Nanophthalmos
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Nanophthalmos is a rare genetic disease included in the spectrum of developmental eye disorders characterized by a small eye secondary to compromised growth[1]. Nanophthalmos is derived from the Greek word nano, meaning dwarf, and nanophthalmic eyes typically present with very high to extreme axial hyperopia without other obvious defects in the ocular structure[2] [3].
Classification
Warburg (1981, 1993) defined nanophthalmos or pure microphthalmos as a developmental arrest of ocular growth not associated with other ocular malformations. Later, it was underscored that nanophthalmos was a form of total or complete microphthalmos, where both anterior and posterior segments are shortened. Nanophthalmos, is characterized by short axial length (≤18-20 mm), microcornea, high hypermetropaia (+8D to +25D), relative anterior or posterior microphthalmos and global reduction in ocular volume[4] [5].
Syndromic VS Non-syndromic nanophthalmos
Nanophthalmos may present as an isolated disorder or be part of a syndrome. These syndromes include: “nanophthalmos, retinitis pigmentosa, foveoschisis and optic drusen syndrome”[6] [7], “oculo-dento-digital syndrome (ODD syndrome)”[8], “autosomal dominant vitreoretinochoroidopathy with nanophthalmos (ADVIRC)”[9] and “Kenny-Caffey syndrome”[10].
Genetics/Inheritance
Most non-syndromic cases of nanophthalmos are sporadic[5], but autosomal dominant and recessive modes of inheritance have been described[3][11] [12].
Autosomal dominant non-syndromic nanophthalmos
Two loci for autosomal dominant non-syndromic nanophthalmos (NNO1 and NNO3) have been identified[11][13]. The NNO1 (OMIM #600165) locus maps to chromosome 11p, while the NNO3 locus (OMIM #611897) maps to chromosome 2q11-q14. Regarding the NNO1 locus, no gene has been cloned, but an association between this locus and a more severe, late-stage phenotype of angle-closure glaucoma/narrow occludable anterior-chamber angles[11] has been reported. Li et al (2008) excluded the three known eye development genes that map inside NNO3 interval (Gli2, Inhibin beta B and En-1 genes). Thus, a novel gene has yet to be identified for that locus as well[13].
Autosomal recessive non-syndromic nanophthalmos
Autosomal recessive non-syndromic nanophthalmos (NNO2) (OMIM #609549) is caused by mutations in the membrane-type frizzled related protein (MFRP) gene (OMIM #606227) on chromosome 11q23[12] and also by mutations in the PRSS56 gene (OMIM #613858) on chromosome 2q37.1[14] Sundin et al (2005) performed linkage analysis using DNA samples from 16 members of an Amish-Mennonite kindred who turned out to be connected to the family originally reported by Cross and Yoder (1976), including five individuals with nanophthalmos[3][12]. Mutations in the MFRP gene were identified as a cause of classic non-syndromic recessive nanophthalmos in this group of individuals[12].
The MFRP gene has 13 exons, which translate into a 579 amino acid protein. This resulting protein comprises three conserved domains: a transmembrane domain with homology to the frizzled family of proteins, containing two cubillin sub-units; a low density lipoprotein receptor A; and a cysteine-rich domain, that can bind to wingless type proteins (WNTs), which might be involved in eye development by mediating cell growth[15].
The PRSS56 gene, first described by Gal et al (2011)[16], is linked to autosomal recessive isolated posterior microphthalmos. Orr et al (2011) linked the gene to autosomal recessive isolated nanophthalmos. This gene has 13 exons and EST database analysis suggests that it is expressed in embryonic tissue, testis, brain and eye. Additionally, the GeneCards database data shows that it is expressed broadly at lower levels and slightly higher in a variety of tissues including testis, lymph node, brain, retina, and smooth muscle. It may also be expressed in the pineal gland according to BioGPS. RT-PCR of human eye showed abundant PRSS56 expression in the neural retina, cornea, sclera, and optic nerve. In the mouse eye and retina Prss56 expression begins at embryonic day 17, increases with development, and is maintained at a high level in adulthood. Moreover, high levels of PRSS56 expression are observed in ganglion cells. The coded protein has 603 aminoacids and is predicted to contain a serine protease enzymatic domain[14][17].
Pathophysiology
The MFRP protein is selectively expressed in the retinal pigment epithelium (RPE) and the ciliary body, with low expression in the brain. MFRP concentrates towards the apical side of the RPE cells, with virtually none in Bruch’s membrane. Patients completely lacking MFRP protein have no identifiable pathology outside the eye. In the fetal eye, at 7 weeks gestation, no MFRP signal is detected in the RPE. A distinct signal is observed in the RPE at 20 weeks gestation which indicates that MFRP expression begins relatively late during eye development[1].
Functionally, MFRP seems to be essential for the eye to reach its full size at birth, and also for correct emmetropization, since it is associated with regulation of ocular axial growth[1]. Heterozygote MFRP carriers without nanophthalmos described by Sundin et al (2005) showed no hyperopia; however, ocular features like corneal curvature and anterior chamber depth revealed semidominance as they were significantly altered when compared with the general population[12]. There is some controversy regarding the role of MFRP in retinal function. While Sundinet al (2008) stated that the MFRP protein is not essential for retinal function, Ayala-Ramirez et al (2006) claimed that it may be necessary for photoreceptor maintenance, considering the severe rod-cone dystrophy phenotype observed in their patients[1][6]. Neri et al (2012) showed progressive rod-cone degeneration as the only significant finding in a patient followed-up for 30 months[18].
Histopathology
Many cases of nanophthalmos complicate with uveal effusion with or without exudative retinal detachment. The reason for such phenomena is thought to be blockage of outflow from the vortex venous system secondary to a thickened sclera. Yamani et al (1999) showed that all three scleral layers in eyes with nanophthalmos had abnormal collagen fibrils that were frayed and split. The degree of abnormality increased inwards, with less abnormal episclera and stroma, and considerable changes in lamina fusca. Ultrastructural studies also demonstrated a larger range of collagen fibril diameters with normal fibril banding periodicities in eyes with nanopthalmos. The authors suggest that the frayed fibrils contribute to scleral inelasticity which causes sequestration of extracellular fluid and consequently choroidal congestion, choroidal detachment, and/or exudative retinal detachment[19].
Fukuchi (2009) showed that scleral proteoglycans were qualitatively identical in nanophthalmic and control tissues, but very different quantitatively. Nanophthalmic eyes had lower amounts of chondroitin sulfate, which could be partially responsible for fluid retention[20].
Diagnosis
Nanophthalmos is usually characterized by bilateral and symmetrical small eyes, associated with: shortened axial length (generally 18-20 mm or less; at least two standard deviations below age-matched controls), high corneal curvature, high lens/eye volume ratio with narrow iridocorneal angle, high hyperopia (ranging from +8.00 to +25.00) and scleral thickening[1]. Patients with nanophthalmos usually have small eyes, deeply-set in the orbit (enophthalmos) and covered by narrow palpebral fissures. Bilateral mild ptosis may be present as well[2][3][11].
Refractive analysis displays high to extreme hyperopia in patients with nanophthalmos[2][3]. In children, both eyes usually exhibit extreme axial hyperopia but are highly functional; correction with glasses that resemble aphakic spectacles usually results in moderate to good visual acuities. Evaluation of the total axial length demonstrates reduced values as well as smaller equatorial and transverse diameters resulting in a reduced total ocular volume[4][21].
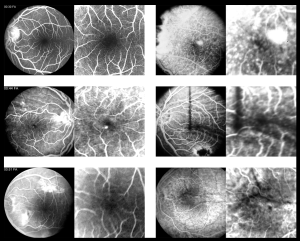
Visual acuity (VA) is primarily affected by high hyperopia, present since birth, which may cause bilateral amblyopia. Secondarily, several ocular complications (more commonly observed in adults)[1] can also limit the patients’ vision. These complications include: angle closure glaucoma [prominent iris convexity, due to high lens/eye volume ratio, eventually leads to peripheral anterior synechiae (PAS) and blocks the outflow of aqueous humor], retinal folds and/or exudative detachments, and cystic macular edema[12]. Best-corrected visual acuity (BCVA) is rarely above 20/40 at any point in nanophthalmic patients’ lives. Walsh et al (2007) reported an underdeveloped foveal avascular area in 4 adult nanophthalmos patients (54 to 70 years) that could further contribute for the reduced VA[23] (see Figure).
Slit-lamp examination discloses a transparent cornea with diameters ranging from 9 to 11.5 mm, spanning from the microcornea range to normal values. Keratometric evaluation reveals higher, regular, corneal curvatures with or without astigmatism. A shallow anterior chamber and narrow iridocorneal angle result from a normal or thicker than usual transparent lens that occupies a disproportionately large percentage of intraocular volume. As a consequence, the presence of a prominent iris convexity and impending angle closure with peripheral anterior synechiae (that may eventually form) may be observed beyond the fourth decade[21][24].
Fundus examination may reveal a normal posterior segment. The discs may be normal or appear crowded, or display the infrequent finding of disc drusen[12]. A variety of macular and peripheral retinal findings have been described; these include varying degrees of macular hypoplasia, rudimentary foveal avascular zone[23], foveal schisis-like changes[25], foveal cysts[26], macular folds, and small yellowish deposits in the mid-periphery[12] similar to flecks, which can be detected as bright spots with red-free scanning imaging, or as dark spots with autofluorescence imaging, with associated RPE mottling and atrophy[18].
Additional reported retinal manifestations include a retinitis pigmentosa-like phenotype[27], choroidal effusions (spontaneous or post-surgical)[28] [29] and nonrhegmatogenous retinal detachments[27]. On distant direct ophthalmoscopic examination or indirect ophthalmoscopy without using a lens, a peculiar glow can be seen and the retinal vessels may be visible.
Structural analysis using optical coherence tomography (OCT) may reveal absence of foveal pit, diffuse macular thickening, rudimentary foveal avascular zone[23], schisis of the outer retinal layers with discrete bridging elements at the fovea, or evidence of macular cysts or cystic-like changes. Jackson et al (2012) described two cases of nanophthalmos with retinal folds where only the layers anterior to the external limiting membrane were affected. In those cases the innermost layers were normal, whereas the inner nuclear, outer plexiform, and outer nuclear layers appeared thickened. This is different from previous reports of folds where the whole neurosensory retina was affected. These findings defy the original thoughts of different embryological origin of RPE and neurosensory retina as cause for the macular folds. These two cases could simply be different, or the SD OCT used allowed a better evaluation. The authors also suggest retinal vessels’ tortuosity as the potential cause for the folds, since they travel more anteriorly in the neurosensory retina[30]. One case of retinal pigment epithelium detachment has been reported in a patient with nanophthalmos[31].
Electrophysiological testing (ERG) varies from normal responses to variable degree of photopic and scotopic dysfunction[12][26].
Additional clinical findings may include nystagmus and strabismus, usually non-accommodative esotropia[32].
Glaucoma and nanophthalmos
Patients with nanophthalmos are prone to primary angle closure glaucoma in adulthood. This is the result of the disproportion between the normal increase in lens volume and a smaller anterior segment[33]. Diagnosis and monitoring for glaucoma in nanophthalmos are rather difficult due to a small disc, where even a small cup may be a sign of glaucoma. Additionally, visual field testing can be inaccurate because many patients have high plus lenses and reduced BCVA. In these cases SD OCT may detect subtle wedge-shaped loss of nerve fiber layer. HRT may assist in monitoring the deterioration of disc parameters. Stereo fundus photos can offer reliable documentation of disc morphology[34]. These patients are also predisposed to malignant glaucoma.
Differential diagnosis
An important diagnostic consideration in the differential of nanophthalmos is posterior microphthalmos. In posterior microphthalmos, anterior segment parameters are normal or slightly smaller though the axial length is small and the refraction is hyperopic. The posterior segment commonly shows reduction of capillary free zone, crowded optic disc, elevated papillomacular fold, chorioretinal folds, fine retinal folds, uveal effusion syndrome, pigmentary retinopathy and sclerochoroidal thickening on ultrasound.
Management
Nanophthalmos patients pose a continuous therapeutic challenge to the ophthalmologist. Early diagnosis along with recognition and management of the complications are crucial to maximize visual outcome. Depending on the patient’s age, a series of problems may need to be addressed in a structured manner.
Pediatric & Strabismus Considerations
In children, refractive errors must be fully corrected and complete cycloplegic retinoscopy values should be given to avoid unilateral and bilateral amblyopia. Refractive correction with spectacles should be started as early as possible. Some patients may present with incomplete accommodation. In such cases, bifocals or progressive power lenses with +3,00 adds for near vision may allow better performance for near activities. Contact lenses (soft or rigid gas permeable lenses) represent a great alternative for the esthetically non-appealing aphakic strength glasses. Appropriate evaluation of the corneal curvatures and assessment of corneal diameters are essential to achieve the best possible fit. When unilateral amblyopia is identified, patching can be a safer alternative to atropine penalization due to anatomical crowding of the anterior segment.
Strabismus surgery should be carried out in non-accommodative esotropia cases to allow binocular vision and prevent amblyopia.
Glaucoma Considerations
In adults, the nanophthalmic eye poses a different set of problems such as: angle-closure glaucoma, complex cataract surgery and exudative retinal detachments. Intraocular pressure (IOP) response to medical treatment is poor, even when initiated in a timely fashion. Many surgical procedures have been tried to prevent/treat glaucoma in nanophthalmos including: Nd YAG laser iridotomy, iridoplasty, trabeculectomy, sclerotomy and lens extraction[33].
If narrow occludable anterior-chamber angles with normal intraocular pressure (IOP) values are detected, iridotomy should be considered to eliminate the risk of pupillary block before the occurrence of PAS[35]. Another option is peripheral iridoplasty. Nonetheless, efficacy of iris procedures can be temporary. The risk of PAS persists in phakic nanophthalmic patients. Lens extraction can be a helpful tool in glaucoma management of nanophthalmic patients. In cases of persistently high IOP levels in pseudophakic/aphakic eyes, filtration surgery should be considered[36].
Cataract Surgery Considerations
In the first report of cataract surgery in nanophthalmos, Singh et al (1982) recommended deferral due to their disastrous results[37]. Since then, many breakthroughs have been made with cataract surgery, such as phacoemulsification. Appropriate choice of IOL for a small anterior segment can be challenging and advanced procedures still may lead to post-operative complications. Steijns et al (2012) found cataract surgery to be relatively safe in nanophthalmic eyes. Cataract surgery was performed in 43 eyes of 32 patients (>90% with phacoemulsification), with no complications in 71.1% of cases and improvement of BCVA in 69.8% of cases. Complications occurred in 12 eyes; the most prevalent were uveal effusion (9.3%) and cystoid macular edema (7.0%)[38]. Subsequent studies report similar complication distributions and rates [39] [40].
Surgical pearls to improve cataract surgery outcomes include: anterior vitrectomy, medical dehydration of the vitreous and scleral lamellar resection. Wu et al (2004) performed cataract surgery in 12 eyes of eight nanophthalmic patients. The reported cumulative complications in four eyes included: absence of response to prednisone with subsequent phthisis, broken intraocular lens (IOL) haptic with vitreous loss, choroidal hemorrhage, glaucoma progression and severe iritis. The authors found phacoemulsification can be fairly safe overall, with or without scleral lamellar resection[41].
Here are some videos regarding cataract surgery in patients with nanophthalmos that may be helpful:
- https://www.aao.org/education/clinical-video/femtosecond-cataract-surgery-nanophthalmos
- https://www.aao.org/education/annual-meeting-video/preop-intraop-postop-considerations-nanophthalmos
- https://www.aao.org/education/annual-meeting-video/scleral-windows-indications-techniques
Overall Eye Surgery Considerations
As consequence of small size and high levels of ocular comorbidity, surgery, in general, on nanophthalmic eyes has less favorable results and higher complication rates when compared to the general population[42]. A sudden decompression of the globe following any kind of surgery, including laser iridotomy, may result in rapidly progressing choroidal effusion. This may lead to secondary retinal detachment, intraocular hemorrhage and malignant glaucoma[35]. Despite the high rates of complications, there is controversy regarding the need for prophylactic sclerostomies. Many authors consider it wise to perform two to four sclerostomies before opening the eye[37], especially if prior history of uveal effusion is present[41].
Choroidal Considerations
Yamani et al (1999) reported the successful treatment of a choroidal detachment by full-thickness sclerectomies in all four quadrants. This procedure resulted in deepening of the anterior chamber, resolution of the choroidal detachment, with subsequent improvement of visual acuity[19].
Oculoplastic Considerations
The nanophthalmic features of enophthalmos and possible mild ptosis can compromise the physical appearance of patients, particularly during childhood. This is due to the reduced ocular volume that is critical for stimulating orbitofacial growth[43][44]. Therefore, oculoplastic management is focused on orbital expansion and elective blepharoplasty aimed at improving oribtofacial growth and appearance.
Orbital expansion
The expansion of the orbital socket can be guided by the axial length of the eye. If the axial length is < 16 mm, the orbital growth is likely to be reduced resulting in facial asymmetries. Therefore, earlier socket expansion is recommended to promote adequate orbital and facial growth during childhood. If the axial length is > 16 mm, the orbit likely has sufficient stimulation, and expansion therapy can be delayed or unnecessary[43].
Ocular conformers
The use of ocular conformers is generally for mild to moderate cases of microphthalmia. The conformer is a clear acrylic shell that is fitted into the eye socket prior to the placement of a prosthesis, which is used in cases where the affected eye has no light perception[45]. In the case of nanophthalmos, where the eyes are highly functional, the use of only clear conformers would be necessary for stimulating orbitofacial growth while preserving vision to avoid amblyopia. The frequency (weekly to monthly) of conformer changes as well as the total duration can vary by case.
Orbital Implants
In cases of severe micro-orbitism with poor cosmesis, and failure with increased-size conformers, orbital implantations are indicated for treatment. In a study by Wavreille et al., they implanted balloons into orbits to temporally expand them until they achieved symmetry. Other studies have underscored the ease and effectiveness of hydrogel implants or pellets for orbital expansion with variable success[46][47]. However, it is important to note that these studies were targeted at cases of anophthalmos or severe microphthalmia, not nanophthalmos. Similarly, the use of dermis-fat grafts has been used for improving micro-orbitism secondary to anophthalmia instead of nanophthalmos[48][49].
Soft tissue interventions
Another physical deformity secondary to nanophthalmos is mild bilateral ptosis. However, it is important to note that ptosis is due to a lack of support to the eyelids secondary to a defective globe. This suggests a pseudoptosis rather than an aponeurotic or myogenic cause of ptosis[50]. Therefore, unless the visual axis is affected, treatment for ptosis in nanophthalmos is focused on cosmetic rather than functional purposes. Upper lid blepharoplasty is the choice of correction[51][52].
Prognosis
Nanophthalmos has a broad clinical spectrum. This broad clinical spectrum translates to a broad spectrum for patients’ quality of life. Amblyopia can be prevented or minimized if high hyperopia is detected early in life. Close monitoring can help with identification and management of additional ocular complications before they become problematic.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Sundin OH, Dharmaraj S, Bhutto IA, et al. Developmental basis of nanophthalmos: MFRP is required for both postnatal ocular growth and postnatal emmetropization. Ophthalmic Genet. 2008; 19(1):1-9.
- ↑ 2.0 2.1 2.2 O’Grady RB. Nanophthalmos. Am J Ophthalmol. 1971; 71:1251-1253.
- ↑ 3.0 3.1 3.2 3.3 3.4 Cross HE, Yoder F. Familial nanophthalmos. Am J Ophthalmol. 1976; 81:300-306.
- ↑ 4.0 4.1 Warburg M. Classification of microphthalmos and coloboma. J Med Genet. 1993; 30:664-669.
- ↑ 5.0 5.1 Warburg M. Genetics of microphthalmos. Int Ophthalmol. 1981; 4(1-2), 45–65.
- ↑ 6.0 6.1 Ayala-Ramirez R, Graue-Wiechers F, Robredo V, Amato-Almanza M, Horta-Diez H, Zenteno JC. A new autosomal recessive syndrome consisting of posterior microphthalmos, retinitis pigmentosa, foveoschisis, and optic disc drusen is caused by a MFRP gene mutation. Mol Vis. 2006; 12:1483-1489.
- ↑ Zenteno J, Buentello-Volante B, Ayala-Ramirez R, Villanueva-Mendoza C. Homozygosity mapping identifies the Crumbs homologue 1 (Crb1) gene as responsible for a recessive syndrome of retinitis pigmentosa and nanophthalmos. Am J Med Genet. 2011; Part A, 155A(5), 1001–6.
- ↑ Sugar HS, Thompson JP, Davis JD. The oculo-dento-digital dysplasia syndrome. Am J Ophthalmol. 1966; 61(6):1448-1451.
- ↑ Yardley J, Leroy BP, Hart-Holden N, et al. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest Ophthalm Vis Sci. 2004; 45:3683-3689.
- ↑ Bergada I, Schiffrin A, Abu Srair H, et al. Kenny syndrome: description of additional abnormalities and molecular studies. Hum Genet. 1988; 80:39-42.
- ↑ 11.0 11.1 11.2 11.3 Othman MI, Sullivan SA, Skuta GL, et al. Autosomal dominant nanophthalmos (NNO1) with high hyperopia and angle-closure glaucoma maps to chromosome 11. Am J Hum Genet. 1998; 63:1411-1418.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 Sundin OH, Leppert GS, Silva ED, et al. Extreme hyperopia is the result of null mutations in MFRP, which encodes a frizzled-related protein. Proc Natl Acad Sci. 2005; 102:9553-9558.
- ↑ 13.0 13.1 Li H, Wang JX, Wang CY, et al. Localization of a novel gene for congenital nonsyndromic simple microphthalmia to chromosome 2q11-14. Hum Genet. 2008; 122:589-593.
- ↑ 14.0 14.1 Nair KS, Hmani-Aifa M, Ali Z, et al. Alteration of the serine protease PRSS56 causes angle-closure glaucoma in mice and posterior microphthalmia in humans and mice. Nat Genet. 2011; 43(6):579-84.
- ↑ Wang P, Yang Z, Li S, Xiao X, Guo X, Zhang Q. Evaluation of MFRP as a candidate gene for high hyperopia. Mol Vis. 2009; 15:181-186
- ↑ Gal A, Rau I, El Matri L, et al. Autosomal-recessive posterior microphthalmos is caused by mutations in PRSS56, a gene encoding a trypsin-like serine protease. Am J Hum Genet. 2011; 11;88(3):382-90.
- ↑ Orr A, Dubé M, Zenteno J, et al. Mutations in a novel serine protease PRSS56 in families with nanophthalmos. Mol Vis. 2011; 17, 1850–61.
- ↑ 18.0 18.1 Neri A, Leaci R, Zenteno J, Casubolo C, Delfini E, Macaluso C. Membrane frizzled-related protein gene-related ophthalmological syndrome: 30-month follow-up of a sporadic case and review of genotype-phenotype correlation in the literature. Mol Vis. 2012; 18, 2623–32.
- ↑ 19.0 19.1 Yamani A, Sugino I, Wanner, M. Abnormal collagen fibrils in nanophthalmos: a clinical and histologic study. Am J Ophthalmol. 1999; (973), 106–108.
- ↑ Fukuchi T, Sawada H, Seki M, Oyama T, Cho H, Abe H. Changes of scleral sulfated proteoglycans in three cases of nanophthalmos. Jpn J Ophthalmol. 2009; 53(2), 171–5.
- ↑ 21.0 21.1 Kimbrough RL, Trempe CS, Brockhurst RJ, Simmons RJ. Angle closure glaucoma in nanophthalmos. Am J Ophthalmol. 1979; 88(3 Pt 2):572-579
- ↑ Walsh MK, Goldberg MF. Abnormal foveal avascular zone in nanophthalmos. Am J Ophthalmol. 2007 Jun;143(6):1067-1068.
- ↑ 23.0 23.1 23.2 Walsh MK, Goldberg MF. Abnormal foveal avascular zone in nanophthalmos. Am J Ophthalmol. 2007; 143:1067-1068.
- ↑ Singh OS, Sofinski SJ. Nanophthalmos: guidelines for diagnosis and therapy. Principles and Practice of Ophthalmology: Clinical Practice, Vol 3; Chapter 135. Albert DM, Jakobiec FA editors. Philadelphia: WB Saunders. 1994; 1528-1540.
- ↑ MacKay CJ, Shek MS, Carr RE, Yanuzzi LA, Gouras P. Retinal degeneration with nanophthalmos, cystic macular degeneration, and angle closure glaucoma: a new recessive syndrome. Arch Ophthalmol. 1987; 105:366-371.
- ↑ 26.0 26.1 Mukhopadhyay R, Sergouniotis P, Mackay D, et al. A detailed phenotypic assessment of individuals affected by MFRP-related oculopathy. Mol Vis. 2010; 16:540-548
- ↑ 27.0 27.1 Hermann P. Le syndrome: microphtalmie-retinite pigmentaire-glaucome. Arch Ophtalmol Rev Gen Ophtalmol. 1958; 18(1):17-24
- ↑ Brockhurst RJ. Nanophthalmos with uveal effusion. A new clinical entity. Arch Ophthalmol. 1975; 93:1989-99.
- ↑ Huang S, Yu M, Qiu C, Ye T. The management of secondary glaucoma in nanophthalmic patients. Yan Ke Xue Bao. 2002; 18:156-9.
- ↑ Jackson T, Yang Y, Shun-Shin G. Spectral domain optical coherence tomography findings in retinal folds associated with posterior microphthalmos. J AAPOS. 2012; 16(4), 389–91.
- ↑ Rao A, Padhi TR, Jena S, Mandal S, Das T. Atypical features of nanophthalmic macula--a spectral domain OCT study. BMC Ophthalmol. 2012; 12:12.
- ↑ Sener EC, Mocan MC, Sarac OI, Gedik S, Sanac AS. Management of strabismus in nanophthalmic patients: a long-term follow-up report. Ophthalmology. 2003; 110:1230-1236.
- ↑ 33.0 33.1 Sharan S, Grigg J, Higgins, R. Nanophthalmos: ultrasound biomicroscopy and Pentacam assessment of angle structures before and after cataract surgery. J Cataract Refractive Surg. 2006; 32(6), 1052–5.
- ↑ Nouri-Mahdavi K, Nilforushan N, Razeghinejad M, Amini H, Perera, S. Glaucoma in a patient with nanophthalmos. J Ophthalmic Vis Res. 2011; 6(3), 208–14.
- ↑ 35.0 35.1 Yalvac I, Satana B, Ozkan G, Eksioglu U, Duman S. Management of glaucoma in patients with nanophthalmos. Eye. 2008; 22(6), 838–43.
- ↑ Bron A. Les indications chirurgicales dans la nanophtalmie. J Fr Ophtalmol. 2007; 30,2, 186-88
- ↑ 37.0 37.1 Singh OS, Simmons RJ, Brockhurst RJ, Trempe CL. Nanophthalmos: a perspective on identification and therapy. Ophthalmology. 1982; 89(9):1006-12.
- ↑ Steijns D, Bijlsma W, Van der Lelij A. Cataract Surgery in Patients with Nanophthalmos. Ophthalmology. 2012; 1–5.
- ↑ Yosar JC, Zagora SL, Grigg JR. Cataract Surgery in Short Eyes, Including Nanophthalmos: Visual Outcomes, Complications and Refractive Results. Clin Ophthalmol. 2021;15:4543-4551
- ↑ Hammer M, Teich L, Friedrich M, Reitemeyer E, Britz L, Khoramnia R, Yildirim TM, Auffarth GU. Cataract surgery in the extremely small eye: morphology, comorbidities and outcomes in 300 eyes. Br J Ophthalmol. 2025 Feb 27:bjo-2024-326998.
- ↑ 41.0 41.1 Wu W, Dawson D, Sugar A, et al. Cataract surgery in patients with nanophthalmos: results and complications. J Cataract Refract Surg. 2004; 30(3), 584–90.
- ↑ Wladis EJ, Gewirtz MB, Guo S. Cataract surgery in the small adult eye. Surv Ophthalmol. 2006; 51(2):153-61.
- ↑ 43.0 43.1 Microphthalmos. EyeWiki. https://eyewiki.aao.org/Microphthalmos#cite_note-:7-10. Published January 12, 2023. Accessed March 5, 2023.
- ↑ Farkas LG, Posnick JC, Hreczko TM, Pron GE. Growth patterns in the orbital region: a morphometric study. Cleft Palate Craniofal J. 1992;29(4):315-318.
- ↑ Changal N, Khandekar RB. Eye Conformers as Socket Expanders in Children: Experience at a Tertiary Eye Hospital in Central Saudi Arabia. Cureus. 2021;13(2):e13465
- ↑ Wavreille O, François Fiquet C, Abdelwahab O, et al. Surgical and prosthetic treatment for microphthalmia syndromes. Br J Oral Maxillofac Surg. 2013;51(2):e17-e21.
- ↑ Schittkowski MP, Guthoff RF. Injectable self inflating hydrogel pellet expanders for the treatment of orbital volume deficiency in congenital microphthalmos: preliminary results with a new therapeutic approach. Br J Ophthalmol. 2006;90(9):1173-1177.
- ↑ De Ruiter BJ, Lesko RP, Knudsen MG, et al. An age-related algorithm for management of micro-orbitism from anophthalmia: a systematic review with supplemental case reports. Orbit. 2022;41(4):397-406.
- ↑ Dermis Fat Graft. EyeWiki. https://eyewiki.aao.org/Dermis_Fat_Graft. Published October 21, 2022. Accessed March 5, 2023.
- ↑ Pavone P, Cho SY, Praticò AD, Falsaperla R, Ruggieri M, Jin DK. Ptosis in childhood: A clinical sign of several disorders: Case series reports and literature review. Medicine (Baltimore). 2018;97(36):e12124.
- ↑ Older JJ. Upper lid blepharoplasty and ptosis repair using a transcutaneous approach. Ophthalmic Plast Reconstr Surg. 1994;10(2):146-149.
- ↑ Jubbal KT, Kania K, Braun TL, Katowitz WR, Marx DP. Pediatric Blepharoptosis. Semin Plast Surg. 2017;31(1):58-64

