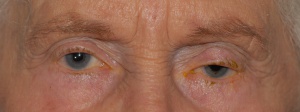
Trabeculectomy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Summary
Glaucoma is a disease in which the optic nerve is damaged, leading to progressive and irreversible loss of vision. Glaucoma can develop at any intraocular pressure (IOP), but elevated intraocular pressure is one of the major risk factors for the development and progression of glaucoma. Most treatments for glaucoma are targeted at lowering the intraocular pressure, either by decreasing the formation of aqueous fluid in the eye, or, as in the case of glaucoma filtration surgery, by increasing the outflow of fluid from the eye. Trabeculectomy is a filtering surgery where an ostium is created into the anterior chamber from underneath a partial thickness scleral flap to allow for aqueous flow out of the eye. The aqueous flows into the subconjunctival space, usually leading to an elevation of the conjunctiva, referred to as a filtering bleb[1]. There are several suggested routes for the aqueous after reaching the filtering bleb. These routes include filtration through the conjunctiva into the tear film, absorption by vascular or perivascular conjunctival tissue, flow through lymphatic vessels near the margins of the surgical area, and drainage through aqueous veins[2]Trabeculectomy is performed for treatment of glaucoma inadequately controlled by maximally tolerated medical therapy. This review will discuss careful patient selection through history and clinical examination, surgical technique of trabeculectomy with potential advantages and disadvantages of antifibrosis drugs commonly employed at the time of filtration surgery, and management of complications in the early postoperative period.
Patient Selection
History
In evaluating a patient for glaucoma filtration surgery, it is important to take a detailed medical and surgical history. Particular points of importance include:
- History of prior ocular inflammation or infection.
- History of prior ocular procedures.
- Intraocular pressure range.
- History of bleeding disorders or consumption of anticoagulative medications.
- History of ocular trauma
Examination
A thorough ophthalmological examination is also recommended prior to trabeculectomy surgery. Important aspects of the physical examination to note include:
- Visual acuity.
- Lid hygiene and presence of severe blepharitis.
- Any areas of conjunctival scarring, irregularity or hyperemia.
- Any areas of scleral thinning.
- Gonioscopic findings.
- Level of Intraocular pressure.
- Any evidence of prior intraocular surgery.
- Signs of prior intraocular inflammation.
- Amount of glaucomatous optic nerve damage.
- Amount of glaucomatous visual field loss.
- Presence of a cataract which may mandate a combined procedure
Laboratory test
A general medical evaluation should be obtained prior to ocular surgery, and any recommended pre-operative laboratory testing should be performed.
Indications
Trabeculectomy can be considered when surgical risks are outweighed by the potential benefits. Patients are candidates for trabeculectomy if their intraocular pressures put them at significant risk for progressive glaucoma damage resulting in visual disability. While the CIGTS study found that visual prognosis was comparable in patients initially treated with medicines and surgery, most physicians try medical therapy prior to proceeding to surgery. However, if the intraocular pressure is not adequately controlled on medications, or if there are significant barriers to using medications regularly (cost, compliance, ocular or systemic side effects, inconvenience, physical impairment, or other psychosocial issues), surgical intervention should be conidered. Laser surgery can be performed if only modest IOP lowering is required particularly if the glaucoma damage is not advanced. However, trabeculectomy is indicated if glaucoma damage is moderate to advanced, if the rate of progression has been rapid, if prior laser has been unsuccessful, or if the there is significant risk of future progression that will result in a symptomatic disability,
Contraindications
Glaucoma surgery is contraindicated in patients who are unlikely to develop visual disability from glaucoma within their lifetime. Patients with limited life expectency will probably not benefit from trabeculectomy unless it is performed to control pain. As with any surgical procedure, the patient must be in stable enough medical condition to undergo a surgical procedure. Caution should be taken in patients on anticoagulant therapy as the bleeding complications are increased. Consultation with the patient's primary care physician regarding whether the anticoagulants can be stopped may be appropriate. Extensive scarring of the superior conjunctiva can preclude the creation of a conjunctival flap; glaucoma drainage implant surgery or cyclodestructive procedures may provide better choices.
Surgical Technique
- Trabeculectomy surgery can be performed under local or general anesthesia. - A traction suture (placed partial thickness through the peripheral clear cornea) is often used for adequate exposure of the surgical site (superior quadrants of the eye). - A conjunctival peritomy is created to expose the superior bare sclera. This peritomy may be limbal based or fornix based.
- Gentle cautery is used to achieve hemostasis.- A partial thickness scleral flap is created in the superior sclera, hinged at the limbus. There are multiple variations in shapes (rectangular, triangular, trapezoidal) and size of this flap. The scleral flap is dissected forward until the bluish gray zone at the limbus is exposed.
- Dissection of the subconjunctival space to provide a pathway for aqueous flow is then performed.- A paracentesis is created in the peripheral clear cornea prior to creating an ostomy into the anterior chamber from underneath the scleral flap. Viscoelastic material may be injected to stabilize the intraocular pressure as the ostomy is created to avoid anterior chamber shallowing.- There are several ways to create the ostomy underneath the scleral flap. A blade is first used to enter the anterior chamber from just behind the hinge of the scleral flap. The entrance into the anterior chamber is then enlarged using the blade, vannas scissors, and/or a Kelly punch device. - Often, a peripheral iridectomy is created at this point, using forceps to pull the peripheral iris through the ostomy site, and scissors to create the iridectomy. In pseudophakic eyes, some surgeons omit this surgical step.- Sutures (usually 9-0 or 10-0 nylon) are then used to close the scleral flap. The sutures may be placed in an interrupted manner, or releasable sutures may be placed. The flap should be closed tightly enough to allow the anterior chamber to remain formed, but loosely enough to allow for aqueous drainage at the edges of the scleral flap. - The conjunctival peritomy is then closed using absorbable or nylon sutures.
- A subconjunctival injection of corticosteroids may be given at the end of the surgical procedure.
| Limbus-Based vs. Fornix-Based Trabeculectomy |
|---|
| 1. IOP control is comparable with both techniques |
| 2. Fornix - Based |
|
| 3. Limbus - Based Advantage: more secure wound closure |
|
Use of antimetabolites
Antimetabolites are usually used during trabeculectomy surgery to prevent bleb failure due to scarring by the wound healing process. The most commonly used antimetabolites are 5-fluorouracil (5-FU) or mitomycin C (MMC).- 5-Fluorouracil is a pyrimidine analogue which blocks DNA synthesis. This can be applied to the surgical site by soaking a surgical sponge and placing it onto the scleral surgical site prior to creation of the ostomy, before or after creation of a scleral flap. The sponge is removed after a variable amount of time (depending on the patient's underlying characteristics or surgeon’s preference, the sponge may be removed after 30 seconds to 5 minutes). Alternatively, 5-FU can be injected into the subconjunctival space intraoperatively or in the early postoperative period.- MMC is a DNA cross-linker which inhibits fibroblast proliferation. This is applied to the surgical site by being soaked onto a surgical sponge and placed onto the scleral surgical site prior to creation of the ostomy, before or after formation of a partial thickness scleral flap. The sponge is removed after a variable amount of time (depending on the patient's underlying characteristics or surgeon’s preference, the sponge may be removed after 30 seconds to 5 minutes) followed by irrigation of the subconjunctival space. Alternatively, the MMC may be injected preoperatively into the subconjunctival space and then irrigated.
| 5-FU |
|---|
|
Advantages Inexpensive No dilution or dosage calculations required Stable at room temperature Better safety margin than MMC Disadvantages Less effective than MMC Multiple injections High incidence of corneal toxicity |
| MMC |
|---|
|
Advantages More potent than 5-FU Results in lower intraocular pressures Disadvantages ExpensiveReconstituted from powder Not stable at room temperature Possibly more complications |
Surgical follow up
After glaucoma filtration surgery, the patient is seen the first post-operative day. Topical corticosteroids are prescribed to be used four times daily up to every two hours. A topical antibiotic is prescribed to be used four times daily for the first week. Depending on the patient’s clinical state and intraocular pressure, the follow up interval can vary. A common routine is to see the patient one week post-operatively then every one to two weeks thereafter for the first two months post-operatively. During these follow up visits, the intraocular pressure may be lowered by release of the scleral flap sutures, either by laser suture lysis or by removal of releasable sutures. The patient should not place pressure on the operative eye or perform vigorous physical activity or lifting during the post-operative period. The patient is advised to use an eye shield at night to avoid accidental rubbing of the eye during sleep.
Outcomes
In a study evaluating the results of trabeculectomy without antimetabolites for the treatment of primary open angle glaucoma, the long term IOP control was found to be 48% and 40% at 3 and 5 years, respectively. The definition of IOP control was defined as IOP < 21 mm Hg and reduction of IOP at least 20% from baseline.[3] In the Collaborative Initial Glaucoma Treatment Study, which was designed to evaluate whether medical therapy or trabeculectomy with 5FU is the better initial treatment for patients with open-angle glaucoma, the IOP was maintained at 14-15mmHg on average over 5 years follow up in the surgical arm.[4] In the Tube vs. Trabeculectomy Study, which was designed to evaluate the safety and efficacy of Baerveldt-350 tube shunt implant compared to trabeculectomy with MMC in eyes with prior cataract or trabeculectomy surgery, the average intraocular pressure was 12.7, 12.1 and 13.3 at 1 year, 2 years and 3 years, respectively, after trabeculectomy, with or without glaucoma medications. The failure rates in the trabeculectomy group were 13.9% at 1 year,28.2% at 2 years, and 30.7% at 3 years. Failure was defined as persistent hypotony (IOP less than or equal to 5mmHg) or IOP > 21mmHg or IOP not reduced by 20% below baseline.[5]Risk factors for failure of trabeculectomy include previous intraocular surgery, neovascular or uveitic glaucoma, black race, and young age.[6]
Complications
In the early postoperative period, the most common complications faced by the glaucoma surgeon involve either elevated IOP or hypotony. Other postoperative complications are listed in Table 1 and Table 2. Hypotony with deep anterior chamber:Early postoperative hypotony can be monitored if it is not associated with a flat anterior chamber, excessive inflammation, wound leak or posterior pole abnormalities. If this hypotony persists and leads to macular folds or choroidals, a bleb revision may be necessary. Autologous blood injection into the bleb has also been studied as a mechanism to decrease bleb filtration.[7]
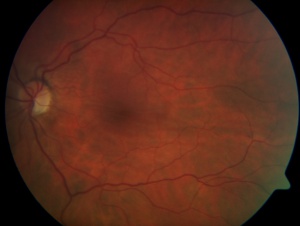
Chorioretinal Folds Secondary to Hypotony Maculopathy from an Overfiltering Bleb
Hypotony with shallow anterior chamber:Prolonged shallow anterior chamber may be associated with reduced corneal endothelial cell count and peripheral anterior synechiae formation. If the shallow anterior chamber is less than Grade 2, it can be monitored closely. However, a shallow anterior chamber Grade 2 or Grade 3, or a shallow anterior chamber that persists beyond the first week, often is an indication for reformation of the chamber with viscoelastic. If a conjunctival hole or wound leak is present, a pressure patch or bandage contact lens may be used as initial measures to tamponade the leak. A bleb revision is necessary if the wound leak or the hypotony persists.[2]
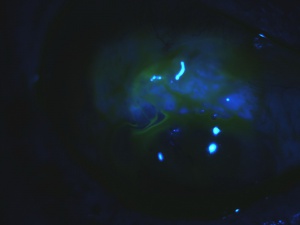
Bleb Leak
Choroidal Effusions
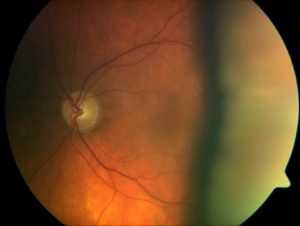
Overfiltering Bleb
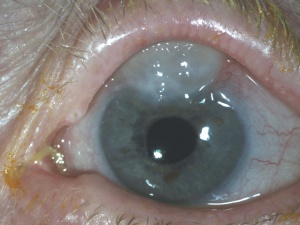
Ptosis from Overfiltering Bleb
Photos Courtesy of Sarwat Salim, MD, FACS, University of Tennessee
Elevated intraocular pressure and deep anterior chamber:An elevated IOP with deep anterior chamber indicates inadequate filtration due to obstruction of the fistula or inadequate flow from the scleral flap. If obstruction of the fistula by the peripheral iris is noted, argon laser may be used to contract the iris away from the ostomy site. Alternatively, a needle or cannula may be used to mechanically pull the iris away from the ostomy site through the anterior chamber. If the ostium is blocked by a large blood clot, TPA can be considered if there are no contraindications present. If there is a low or flat bleb with insufficient flow from the scleral flap, the flap sutures can be lysed using the argon laser, or removed if releasable sutures are used. If these measures do not succeed in lowering IOP, glaucoma medications should be resumed, and further glaucoma surgery is necessary if IOP cannot be lowered to an acceptable level. [2]Elevated IOP and shallow anterior chamber:An early postoperative elevated IOP and shallow anterior chamber after trabeculectomy is due to either suprachoroidal hemorrhage, pupillary block, or aqueous misdirection. The IOP should be managed medically while the underlying cause is being evaluated and treated.[2]
Table 1: Early postoperative complications after trabeculectomy surgery with 5-FU in the CIGTS study[8]
| Complication | Percentage of Patients | Complication | Percentage of Patients |
|---|---|---|---|
| Wound lead | 6 | Iris prolapse | 1.1 |
| Anterior chamber bleeding | 10 | Serous choroidal detachment | 11 |
| Suprachoroidal hemorrhage | 0.7 | Hypotony | 0.9 |
| Corneal epithelial defect | 0.9 | Monocular diplopia | 0.2 |
| Cystoid Macular Edema | 0.2 | Shallow or flat anterior chamber | 13 |
| Malignant glaucoma | 0.4 | New synechiae or adhesions | 5 |
| Encapsulated bleb | 12 | Dellen | 4 |
| Ptosis | 12 |
Table 2: Postoperative complications after trabeculectomy with MMC in the Tube vs. Trabeculectomy Study[5]
| Early Complication | Percentage of Patients | Complication | Percentage of Patients |
|---|---|---|---|
| Choroidal effusion | 13 | Persistant corneal edema | 6 |
| Shallow or flat anterior chamber | 10 | Dysesthesia | 8 |
| Wound leak | 11 | Encapsulated bleb | 6 |
| Hyphema | 8 | Choroidal effusion | 4 |
| Malignant glaucoma | 1 | Cystoid macular edema | 1 |
| Suprachoroidal hemorrhage | 3 | Hypotony maculopathy | 4 |
| Vitreous hemorrhage | 1 | Bleb leak | 5 |
| Decompression retinopathy | 1 | Endophthalmitis/blebitis | 3 |
| Cystoid macular edema | 1 | Chronic or recurrent iritis | 1 |
| Retinal detachment | 1 | ||
| Corneal ulcer | 1 |
References
- ↑ Jinza K, Saika S, Kin K, et al. Relationship between formation of a filtering bleb and an intrascleral aqueous drainage route after trabeculectomy: Evaluation using ultrasound biomicroscopy. Ophthalmic Res 2000; 32: 240-3.
- ↑ 2.0 2.1 2.2 2.3 Allingham RR, Damji KF, Freedman S, et al. “Filtering surgery.” Shields’ Textbook of Glaucoma. Ed. Jonathen Pine and Joyce Murphy. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins, 2005. 568-95.
- ↑ Nouri-Mahdavi K, Brigatti L, Weitzman M, et al. Outcomes of trabeculectomy for primary open-angle glaucoma. Ophthalmol 1995; 102: 1760-9.
- ↑ Feiner L and Piltz-Seymour JR. Collaborative initial glaucoma treatment study: a summary of results to date. Curr Opin Ophthalmol 2003; 14(2): 106-11.
- ↑ 5.0 5.1 Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol 2009; 148(5):670-84.
- ↑ Broadway DC and Chang L. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma, 2001; 10(3): 237-49.)
- ↑ Okada K, Tsukamoto H, Masumoto M, et al. Autologous blood injection for marked overfiltration early after trabeculectomy with mitomycin C. Acta ophthalmol Scand 2001; 79(3): 305-8.
- ↑ Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol 2005; 140(1):16-22.