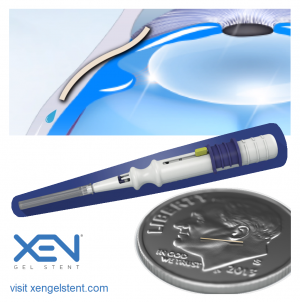
Xen Glaucoma Treatment System
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Background
Glaucoma is the number one cause of irreversible blindness both in the United States and worldwide.[1][2] The decrease in quality of life associated with glaucoma can be profound and may occur earlier than previously thought, highlighting the importance of early diagnosis and treatment.[3] Elevated intraocular pressure (IOP) is a major risk factor for the development and progression of glaucoma,[4] and reduction of IOP is the only proven method to prevent and treat glaucoma.[5][6][7][8] Current guidelines from the American Academy of Ophthalmology Preferred Practice Pattern recommend lowering the IOP to a target level, which is a value or range of values at which the rate of disease progression will be slowed sufficiently to avoid functional impairment from the disease.[9]
Glaucoma treatments include various topical and oral medications, cyclodestructive and trabecular meshwork-directed laser procedures, and various surgical techniques designed to improve trabecular meshwork outflow or divert aqueous to the subconjunctival space. Subconjunctival drainage of aqueous humor, resulting in bleb formation, has been a cornerstone of glaucoma surgery for more than a century.[10] However, the ‘gold standard’ trabeculectomy[11] has significant short-term risks like near 50% rate of transient perioperative complications,[12] as well as long-term risks of failure, reported to be as high as 50% at 5 years.[13] Because of potentially serious complications associated with traditional glaucoma surgeries (e.g., trabeculectomy and tube-shunt drainage devices)[4], a wave of less invasive glaucoma surgical devices have emerged in recent years with a goal of safer, earlier surgical intervention. As with all glaucoma surgeries, these devices can offer lower and less variable IOP on fewer medications, thereby addressing both under-treatment and noncompliance issues.[11]
A number of criteria have been proposed for minimally invasive glaucoma surgery (MIGS) including minimal tissue disruption, ab-interno implantation, short surgical time, simple instrumentation, and fast postoperative recovery.[14] The Xen gel implant is a microstent that can lowers IOP via subconjunctival drainage without the tissue disruption of a trabeculectomy or traditional glaucoma drainage implant.[4] [15] FDA approved in 2016, the Xen gel stent is the first ab interno procedure which bypasses the diseased trabecular meshwork to drain aqueous fluid from the anterior chamber to a subconjunctival bleb.[16]
Xen gel stent
The Xen gel stent (Allergan Inc., CA, USA) is a 6-mm hydrophilic[15] flexible tube with a 45-micron lumen. The implant is made of porcine collagen-derived gelatin cross-linked with glutaraldehyde[4], which is noninflammatory[17] and causes minimal extraocular fibrotic or vascular response to the implant material[11]. The Xen decreases IOP by creating a permanent drainage shunt from the anterior chamber (AC) to the subconjunctival space through a scleral channel.[15] The device hydrates within 1-2 minutes of contact with aqueous humor, bending and conforming to tissue.[4] This flexibility mitigates many of the issues seen with synthetic materials such as migration, erosion and corneal endothelial damage.[17]
The design of the Xen gel stent is based upon the principles of laminar fluid dynamics.[15] The Hagen–Poiseuille equation was used to calculate the required internal dimensions of a tube that (1) would prevent hypotony at an average aqueous humor production of 2–3 μl/min and (2) would provide a steady-state IOP floor of approximately 6–8 mmHg. In effect, the primary flow resistance of the tube itself is designed to prevent hypotony.[11][18]
Three Xen models were initially designed[11], with 45, 63, and 140 μm internal lumen diameters for varying levels of IOP control. The smallest one, XEN45, was approved by the FDA in 2016 and hereafter is referred to as Xen.[15] The tube length of 6 mm is of suitable length for passage from the AC, through the trabecular meshwork and sclera, and into the subconjunctival space at an optimal distance from the limbus.[4] The external diameter of the Xen is 150 μm and the inner diameter is 45 μm. Implantation of the Xen is performed with a sterilized, single-hand inserter containing a 27G needle that is preloaded with one gelatin stent.[19]
Patient Selection
Indications
The Xen gel stent was approved by the FDA in 2016 for use in primary open angle glaucoma and pseudoexfoliative or pigmentary glaucoma with open angles that are unresponsive to maximum tolerated medical therapy, as well as refractory open angle glaucoma (OAG) that has failed previous surgical treatment.[20] In Europe, the Xen gel stent is indicated to reduce IOP in patients with OAG who have failed previous medical treatments.[21] Patients with open angles requiring combined phacoemulsification and glaucoma surgery are also suitable patients for Xen implantation.[22]
Contraindications
The Xen gel stent is generally contraindicated in patients with the following conditions:[15][23]
- Angle closure glaucoma, where the angle has not been surgically opened
- Previous glaucoma shunt or valve in target quadrant
- Conjunctival scarring or other pathology in the target quadrant (e.g. pterygium)
- Active iris neovascularization or neovascularization of the iris within 6 months of the surgical date
- Active ocular inflammation (e.g., blepharitis, conjunctivitis, keratitis, uveitis)
- Anterior chamber intraocular lens
- Intraocular silicone oil
- Vitreous in the anterior chamber
- Impaired episcleral venous drainage (e.g., Sturge–Weber, nanophthalmos)
- History of dermatologic keloid formation
- Known or suspected allergy or sensitivity to drugs required for the surgical procedure or any of the device components (e.g., porcine products or glutaraldehyde)
Pre-Operative Considerations
Studies on predictive values in Xen have indicated that higher baseline IOP was significantly associated with greater mean IOP reduction at 12 months (p<0.001). Other baseline characteristics, such as age, baseline number of glaucoma medications, central corneal thickness, and corneal hysteresis were not associated with 12-month success.[24]
The Xen gel stent has some advantages over traditional surgeries, such as trabeculectomy and tube shunts, including:[25]
- Less invasive surgical technique, not requiring an extensive conjunctival dissection
- Pre-loaded injector
- Device that is soft and flexible when wet
- Shorter surgical and recovery times
- Potentially fewer side effects, e.g. prevention of chronic hypotony by an intrinsic flow-limiting design[11]
- Retains postoperative options, allowing physicians to use additional IOP-reduction techniques that could be required at a later time [17]
Surgical Procedure
The Xen gel stent comes preloaded in an injector device, which is a sterilized, single-hand plastic inserter containing a 27-gauge sharp beveled needle.[26] While ab externo approaches exist, the device is FDA-approved to be implanted ab interno, meaning from within the AC. The steps for each approach are described below.
Ab Interno Approach
The superior nasal conjunctiva is marked 2.5 to 3.0 mm posterior the limbus. Intraoperative mitomycin C (MMC) is injected subconjunctivally in order to reduce postoperative scar tissue formation. Clear corneal incisions (main and side-port) are created and the anterior chamber is filled with a cohesive viscoelastic. The needle is inserted through the main corneal incision and directed across the anterior chamber (AC) towards the superonasal quadrant. A goniolens can be used to assess positioning in the angle, ideally entering just above trabecular meshwork to avoid bleeding and to stay clear of the iris and endothelium. The needle is advanced through the sclera into the subconjunctival space, while the eye is stabilized using a second instrument in the side-port incision. Once the bevel is clearly visualized exiting the sclera into the subconjunctival space, the gelatin stent is released and the injector is removed from the eye. Approximately 1 mm of the implant remains in the AC, 3 mm pass through the scleral wall, and 2 mm emerge under the conjunctiva. The viscoelastic is then washed out of the anterior chamber creating an early bleb and confirming patency of the device.
These steps are summarized in more detail below:
- Cataract surgery may be performed before Xen implantation, if planned. Following intraocular lens implantation, miotic drugs may be used to facilitate Xen implantation.[15]
- Topical anesthesia is applied.
- The intended area of placement in the supero-nasal quadrant is marked, which is approximately 3 mm from the limbus.[15]
- 0.05–0.2 ml MMC (0.1–0.2 mg/ml) is injected with a 30-gauge needle in the superonasal quadrant and massaged over the area of anticipated Xen implant insertion or a conjunctival flap is made and MMC applied on sponges (off-label use).[4] Administration of MMC also can be performed after Xen implantation.
- Two self-sealing corneal incisions are made; a larger incision (e.g., 1.8 mm) inferotemporally or temporally and a smaller paracentesis incision (e.g., 1.0 mm) superotemporally or superonasally.
- A cohesive viscoelastic is used to fill the AC through the paracentesis.
- The injector tip is placed through the inferotemporal or temporal clear corneal incision, while a second instrument is utilized for counter-traction through a superotemporal or superonasal paracentesis.
- The inserter needle (double-beveled 27 gauge) is directed through the temporal incision and across the AC toward the superonasal quadrant. A mirrored gonioscope can be used to guide this step, but it is not mandatory and can be used at the discretion of the surgeon.[15]
- The Xen gel implant should be placed anterior to Schlemm’s canal between the pigmented and nonpigmented trabecular meshwork to avoid bleeding. This step should be performed under gonioscopic visualization.
- The sharp tip is engaged at or slightly anterior to the trabecular meshwork and advanced through the sclera.[11]
- The needle is tunneled through the sclera coming out subconjunctivally 2-3 mm from the limbus, as previously marked, using a second instrument to provide countertraction via the side port.
- A sliding mechanism is pushed forward to initially deploy the stent and then to retract the needle into the hub,[11] without drawing the implant back.[15]
- The injector is withdrawn, and placement of the Xen is confirmed. The ideal stent placement should leave 2.0 mm of exposed implant in the subconjunctival space (preferentially in a more superficial layer than the sub-Tenon's space), 1.0 mm in the AC, and 3.0 mm tunneled through sclera.
- Viscoelastic is removed from the AC.
- Bleb morphology and function may be obtained by forced infusion of fluid through the paracentesis at the end of the procedure.[15]
Ab Externo Approach
Ab externo placement can be performed with or without conjunctival dissection. In the first technique, a closed ab externo approach, the conjunctiva is displaced anteriorly, and the needle pierces the sclera at 2.5 mm from the limbus and tunnels through the sclera before entering the AC, where the stent is then released. MMC is injected subconjunctivally before or after implantation.
If conjunctiva is opened, the stent is deployed 2.5 mm from the limbus and MMC may be applied either via soaked sponges or subconjunctival injection. After placement of the device is confirmed, the conjunctiva and Tenon’s layer are sutured in a watertight closure.
In both ab externo techniques, placement in the anterior chamber is confirmed using gonioscopic visualization, and the stent can be gently repositioned using a bent tying forcep if the stent is either too short or too long in the anterior chamber. This repositioning of the stent may be performed through the conjunctiva, if a closed technique is employed.
Compared with ab interno placement, ab externo placement avoids the necessity of viscoelastic injection and allows for more precise positioning of the device.[27]
Tan et al.[28], Ucar and Cetinkaya[29], and Do et al.[30] reported that there were no differences in outcomes between ab-interno and ab-externo approaches of the Xen implantation in terms of the IOP reduction and success rates.
However, many studies have demonstrated that the ab-externo approach implantation could reduce the rate of needling to as low as 11.8%. [29] [30][31][32]
Video Demonstrations
[1] – 2-3 minute 3D animation
[2] – Dr. Paul Singh ab interno approach
[3] – Dr. Jella An ab externo approach
Perioperative Management
Pre-Operative
- Patients are instructed to stop all glaucoma medications on the day of the operation.
Post-Operative
- Patients are seen on postoperative day 1 and followed up at the physician's discretion.
- Postoperative medication regimen varies, but generally includes topical antibiotic prophylaxis for 1 week and topical corticosteroids multiple times each day for at least one month followed by a slow taper.[33][34]
- Close monitoring of implant location and bleb morphology should be performed via slit lamp biomicroscopic examination, which can be complemented by ultrasound biomicroscopy (UBM) or anterior segment optical coherence tomography (AS-OCT), if desired.
Morphologic changes to the developing filtering bleb after surgery may help to predict early treatment failure and guide bleb revision and management. Fea et al. reported that maximal height of the bleb and the total area of cystic hypoehoic spaces were significantly higher in the success group. Increased microcysts and loosely arranged connective tissue with low stromal reflectivity are suggestive of new or increased alternative outflow induced by the stent implantation. In the other hand, bleb wall reflectivity was significantly higher in the failure group.[35]
Post-Operative Needling
The most common bleb-reported complication is bleb fibrosis, requiring post-operative needling of the bleb for lysis of adhesions. Whenever a Xen bleb becomes flat/fibrotic, bleb needling can be performed as an alternative to surgery. Prospective studies have reported needling rates as high as 46.2% within the first 12 months with 39.2% of eyes requiring more than one needling revision in the first 24 months, most frequently within the first three months post-op.[36] Xen needling has been demonstrated to be relatively safe and effective.[37] However, there are reports of Xen fractures and amputations caused by needling.[38] One case series of Xen fractures after needling concluded that because Xen is composed of flexible gelatin, the device can be damaged relatively easily during the bleb needling process.
Higher IOP at post-operative day 1 has been identified as a predictor for future needling following standalone Xen. One prospective study by Midha et al. found that a post-operative IOP > 20 mmHg increases the probability of requiring needling to 80%, while IOP < 10 mmHg decreases the probability of needling to 35%.[39] Additionally, morphologic changes to the developing bleb after surgery may help to predict early treatment failure and guide bleb revision and management. Assessment of bleb morphology through biomicroscopy, in vivo confocal microscopy (IVCM), and anterior segment-optical coherence tomography (AS-OCT) indicated that bleb morphologies in Xen success groups were associated with significantly higher bleb heights and total area of cystic hypoehoic spaces.[40] Increased micro-cysts, loosely arranged connective tissue, and low stromal reflectivity were suggestive of new or increased alternative outflow induced by the stent implantation. On the other hand, bleb wall reflectivity was significantly higher in the failure group.
One study found that there were no factors that were analyzed that increased the risk of post-operative needling including age, sex, glaucoma subtype, number of preoperative glaucoma medications, preoperative medicated IOP, and preoperative unmedicated IOP. [41] Another study demonstrated a significant correlation between the number of needling procedures and a 1-day, 1-week and 1-month postoperative IOP. [42]
The main complication of bleb needing is implant fracture due to unintentional damage to the soft implant. However, fractures have not been reported to impair the efficacy of the draining mechanism itself.[43]
Complications
Most reported intra- and post-operative complications are minor and inherent to the surgical technique. However, more rare and serious complications have been reported. A critical point is the final placement of the Xen device.
Intra-Operative Complications
- Incorrect placement of the Xen
- Posterior placement of the implant, especially through ciliary body, resulting in bleeding and/or hypotony
- Subconjunctival or AC bleeding during the implantation
- Stent breaking into multiple pieces during placement into the subconjunctival space[44]
- Conjunctival leakage, due to penetration with the needle
- Cyclodialysis cleft secondary to Xen insertion[45]
Post-Operative Complications
A systematic review and meta-analysis conducted by Chen et al. included 56 studies and noted the most frequent complications[46].
- Transient hypotony (9.59%)
- Hyphema (5.53%)
- IOP spikes (2.11%)
- Choroidal effusions (1.31%)
- Implant occlusion (0.93%)
- Macular edema (0.91%)
- Implant malposition (0.88%)
- Shallow AC (0.88%)
- Bleb leakage (0.68%)
- 2+ Snellen lines vision loss lasting 1+ month (0.34%)
- Endophthalmitis (0.15%)
- Corneal edema (0.29%)
- Additional Glaucoma Surgery (2.4-15.3%)
- Require needling (30.7-43%)
- Wound leak (1.6 - 9.2%)[13]
- Suprachoroidal hemorrhage[47]
- Malignant glaucoma[48]
Late Complications
- Device erosion and exposure of implant[49]
- Implant migration: Dislocation into AC, Xen-iris touch[13][44]
- Bleb leak or dehiscence (may be due to thin or ischemic bleb with overfiltration)
- Reported cases of blebitis and persistent hypotony,[50] suprachoroidal bleeding,[51] and conjunctival perforation with late Seidel-positive bleb leakage[52]
- Endophthalmitis[45][53]
Outcomes
Efficacy and Safety Profile with Xen
The Xen was introduced relatively recently, thus long-term results are yet to be published. Studies with 1-year follow-up have found it to be efficacious in lowering IOP significantly and reducing the number of hypotensive medications used, with minimal complications or serious side effects.[4] Most studies document an IOP reduction of >29% (greater than the reduction demonstrated by isolated phacoemulsification in patients with primary open-angle glaucoma) and a significant reduction in the number of IOP-lowering medications.
In the meta analysis mentioned above by Chen et al., the mean difference in IOP before and after Xen was -7.80 (-8.21, -7.38; p < 0.00001) and -8.35 (-9.82, -6.88; p < 0.00001) when combined with phacoemulsification. The average number of drops decreased by -1.97 (-2.19, -1.75; p < 0.00001) when xen was performed alone, and -1.86 (-2.11, -1.60; p < 0.00001) when combine with phacoemulsification [46].
Grover et al. evaluated the IOP-lowering effect and safety of the Xen gel stent in patients with refractory glaucoma (previously failed filtering or cycloablative procedure and/or uncontrolled IOP on maximally tolerated medical therapy).[54] In 76.3% of patients there was ≥20% reduction of IOP on same or fewer medications. The mean (SD) medication use reduced from 3.5 (1) at baseline to 1.7 (1.5) at 12-month follow-up. There was 75% probability of success reported at 12 months (Kaplan–Meier analysis). Visual recovery post-surgery was rapid with most patients experiencing either no change in vision or improvement in BCVA. This study clearly demonstrates the effectiveness and good safety profile of Xen gel stent in refractory glaucoma.
In Galal et al evaluated 13 eyes with primary open angle glaucoma underwent Xen implantation with subconjunctival mitomycin C (MMC).[55] Complete success (IOP reduction ≥20% from preoperative baseline at 1 year without any glaucoma medications) was achieved on 41.7% of patients, while qualified success rate (IOP reduction of ≥20% at 1 year with medications) was 66.7%. IOP reduction at 12 months was 16 ± 4 to 12 ± 3 mmHg (p ≤ 0.01) and medication reduction at 12 months was 1.9 ± 1 to 0.3 ± 0.49 (p = 0.003).
In a prospective, non-RCT by De Gregorio et al, 41 eyes of 33 patients underwent a Xen gel stent procedure combined with cataract surgery.[56] Outcomes of this study showed that the XEN45 implant was statistically effective in reducing IOP and medications after 12 months. The complete success rate (≤18 mmHg without medications) after 12 months was achieved in 80.4% (33 of 41 eyes) and a qualified success (≤18 mmHg with medications) in 97.5% (40 of 41 eyes). IOP reduction at 12 months was 22.5 ± 3.7 to 13.1 ± 2.4 mmHg (p < 0.05) and medication reduction at 12 months was 2.5 ± 0.9 to 0.4 ± 0.8 (p < 0.05).
In a prospective, multicenter, non-RCT, Reitsamer et al reported 24 months follow-up of 174 eyes (79.8% of 218 total eyes initially enrolled).[57] Of these, 65.8% achieved complete success, defined as ≥20% reduction in IOP without secondary surgical intervention. 48.4% achieved ≥30% reduction in IOP without secondary surgical intervention. 41.1% of eyes underwent at least one needling. This study included both standalone Xen implantations and those that combined phacoemulsification with Xen implantation, and the results were similar for both of these treatment arms. In a multicenter retrospective chart review by the same group, the three-year effectiveness and safety was evaluated implanted ab interno. Results showed mean IOP decreased from 20.7 mmHg on 2.5 medications at baseline to 13.9 mmHg and 1.1 medications. Results appeared comparable when implantation was performed with or without pseudoemulsification. However, 7.1% of eyes had intraoperative complications, 14.6% experienced 46 postoperative adverse events, and 12.3% required secondary surgical intervention. [58]
In a prospective multi-centered study, Lenzhofer et al reported 4-year results of 64 eyes that received XEN63 Gel Microstent for treatment of uncontrolled open angle glaucoma.[59] The average baseline IOP with optimal medical before the Xen implantation was 22.5 ± 4.2 mmHg (n = 34, p <0.001). At 4 years after Xen implantation, the average IOP was reported to be 13.4 ± 3.1 mmHg, a 40% decrease from baseline. 25% (12/53) eyes achieved complete surgical success after 4 years and 53% (28/53) achieved qualified success. 53.1% of eyes underwent needling. Notably, the generalizability of the results is limited because MMC was not used and the device under investigation is longer than the FDA-approved XEN45.
Ab Interno vs. Ab Externo Approaches
Early studies comparing outcomes of ab interno and ab externo techniques report similar safety and efficacy profiles, including similar incidences of bleb revision and secondary surgery.[60] The ab externo approach appears to be favored in the literature for its slightly greater IOP and glaucoma medication reduction rates.
Recent multicenter retrospective cohort studies have found a significantly greater percentage of IOP reduction at 12 months in the ab externo open conjunctival group compared to the ab interno closed conjunctival group (43.1% vs. 24.8%, respectively; P = 0.02). Postoperative needling rates were also higher in the ab externo group compared with the ab interno group (36.1% vs. 11.8%, P = 0.001).[61]
Xen Gel Stent vs. Trabeculectomy
An international multicenter retrospective study has been published that compares the efficacy, safety, and risk factors for failure of standalone Xen gel stent implantation versus trabeculectomy. In this study, 354 eyes with uncontrolled glaucoma and no prior incisional filtering surgery underwent microstent implantation (n = 185) or trabeculectomy (n = 169), both with adjunctive MMC. The results failed to demonstrate any difference in efficacy, risk of failure, and safety profile between the 2 surgical procedures.[15]
Chen et al. noted that trabeculectomy decreased IOP significantly more than Xen implant alone by 3.04 mmHg (0.70, 5.38) in a comparison of 3 studies.[46]
Schlenker et al. reported a median preoperative IOP of 24 mmHg on three medications for both the Xen45 and trabeculectomy groups.[62] At 12 months, the IOP in both groups decreased to 13.0 mmHg off all IOP-lowering medications, without any statistically significant difference between groups. There was no difference in the HRs of failure between the two procedures. At last follow-up, a larger proportion of XEN45 patients were completely off medications (75.7 vs. 67.0%), but this was not statistically significant.[11] The trabeculectomy group had more postoperative interventions (mostly laser suture lysis) and complications (bleb leak or dehiscence). Bleb needling was slightly higher for the Xen gel stent group (43% vs. 31%), but this difference was not statistically significant.
A retrospective cohort study by Cepelli et al. showed that over a 3-year period, trabeculectomy was superior to Xen gel implant for all survival curves. However, when considering complete success, defined as no drops required post-operatively, trabeculectomy was only statistically significantly superior in groups with an IOP of 6-12mmHg. When qualified success was considered, defined as post-operative drops required, trabeculectomy was superior to Xen gel for all criteria. However, a higher number of post-operative flat chamber and bled leakage were observed in the trabeculectomy group [63].
Xen Solo vs. Xen Combo
Multiple studies have been published to assess the safety of Xen implantation as a standalone procedure (Xen solo) vs in combination with phacoemulsification (Xen combo). The findings are mixed and appear to vary at least in part due to differences in data analysis methodology. In the previously mentioned prospective study by Reitsamer et al, standalone Xen and Xen performed in combination with cataract surgery had similar IOP-lowering results at 24 months.[57]
In a retrospective study comparing Xen solo and Xen combo, outcomes at 12 months failed to detect a difference in IOP or medication reduction. In this study, 200 Xen solo eyes improved their baseline IOP from 31.5 ± 8.4 mmHg to 14.3 ± 4.2 mmHg compared to 35.7±12.0 mmHg to 13.9±2.5 in the Xen combo eyes. The number of glaucoma medications in the Xen solo group dropped from 3.1± 1.0 to 0.3 ± 0.7, while medications in the Xen combo group dropped from 3.3 ± 1.0 to 0.4 ± 0.7. There was no statistical difference between XEN solo and XEN combo.[64]
In a prospective interventional case series comparing Xen solo with Xen combo at 12 months, Mansouri et al reported a median IOP reduction of 40% in the Xen solo treatment arm compared to 22.9% in the Xen combo arm. 81% of Xen solo eyes achieved IOP reduction ≥20% from medicated baseline IOP, whereas 56.1% of Xen combo eyes achieved IOP reduction ≥20% (p= 0.04). At 12 months, 57.7% of all eyes achieved IOP < 16 on no medications, and there was no difference between the Xen solo and Xen combo groups. 37% of all eyes required needling.[65]
In a prospective multicenter trial, Fea et al reported that the mean IOP in Xen solo eyes improved from 21.4 mmHg to 15.8 mmHg at 12-month follow-up, whereas Xen combo eyes’ mean IOP improved from 25.0 to 15.4 mmHg. There was no statistical difference between the two groups’ IOP improvement at any time after post-operative week 1.[48]
Xen in Pediatric Glaucoma
In 2020, an interventional case series reported three eyes of three pediatric patients with congenital glaucoma who received Xen gel stent implant. Two of the eyes failed trabeculotomy and the third eye was a primary Xen implantation. All three eyes had controlled IOP post-operatively without the use of topical medication up to 24 months of follow-up.[66] An additional retrospective study on childhood glaucoma and Xen looked at Xen-augmented Baerveldt implantation, finding encouraging results for refractory pediatric glaucoma.[67]
Conclusion
In the rapidly evolving era of less invasive glaucoma surgeries, Xen gel stent offers a new means of lowering IOP for patients with advanced open angle glaucoma. Most published studies are reporting early follow-up, so the long-term efficacy and safety are yet to be demonstrated. However, many trials with a 1-year follow-up have reported acceptable safety and good efficacy in comparison to traditional filtering surgery.[4][19] Longer follow-up periods will be needed to assess the durability and long-term safety of Xen, especially in comparison with existing traditional glaucoma surgeries.[11]
References
- ↑ Quigley HA, Broman AT. The number of people with glaucoma world wide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-267.
- ↑ Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and metaanalysis. Ophthalmology 2014;121:2081 – 2090.
- ↑ Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Chaudhary A, Salinas L, Guidotti J, Mermoud A, Mansouri K. XEN Gel Implant: a new surgical approach in glaucoma. Expert Rev Med Devices. 2018;15(1):47-59. doi:10.1080/17434440.2018.1419060
- ↑ Boland MV, Ervin AM, Friedman DS, et al. Comparative effective- ness of treatments for open-angle glaucoma: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:271–279.
- ↑ Kass M , Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713.
- ↑ Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression. Arch Ophthalmol. 2002;120:1268–1279.
- ↑ Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953.
- ↑ American Academy of Ophthalmology Preferred Practice Patterns Committee GP. Ophthalmology. Chicago (IL): American Academy of Ophthalmology; 2010. Preferred practice pattern: primary open-angle glaucoma.
- ↑ Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40(8):1301–1306.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 Green W, Lind JT, Sheybani A. Review of the Xen Gel Stent and InnFocus MicroShunt. Curr Opin Ophthalmol. 2018;29(2):162-170. doi:10.1097/ICU.0000000000000462
- ↑ Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2005;140:16–22.
- ↑ 13.0 13.1 13.2 Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow- up. Am J Ophthalmol. 2012;153:789–803.
- ↑ Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 De Gregorio A, Pedrotti E, Stevan G, Bertoncello A, Morselli S. XEN glaucoma treatment system in the management of refractory glaucomas: a short review on trial data and potential role in clinical practice. Clin Ophthalmol. 2018;12:773-782. Published 2018 Apr 30. doi:10.2147/OPTH.S146919
- ↑ American Academy of Ophthalmology. FDA approves Xen gel stent for glaucoma. https://www.aao.org/headline/fda-approves-xen-gel-stent-glaucoma. Accessed May 20, 2018.
- ↑ 17.0 17.1 17.2 American Academy of Ophthalmology. FDA approves Xen gel stent for glaucoma. https://www.aao.org/headline/fda-approves-xen-gel-stent-glaucoma. Accessed May 20, 2018.
- ↑ Sheybani A, Reitsamer H, Ahmed IIK. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2015;56:4789–4795.
- ↑ 19.0 19.1 Fea AM, Durr GM, Marolo P, Malinverni L, Economou MA, Ahmed I. XEN® Gel Stent: A Comprehensive Review on Its Use as a Treatment Option for Refractory Glaucoma. Clin Ophthalmol. 2020;14:1805-1832. Published 2020 Jun 30. doi:10.2147/OPTH.S178348
- ↑ Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression. Arch Ophthalmol. 2002;120:1268–1279.
- ↑ De Gregorio A, Pedrotti E, Stevan G, Bertoncello A, Morselli S. XEN glaucoma treatment system in the management of refractory glaucomas: a short review on trial data and potential role in clinical practice. Clin Ophthalmol. 2018;12:773-782. Published 2018 Apr 30. doi:10.2147/OPTH.S146919
- ↑ Hohberger B, Welge-Lüßen UC, Lämmer R. MIGS: therapeutic success of combined Xen Gel Stent implantation with cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):621-625. doi:10.1007/s00417-017-3895-3
- ↑ De Gregorio A, Pedrotti E, Stevan G, Bertoncello A, Morselli S. XEN glaucoma treatment system in the management of refractory glaucomas: a short review on trial data and potential role in clinical practice. Clin Ophthalmol. 2018;12:773-782. Published 2018 Apr 30. doi:10.2147/OPTH.S146919
- ↑ Tan NE, Tracer N, Terraciano A, Parikh HA, Panarelli JF, Radcliffe NM. Comparison of Safety and Efficacy Between Ab Interno and Ab Externo Approaches to XEN Gel Stent Placement. Clin Ophthalmol. 2021;15:299-305. Published 2021 Jan 26. doi:10.2147/OPTH.S292007
- ↑ Kerr NM, Wang J, Barton K. Minimally invasive glaucoma surgery as primary stand-alone surgery for glaucoma. Clin Exp Ophthalmol. 2017;45(4):393-400. doi:10.1111/ceo.12888
- ↑ Fea AM, Durr GM, Marolo P, Malinverni L, Economou MA, Ahmed I. XEN® Gel Stent: A Comprehensive Review on Its Use as a Treatment Option for Refractory Glaucoma. Clin Ophthalmol. 2020;14:1805-1832. Published 2020 Jun 30. doi:10.2147/OPTH.S178348
- ↑ Fea AM, Durr GM, Marolo P, Malinverni L, Economou MA, Ahmed I. XEN® Gel Stent: A Comprehensive Review on Its Use as a Treatment Option for Refractory Glaucoma. Clin Ophthalmol. 2020;14:1805-1832. Published 2020 Jun 30. doi:10.2147/OPTH.S178348
- ↑ Tan NE, Tracer N, Terraciano A, Parikh HA, Panarelli JF, Radcliffe NM. Comparison of safety and efficacy between Ab interno and Ab externo approaches to XEN gel stent placement. Clin Ophthalmol. (2021) 15:299– 305. doi: 10.2147/OPTH.S292007
- ↑ 29.0 29.1 Ucar F, Cetinkaya S. Xen implantation in patients with primary open-angle glaucoma: comparison of two different techniques. Int Ophthalmol. (2020) 40:2487–94. doi: 10.1007/s10792-020-01427-z
- ↑ 30.0 30.1 Do A, McGlumphy E, Shukla A, Dangda S, Schuman JS, Boland MV, et al. Comparison of clinical outcomes with open versus closed conjunctiva implantation of the XEN45 gel stent. Ophthalmol Glaucoma. (2021) 4:343– 9. doi: 10.1016/j.ogla.2020.12.003
- ↑ Dangda S, Radell JE, Mavrommatis MA, Lee R, Do A, Sidoti PA, et al. Open conjunctival approach for sub-tenon’s xen gel stent placement and bleb morphology by anterior segment optical coherence tomography. J Glaucoma. (2021) 30:988–95. doi: 10.1097/IJG.0000000000001929
- ↑ Kong YXG, Chung IY, Ang GS. Outcomes of XEN45 gel stent using posterior small incision sub-tenon ab interno insertion (semi-open) technique. Eye. (2021). doi: 10.1038/s41433-021-01635-6
- ↑ Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II. Phacoemulsification combined with a new ab interno gel stent to treat open-angle glaucoma: Pilot study. J Cataract Refract Surg. 2015;41(9):1905-1909. doi:10.1016/j.jcrs.2015.01.019
- ↑ Sheybani A, Dick B, Ahmed II. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–e696.
- ↑ Fea AM, Spinetta R, Cannizzo PML, et al. Evaluation of Bleb Morphology and Reduction in IOP and Glaucoma Medication following Implantation of a Novel Gel Stent. J Ophthalmol. 2017;2017:9364910. doi:10.1155/2017/9364910
- ↑ Midha N, Gillmann K, Chaudhary A, Mermoud A, Mansouri K. Efficacy of Needling Revision After XEN Gel Stent Implantation: A Prospective Study. J Glaucoma. 2020;29(1):11-14. doi:10.1097/IJG.0000000000001394
- ↑ Midha N, Gillmann K, Chaudhary A, Mermoud A, Mansouri K. Efficacy of Needling Revision After XEN Gel Stent Implantation: A Prospective Study. J Glaucoma. 2020;29(1):11-14. doi:10.1097/IJG.0000000000001394
- ↑ Olivari S, Cutolo CA, Negri L, et al. XEN Implant Fracture During Needling Procedure. J Glaucoma. 2019;28(12):1086-1089. doi:10.1097/IJG.0000000000001360
- ↑ Midha N, Rao HL, Mermoud A, Mansouri K. Identifying the predictors of needling after XEN gel implant. Eye (Lond). 2019;33(3):353-357. doi:10.1038/s41433-018-0206-0
- ↑ Fea AM, Spinetta R, Cannizzo PML, et al. Evaluation of Bleb Morphology and Reduction in IOP and Glaucoma Medication following Implantation of a Novel Gel Stent. J Ophthalmol. 2017;2017:9364910. doi:10.1155/2017/9364910
- ↑ Sng CCA, Chew PTK, Htoon HM, Lun K, Jeyabal P, Ang M. Case series of combined XEN implantation and phacoemulsification in chinese eyes: one-year outcomes. Adv Ther. (2019) 36:3519– 29. doi: 10.1007/s12325-019-01127-w
- ↑ Fea AM, Bron AM, Economou MA, Laffi G, Martini E, Figus M, et al. European study of the efficacy of a cross-linked gel stent for the treatment of glaucoma. J Cataract Refract Surg. (2020) 46:441– 50. doi: 10.1097/j.jcrs.0000000000000065
- ↑ Olivari S, Cutolo CA, Negri L, et al. XEN Implant Fracture During Needling Procedure. J Glaucoma. 2019;28(12):1086-1089. doi:10.1097/IJG.0000000000001360
- ↑ 44.0 44.1 Gupta C, Mathews D. XEN® stent complications: a case series. BMC Ophthalmol. 2019;19(1):253. Published 2019 Dec 12. doi:10.1186/s12886-019-1267-y
- ↑ 45.0 45.1 Karimi A, Lindfield D, Turnbull A, et al. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye (Lond). 2019;33(3):469-477. doi:10.1038/s41433-018-0243-8
- ↑ 46.0 46.1 46.2 Chen X-z, Liang Z-q, Yang K-y, Lv K, Ma Y, Li M-y and Wu H-j (2022) The Outcomes of XEN Gel Stent Implantation: A Systematic Review and Meta-Analysis. Front. Med. 9:804847. doi: 10.3389/fmed.2022.804847
- ↑ Suprachoroidal Bleeding After XEN Gel Implantation. Prokosch-Willing V, Vossmerbaeumer U, Hoffmann E, Pfeiffer N. J Glaucoma. 2017 Dec; 26(12):e261-e263.
- ↑ 48.0 48.1 Fea AM, Bron AM, Economou MA, et al. European study of the efficacy of a cross-linked gel stent for the treatment of glaucoma. J Cataract Refract Surg. 2020;46(3):441-450. doi:10.1097/j.jcrs.0000000000000065
- ↑ Arnould L, Theillac V, Moran S, Gatinel D, Grise-Dulac A. Recurrent Exposure of XEN Gel Stent Implant and Conjunctival Erosion. J Glaucoma. 2019;28(3):e37-e40. doi:10.1097/IJG.0000000000001146
- ↑ Sng CC, Wang J, Hau S, Htoon HM, Barton K. XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin Exp Ophthalmol. 2018;46(4):339-345. doi:10.1111/ceo.13087
- ↑ Prokosch WV, Vossmerbaeumer U, Hoffmann E, et al. Suprachoroidal bleeding after XEN gel implantation. J Glaucoma. 2017;26:e261–63.
- ↑ Olate-Pérez Á, Pérez-Torregrosa VT, Gargallo-Benedicto A, et al. Management of conjunctival perforation and late Seidel after XEN® surgery. Manejo de la perforación conjuntival y Seidel tardío poscirugía XEN®. Arch Soc Esp Oftalmol. 2018;93(2):93-96. doi:10.1016/j.oftal.2017.10.002
- ↑ Napoli L, Riva I, Oddone F, Michelessi M, Quaranta L. A rare case of endophthalmitis after bleb needle revision for glaucoma Xen® gel stent. Eur J Ophthalmol. 2021;31(1):NP9-NP12. doi:10.1177/1120672119878016
- ↑ Grover DS, Flynn WJ, Bashford KP, et al. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Am J Ophthalmol. 2017;183:25-36. doi:10.1016/j.ajo.2017.07.023
- ↑ Galal A, Bilgic A, Eltanamly R, Osman A. XEN Glaucoma Implant with Mitomycin C 1-Year Follow-Up: Result and Complications. J Ophthalmol. 2017;2017:5457246. doi:10.1155/2017/5457246
- ↑ De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2018;38(3):1129-1134. doi:10.1007/s10792-017-0571-x
- ↑ 57.0 57.1 Reitsamer H, Sng C, Vera V, et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):983-996. doi:10.1007/s00417-019-04251-z
- ↑ Reitsamer H, Vera V, Ruben S, Au L, Vila-Arteaga J, Teus M, Lenzhofer M, Shirlaw A, Bai Z, Balaram M, Stalmans I. Three-year effectiveness and safety of the XEN gel stent as a solo procedure or in combination with phacoemulsification in open-angle glaucoma: a multicentre study. Acta Ophthalmol. 2022 Feb;100(1):e233-e245. doi: 10.1111/aos.14886. Epub 2021 May 10. PMID: 33973370; PMCID: PMC9290976.
- ↑ Lenzhofer M, Kersten-Gomez I, Sheybani A, et al. Four-year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi-centre study. Clin Exp Ophthalmol. 2019;47(5):581-587. doi:10.1111/ceo.13463
- ↑ Tan NE, Tracer N, Terraciano A, Parikh HA, Panarelli JF, Radcliffe NM. Comparison of Safety and Efficacy Between Ab Interno and Ab Externo Approaches to XEN Gel Stent Placement. “Clin Ophthalmol”. 2021;15:299-305. doi:10.2147/OPTH.S292007
- ↑ Do A, McGlumphy E, Shukla A, Dangda S, Schuman JS, Boland MV, Yohannan J, Panarelli JF, Craven ER. Comparison of Clinical Outcomes with Open Versus Closed Conjunctiva Implantation of the XEN45 Gel Stent. Ophthalmol Glaucoma. 2020 Dec 13:S2589-4196(20)30321-5. doi: 10.1016/j.ogla.2020.12.003.
- ↑ Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, Safety, and Risk Factors for Failure of Standalone Ab Interno Gelatin Microstent Implantation versus Standalone Trabeculectomy [published correction appears in Ophthalmology. 2018 Mar;125(3):463]. Ophthalmology. 2017;124(11):1579-1588. doi:10.1016/j.ophtha.2017.05.004
- ↑ Cappelli F, Cutolo CA, Olivari S, et al Trabeculectomy versus Xen gel implant for the treatment of open-angle glaucoma: a 3-year retrospective analysis BMJ Open Ophthalmology 2022;7:e000830. doi: 10.1136/bmjophth-2021-000830
- ↑ Hengerer FH, Kohnen T, Mueller M, Conrad-Hengerer I. Ab Interno Gel Implant for the Treatment of Glaucoma Patients With or Without Prior Glaucoma Surgery: 1-Year Results. J Glaucoma. 2017;26(12):1130-1136. doi:10.1097/IJG.0000000000000803
- ↑ Mansouri K, Guidotti J, Rao HL, et al. Prospective Evaluation of Standalone XEN Gel Implant and Combined Phacoemulsification-XEN Gel Implant Surgery: 1-Year Results. J Glaucoma. 2018;27(2):140-147. doi:10.1097/IJG.0000000000000858
- ↑ Smith OU, Grover DS, Emanuel ME, Godfrey DG, Fellman RL. XEN Gel Stent in Pediatric Glaucoma. J Glaucoma. 2020;29(4):e19-e22. doi:10.1097/IJG.0000000000001453
- ↑ Arad T, Hoffmann EM, Prokosch-Willing V, Pfeiffer N, Grehn F. XEN-augmented Baerveldt Implantation for Refractory Childhood Glaucoma: A Retrospective Case Series. J Glaucoma. 2019;28(11):1015-1018. doi:10.1097/IJG.0000000000001356