy: a new approach to management of vitreous. Highlights Ophthalmol. 1969;11:304.
- ↑ Machemer R, Buettner H, Norton E, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1972;75(4):813-820.
- ↑ Machemer R, Parel J, Norton E. Vitrectomy: a pars plana approach—technical improvements and further results. Trans Am Acad Ophthalmol Otolaryngol. 1972;76(2):462-466.
- ↑ 4.0 4.1 O’Malley C, Heintz RM. Vitrectomy with an alternative instrument system. Ann Ophthalmol. 1975;7(4):585-588.
- ↑ Machemer R, Hickingbotham D. The three-port micro-cannular system for closed vitrectomy. Am J Ophthalmol. 1985;100(4):590-592.
- ↑ de Juan E Jr, Machemer R, Charles S, Hirose T, Tasman WS, Trese MT. Surgery for stage 5 retinopathy of prematurity. Arch Ophthalmol. 1987;105(1):21.
- ↑ Peyman GA. A pneumovitrector for the diagnostic biopsy of the vitreous. Ophthalmic Surg Lasers. 1996;27(3):246-247.
- ↑ Peyman GA. A miniaturized vitrectomy system for vitreous and retinal biopsy. Can J Ophthalmol. 1990;25(6):285-286.
- ↑ Fujii GY, de Juan E Jr, Humayun MS. Improvements after sheathotomy for branch retinal vein occlusion documented by optical coherence tomography and scanning laser ophthalmoscope. Ophthalmic Surg Lasers Imaging. 2003;34(1):49-52.
- ↑ Fujii GY, de Juan E Jr, Humayun MS, et al. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109(10):1814-1820.
- ↑ Fujii GY, de Juan E Jr, Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109(10):1807-1812; discussion 1813.
- ↑ Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25(2):208-211.
- ↑ Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117(1):93-102.e2.
- ↑ Kunimoto DY, Kaiser RS, Wills Eye Retina Service. Incidence of endophthalmitis after 20- and 25-gauge vitrectomy. Ophthalmology. 2007;114(12):2133-2137.
- ↑ Scott IU, Flynn HW Jr, Dev S, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina. 2008;28(1):138-142.
- ↑ Scott IU, Flynn HW Jr, Acar N, et al. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2011;249(3):377-380.
- ↑ Mason JO III, Yunker JJ, Vail RS, et al. Incidence of endophthalmitis following 20-gauge and 25-gauge vitrectomy. Retina. 2008;28(9):1352-1354.
- ↑ 18.0 18.1 Recchia FM, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS. Small-gauge pars plana vitrectomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2010;117(9):1851-1857.
- ↑ 19.0 19.1 Misra A, Ho-Yen G, Burton RL. 23-gauge sutureless vitrectomy and 20-gauge vitrectomy: a case series comparison. Eye (Lond). 2009;23(5):1187-1191.
- ↑ 20.0 20.1 Yanyali A, Celik E, Horozoglu F, Oner S, Nohutcu AF. 25-gauge transconjunctival sutureless pars plana vitrectomy. Eur J Ophthalmol. 2006;16(1):141-147.
- ↑ Yanyali A, Celik E, Horozoglu F, Nohutcu AF. Corneal topographic changes after transconjunctival (25-gauge) sutureless vitrectomy. Am J Ophthalmol. 2005;140(5):939-941.
- ↑ Okamoto F, Okamoto C, Sakata N, et al. Changes in corneal topography after 25-gauge transconjunctival sutureless vitrectomy versus after 20-gauge standard vitrectomy. Ophthalmology. 2007;114(12):2138-2141.
- ↑ Recchia FM, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS. Small-gauge pars plana vitrectomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2010;117(9):1851-1857.
- ↑ Spitznas M. A binocular indirect ophthalmomicroscope (BIOM) for non-contact wide-angle vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 1987;225:13-15.
- ↑ Peyman GA. A new wide-angle irrigating contact lens for pars plana vitrectomy. Can J Ophthalmol. 1988;23:150.
- ↑ Bovey EH, Gonvers M. A new device for noncontact wide-angle viewing of the fundus during vitrectomy. Arch Ophthalmol. 1995;113:1572-1573.
- ↑ 27.0 27.1 Advanced Visual Instruments. http://www.avi-panoramic.com/index.html
- ↑ 28.0 28.1 28.2 28.3 28.4 Vitrectomy Basics. Vit-Buckle Society. https://vitbucklesociety.org/vitrectomy-basics
- ↑ Villegas VM, Murray TG. Know your retinal surgery toolbox. Retinal Physician. May 1, 2018. https://retinalphysician.com/issues/2018/may/know-your-retinal-surgery-toolbox/
- ↑ Bracha P, Ciulla TA, Baumal CR. Vital dyes in vitreomacular surgery. Ophthalmic Surg Lasers Imaging Retina. 2018;49(10):788-798.
- ↑ Rodrigues EB, Maia M, Meyer CH, Penha FM, Dib E, Farah ME. Vital dyes for chromovitrectomy. Curr Opin Ophthalmol. 2007;18(3):179-187.
- ↑ Maia M, Penha FM, Farah ME, et al. Subretinal injection of preservative-free triamcinolone acetonide and supernatant vehicle in rabbits: an electron microscopy study. Graefes Arch Clin Exp Ophthalmol. 2008;246:379-388.
- ↑ Maia M, Penha F, Rodrigues EB, et al. Effects of subretinal injection of patent blue and trypan blue in rabbits. Curr Eye Res. 2007;32(4):309-317.
- ↑ Malerbi FK, Maia M, Farah ME, Rodrigues EB. Subretinal brilliant blue G migration during internal limiting membrane peeling. Br J Ophthalmol. 2009;93(12):1687.
- ↑ Tadayoni R, Paques M, Girmens JF, Massin P, Gaudric A. Persistence of fundus fluorescence after use of indocyanine green for macular surgery. Ophthalmology. 2003;110:604-608.
- ↑ Maia M, Haller JA, Pieramici DJ, et al. Retinal pigment epithelial abnormalities after internal limiting membrane peeling guided by indocyanine green staining. Retina. 2004;24:157-160.
- ↑ Vanner EA, Stewart MW. Vitrectomy timing for retained lens fragments after surgery for age-related cataracts: a systematic review and meta-analysis. Am J Ophthalmol. 2011;152(3):345-357.e3.
- ↑ Mango CW. My approach to PVR detachment: an American perspective. In: Spandau U et al, eds. Retinal Detachment Surgery and Proliferative Vitreoretinopathy. Springer International Publishing AG; 2018.
- ↑ Stein JD, Zacks DN, Grossman D, Grabe H, Johnson MW, Sloan FA. Adverse events after pars plana vitrectomy among medicare beneficiaries. Arch Ophthalmol. 2009;127(12):1656-1663.
- ↑ Day S, Grossman DS, Mruthyunjaya P, Sloan FA, Lee PP. One-year outcomes after retinal detachment surgery among medicare beneficiaries. Am J Ophthalmol. 2010;150(3):338-345.
- ↑ Chang S. LXII Edward Jackson lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006;141(6):1033-1043.