Microinvasive Glaucoma Surgery (MIGS)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide and is estimated to affect 76 million people worldwide, a number expected to increase to 111.8 million in 2040. About 3 million Americans have glaucoma, which is estimated to have a direct medical cost of $2.9 billion.[1][2][3] Initial therapy for glaucoma typically consists of topical eye drops or laser trabeculoplasty, both of which aim to lower intraocular pressure and have similar efficacy.[4] Historically though, when pharmacologic and/or laser treatment fails to control IOP, incisional surgery such as trabeculectomy or glaucoma drainage device implantation is required. Despite their efficacy, these surgical interventions result in labile postoperative IOPs, require frequent follow-up, prolonged recovery time and offer a risk of serious early and late complications. Therefore, these procedures are often delayed in mild to moderate disease and reserved for patients with more severe disease who warrant aggressive intervention. For decades, this left clinicians and patients with a treatment gap of limited options for patients with mild to moderate disease who had uncontrolled IOP despite medical and laser therapy. Even in patients who respond to medical therapy, there are several barriers to adequate therapy that may limit their long term utility. These barriers include noncompliance with the prescribed regimen, intolerance of medication side effects, financial burden limiting access to medications, or physical inability to reliably self-administer eye drops.[5] However, since the early 2000s, the field of glaucoma has seen the advent of micro invasive glaucoma surgery (MIGS) which has sought to fulfill the unmet need for improved treatment options and close this treatment gap. The phrase ‘MIGS’ was coined by Dr. Ike Ahmed in 2009.[6]
Patients generally are good candidates for MIGS if they have mild to moderate glaucoma, are intolerant or noncompliant with drops, or if their IOP is not controlled with topical eye drops or laser trabeculoplasty. MIGS procedures can also be used to reduce drop dependence at the time of traditional phacoemulsification surgery in patients whose disease may be well controlled. MIGS are often not sufficient treatment for patients with more advanced disease who require a low IOP goal. For these patients, traditional incisional surgeries such as glaucoma drainage implants or trabeculectomy are often still indicated.
MIGS Definition
MIGS procedures constitute a group of surgical interventions which share five characteristics[7]:
- High safety profile: Compared to traditional incisional surgeries, MIGS carry a much lower risk of serious complications such as hypotony, choroidal effusions, or choroidal hemorrhages.
- Minimal disruption of normal anatomy: MIGS allow for enhancement of physiological outflow mechanisms avoiding major alterations in normal ocular anatomy.
- Ab interno approach: MIGS are typically performed ab interno through a traditional clear corneal wound with direct visualization of the anatomical target.
- Efficacy: MIGS should offer meaningful IOP lowering effect. The level of IOP reduction is often inferior to traditional filtering surgery but should be at least 20%. Alternatively, patients who do not experience an IOP decrease should attain the reduction of at least one medication.
- Ease of use for patients and physicians: MIGS should allow for a rapid recovery with minimal additional downtime for patients. They should also be easily incorporated into traditional phacoemulsification surgery.
MIGS Approaches
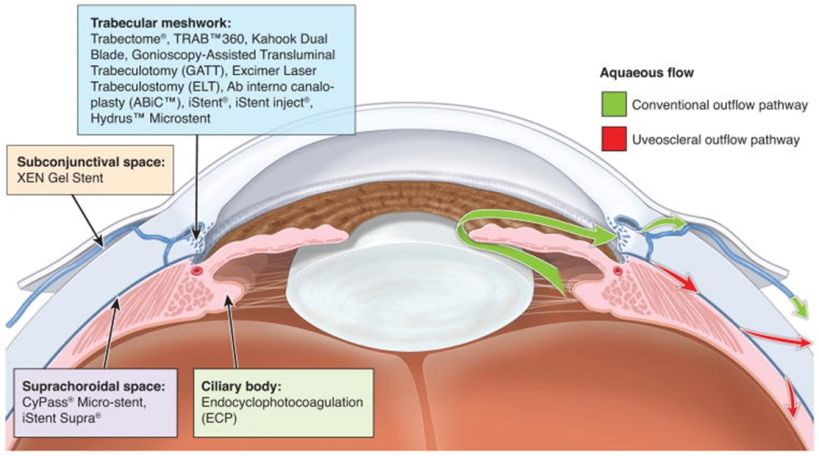
MIGS offer IOP lowering by targeting various aspects of normal aqueous dynamics. The first approaches involve enhancing outflow across the trabecular meshwork and through Schlemm’s canal. The juxtacanalicular trabecular meshwork has traditionally been identified as the site of greatest resistance to aqueous outflow. This resistance can be overcome through bypassing or removing this tissue to lower IOP through increased outflow. Bypass can be achieved by placing a trabecular meshwork bypass stent which allows aqueous to flow directly through the stent from the anterior chamber into Schlemm’s canal. Another approach to bypass the resistance of the trabecular meshwork is goniotomy or trabeculotomy which involves surgical incision and/or excision of this tissue and allows for improved aqueous outflow into Schlemm’s canal. Dilation of Schlemm’s canal through cannulation and expansion with viscoelastic is yet another approach to improve outflow through the normal physiologic aqueous outflow system.
A second group of MIGS approaches seeks to increase outflow via alternate pathways. The uveoscleral outflow pathway can be augmented by accessing the suprachoroidal space with microstent placement. Alternatively, if traditional outflow pathways are unlikely to be improved, aqueous can be shunted into the subconjunctival space through an ab interno, small incision approach.
A third MIGS approach involves decreasing aqueous production by ablation of the ciliary body. This approach is employed during endocyclophotocoagulation in which an endoscopic laser probe is inserted through a clear corneal incision and used to directly visualize and ablate the ciliary body.
MIGS Devices and Outcomes
Trabecular meshwork bypass by stent placement
As previously mentioned, trabecular meshwork bypass can be achieved either through the placement of stents or through surgical incision/excision of the trabecular meshwork tissue. MIGs utilizing stent placement are discussed below, with additional details in the article about Trabecular Meshwork Bypass by Stent.
iStent (1st Generation)
Device Design
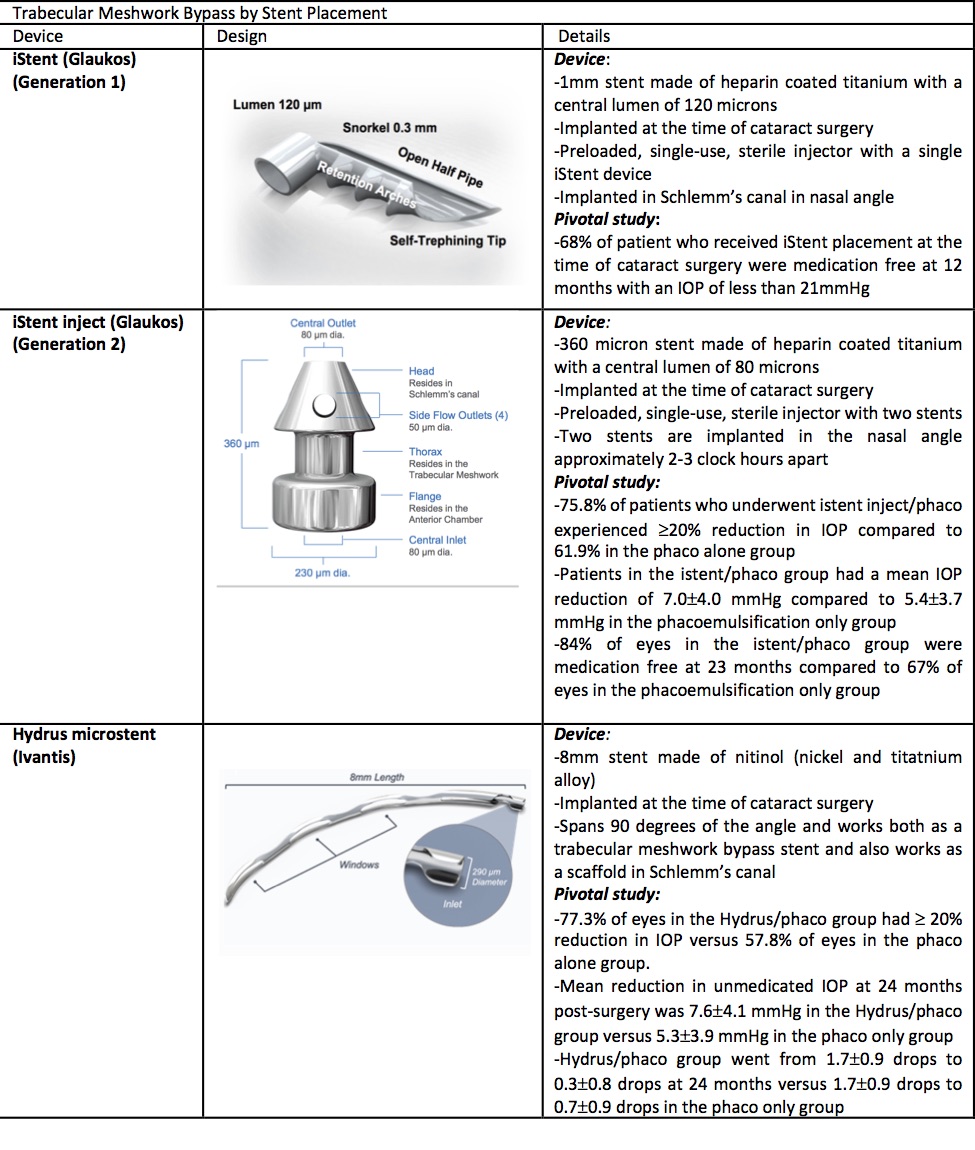
The iStent Trabecular Micro-Bypass Stent (Glaukos, Laguna Hills, CA) received FDA approval in 2012 as the first MIGS implant. The device is a heparin-coated, non-ferromagnetic titanium stent. The device measures 0.3mm in height and 1mm in length. There is a snorkel that is 0.25mm in height with a central lumen of 120 microns which projects into the anterior chamber. The body of the device which is implanted into Schlemm’s canal contains three retention arches to ensure that the device will remain in place.[9] The iStent is preloaded into a single-use sterile injector.
Indication
The device is indicated for the treatment of mild to moderate glaucoma and is approved for implantation at the time of phacoemulsification.
Pivotal trial
The pivotal trial for approval of the iStent compared the effectiveness of phacoemulsification alone to phacoemulsification with iStent placement. It was a prospective, randomized trial that included 240 eyes with mild to moderate glaucoma and intraocular pressure less than or equal to 24 mmHg on one to three drops. Patients were randomized to either phacoemulsification alone or phacoemulsification with iStent placement: 111 patients received iStent placement at the time of phacoemulsification. Patients were followed out to 12 months and the primary endpoint was an IOP ≤21 mmHg without ocular hypotensive medications. At 12 months, 72% of eyes that underwent iStent placement with phacoemulsification had reached the primary endpoint versus 50% of eyes undergoing phacoemulsification alone. Furthermore, 66% of iStent eyes had achieved an IOP reduction greater or equal to 20% compared to only 48% of eyes in the control (phacoemulsification only) group. Of note, both groups had statistically significant intraocular pressure lowering at 1 year and there was no difference in adverse events between the two groups. [10]
iStent inject
Device Design
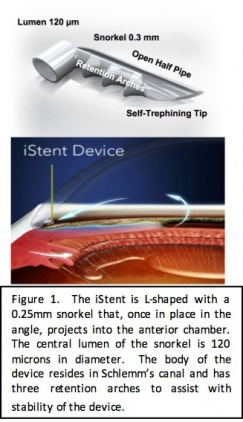
The iStent inject is the second generation trabecular meshwork bypass stent which received FDA approval in June 2018 (Glaukos, Laguna Hills, CA). The iStent inject is the smallest device to be implanted in the human body and, like the first generation iStent, is composed of heparin-coated, non-ferromagnetic titanium. Each stent is 360 microns in height by 230 microns in diameter; consisting of a tapered head with a recessed thorax and a terminal flange. The head resides in Schlemm’s canal, the thorax straddles the trabecular meshwork and the flange resides in the anterior chamber. The central lumen of each stent is 80 microns in diameter. The iStent inject consists of a single-use injector that comes pre-loaded with two devices, which are to be placed 2-3 clock hours apart in the nasal angle at the time of cataract surgery. The iStent inject W is a third generation implant that most notably has a wider flange that resides in the anterior chamber and helps to facilitate implantation while minimizing over-implantation. The outer diameter of the flange is 260 microns.
Indication
The device is indicated for the treatment of mild to moderate glaucoma and is approved for implantation at the time of phacoemulsification.
Pivotal Study
The pivotal trial for approval of the stent compared iStent inject/phacoemulsification to phacoemulsification alone. It was a prospective, single-masked, multi-center study with 505 eyes randomized 3:1 to iStent plus phacoemulsification (n=387) or phacoemulsification alone (n=118). Included patients had a diagnosis of mild to moderate primary open angle glaucoma, were on up to three pressure lowering drops, had a pre-operative intraocular pressure of less than or equal to 24 mmHg and required cataract surgery. Primary endpoints examined were number of patients with 20% or greater reduction in IOP at 24 months and the change in unmedicated IOP at 24 months. At 24 months, 75.8% of patients who received iStent inject at the time of cataract surgery had experienced at least a 20% reduction in IOP compared to 61.9% in the phacoemulsification alone group. Patients who received iStent at the time of phacoemulsification also had a mean IOP reduction of 7.0 ± 4.0 mmHg compared to 5.4 ± 3.7 mmHg in the patients who underwent phacoemulsification alone. Furthermore, 84% of eyes that underwent iStent/phacoemulsification were medication free at 23 months compared to 67% of eye that underwent phacoemulsification alone.[11]
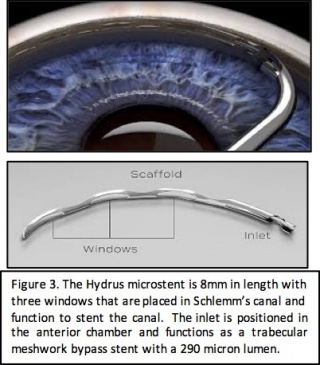
Hydrus microstent
Device Design
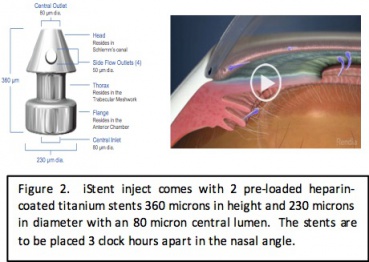
The Hydrus Microstent was developed by Ivantis Inc. and received FDA approval in August 2018. The microstent is 8mm in length and 290 microns in diameter with three windows and an inlet that sits in the anterior chamber. It is made of nitinol which is a flexible, biocompatible titanium and nickle alloy that has been used in cardiac stents. The device spans 90 degrees of the trabecular meshwork. It functions to lower intraocular pressure through two mechanisms: trabecular meshwork bypass stent and scaffold for Schlemm’s canal to maintain patency.
Indication
The device is indicated for the treatment of mild to moderate glaucoma and is approved for implantation at the time of phacoemulsification.
Clinical Outcomes
The HORIZON study was the pivotal study for approval of the Hydrus microstent; it compared phacoemulsification alone to phacoemulsification with Hydrus microstent implantation. A total of 546 patients with primary open angle glaucoma who had visually significant cataracts, IOP between 22-34 mmHg, and who were on between 1-4 hypotensive eye drops were enrolled. 369 eyes underwent phacoemulsification with Hydrus microstent implantation and 187 eyes underwent phacoemulsification alone. The primary endpoint was the proportion of eyes with unmedicated IOP reduction of greater than or equal to 20% at 24 months after surgery. Other secondary endpoints included mean change in IOP from baseline to 24 months and number of hypotensive medications. At 24 months, 77.3% of eyes in the Hydrus group experienced at least a 20% reduction in IOP compared to 57.8% of eyes in the phacoemulsification group alone. Mean change in unmedicated IOP at 24 months post-surgery was -7.6±4.1 mmHg in the Hydrus group compared to -5.3±3.9 mmHg in the phacoemulsification alone group. There was also a significantly greater reduction in hypotensive eye drops in the Hydrus group compared to the phacoemulsification alone groups. Hydrus plus phacoemulsification group went from 1.7±0.9 drops to 0.3±0.8 drops at 24 months after surgery while the phacoemulsification alone group went from 1.7±0.9 drops to 0.7±0.9 drops at 24 months.[11]
Trabecular meshwork bypass by tissue excision
In addition to the above mentioned stents, trabecular meshwork bypass can also be achieved through surgical incision/excision of the trabecular meshwork tissue. General advantages of these MIGS procedures are the ability to bypass the trabecular meshwork and form a direct pathway to Schlemm’s canal for 3-4 or more clock hours. Importantly, no implant is required and these goniotomy/trabeculotomy procedures can be performed as a standalone procedure or combined with simultaneous cataract surgery. General disadvantages are the risk of cleft closure from residual TM leaflets and IOP reduction is limited by episcleral venous pressure and Schlemm’s canal resistance.[12]MIGS utilizing this technique are described below,with additional details in the article about Ab Interno Trabeculectomy and Trabeculotomy.
Kahook Dual Blade Goniotomy
Device Design
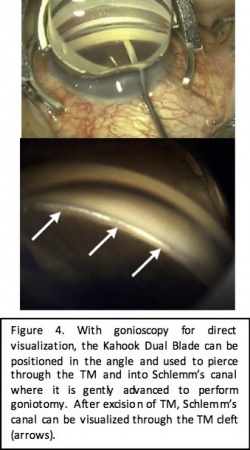
The Kahook Dual Blade (New World Medical, Rancho Cucamonga, CA) is a specialized goniotomy blade that was introduced in the United States in 2015. It was designed for improved excision of the trabecular meshwork during goniotomy. The blade is a single use disposable blade with a sharp tip which is used to pierce the trabecular meshwork, a ramp which stretches the trabecular meshwork and dual parallel blades which create paired parallel incisions in the trabecular meshwork. This design allows for complete excision of the trabecular meshwork and reduces the potential for scarring from residual tissue leaflets. Surgical technique involves an ab interno approach through clear corneal incisions with visualization of the angle using direct gonioscopy and a perpendicular approach to the TM. Treatment ranges between three to five clock hours.
The second-generation KDB GLIDE has been available since October 2020. While the parallel dual blade and ramp design remains the same, new features include a rounded heel, tapered sides and a smaller footplate. These changes improve ease of use as well as optimize fit within the canal.
Indications
Kahook Dual Blade goniotomy can be performed in patients with open angle glaucoma, ocular hypertension and certain patients with angle closure glaucoma. KDB goniotomy can be performed either at the time of cataract surgery or as a stand-alone procedure in phakic, aphakic or pseudophakic patients.
Clinical Outcomes
Several studies have demonstrated the clinical efficacy of Kahook Dual Blade goniotomy for IOP reduction in patients with glaucoma. One such study by Greenwood et al. looked at 1 year outcomes of KDB with phacoemulsification. The study included 71 eyes from a mixed population of patients with mild to severe glaucoma of various types, including primary open angle glaucoma, pigmentary glaucoma, pseudoexfoliation, normal tension glaucoma and angle closure glaucoma. Primary outcome measures were reduction in IOP and proportion of patients achieving a reduction in IOP of more than 20% from baseline. Secondary outcome measures included reduction in hypotensive eye drops. At 6 months, mean IOP decreased from 17.4±5.2 mmHg to 12.8±2.6 mmHg, and 58.3% of patients had an IOP reduction of ≥20% from baseline. The number of hypotensive eye drops decreased from 1.6±1.3 to 0.9±1.0 eye drops.[13] Another study included 52 eyes of patients with open angle glaucoma and visually significant cataracts who underwent phacoemulsification with KDB goniotomy. Outcome measures were mean reduction in IOP, proportion of patients with ≥20% reduction in IOP and reduction in IOP-lowering eye drops. At 1 year post-operatively, eyes that underwent phacoemulsification with KDB goniotomy experienced a significant reduction in mean IOP from 16.8±0.6 mmHg at baseline to 12.4+0.3 mmHg, 57.7% of eyes experienced a 20% reduction in IOP and the number of medications decreased from 1.6±0.2 to 0.8±0.1 eye drops.[14] Another study evaluated 12 month outcomes of phaco-KDB versus KDB alone. Success was defined as ≥20% IOP reduction and/or reduction of at least one glaucoma medication. Phaco-KDB had a success rate of 71.8% and KDB alone of 68.8%. [15]
Trabectome
Device Design
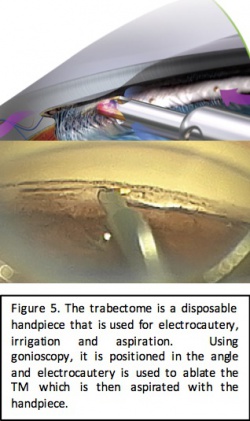
Trabectome is a surgical system developed by NeoMedix (Tustin, CA). It was FDA approved in 2004 and is used to perform an ab interno trabeculotomy. The system works by removing a strip of trabecular meshwork and the inner wall of Schlemm’s canal in order to create a path for the drainage of aqueous humor.[9] The device consists of a single-use, disposable hand piece that performs electrocautery, irrigation, and aspiration. It is connected to a generator with a frequency of 550 kHz that allows adjustments in 0.1 Watt increments and is controlled via a 3-stage foot pedal control that initiates irrigation, aspiration and electrocautery in sequence. Continuous irrigation and aspiration allows for removal of debris and regulation of temperature. Additionally, the tip of the Trabectome is bent at a 90° angle to create a protective triangular footplate and allow for easier insertion into Schlemm’s canal and is coated to facilitate smooth movement within the canal. Ablation may be applied for 60°-120° of trabecular meshwork to allow for re-establishment of the drainage pathway.
Indication
Trabectome is indicated for patients with various types of open angle glaucoma, ocular hypertension and can be used in some patients with angle closure glaucoma. Trabectome can be performed in conjunction with cataract surgery or as a stand-alone procedure. It can be performed in patients who are phakic, pseudophakic or aphakic.
Clinical Outcomes
Maeda et al[12] evaluated the outcomes of stand-alone Trabectome in 80 eyes of 69 patients with or without previous intraocular surgery or laser. A mean preoperative IOP of 26.6±8.1 mmHg was reduced to a mean of 17.4±3.4 mmHg at 6 months post-operatively. Average number of medications also decreased from 4.0±1.4 to 2.3±1.2 drops at 6 months. The study reported no serious complications, including choroidal effusion, choroidal hemorrhage, or infection. Thirteen eyes required subsequent surgeries due to uncontrolled IOP, with 10 patients receiving trabeculectomy and 3 patients receiving repeat Trabectome.[12] In a study completed in 2008 involving 304 eyes in patients with open angle glaucoma, similar results were found with combined phacoemulsification and Trabectome.[16] Mean IOP was found to have decreased from 20.0±6.3 mmHg to 15.5±2.9 mmHg at 1 year post-operative and the average number of topical medications was reduced from 2.65±1.13 to 1.44±1.29 drops. Complications included iris injury in 4 patients and IOP spike in 26 patients.
Gonioscopy Assisted Transluminal Trabeculotomy (GATT)
Surgical approach
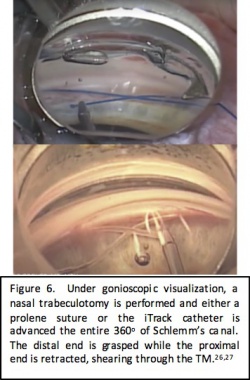
GATT is a form of ab interno trabeculotomy which was initially described by Grover et al. in 2014. Under direct visualization with a gonioscopy lens, a goniotomy is made in the nasal trabecular meshwork which serves as the entry point for the iTrack microcatheter (Ellex iScience, Fremont, CA). The microcatheter is 250 microns in diameter and has an illuminated distal tip to allow for visualization during advancement of the catheter through Schlemm’s canal. A modified technique has also been described using 4-0 nylon suture instead of the microcatheter. Microsurgical forceps are used to advance the microcatheter or suture into Schlemm canal circumferentially 360 degrees. Once it has been passed through the entire canal, the distal end of the catheter is grasped and the proximal end is retracted out of the eye, shearing the trabecular meshwork to create a 360-degree trabeculotomy.
GATT Procedure Gonioscopy-Assisted Transluminal Trabeculotomy
Source: YouTube, Ophthalmology Times, GATT, DSG RLF labelled. https://youtu.be/VXPOZtIhpnk Accessed June 02, 2020.[17]
Surgical Video Demonstrating a suture GATT with a 5-0 Prolene Suture
Source: YouTube, Davinder Grover, MD, MPH https://youtu.be/1laohyDLi2A Accessed June 02,2020. [18]
Indications
GATT can be performed in adult or pediatric patients with open angle glaucoma and a clear cornea. It can be performed as a stand-alone procedure or in combination with cataract surgery. Patients can be phakic, pseudophakic or aphakic.
Clinical outcomes
In their original review, Grover et al. reported on the 6 and 12 month outcomes of GATT performed in adult patients with open angle glaucoma either in combination with phacoemulsification or as a stand-alone procedure. There were 85 eyes of 85 patients included: 57 patients with primary open angle glaucoma showed an average IOP decrease of 11.1 ± 6.1 mm Hg and patients required 1.1 fewer drops at 1 year post-operatively.[19] For the 28 patients with other forms of open angle glaucoma, IOP decreased an average of 19.9 ± 10.2 mm Hg and patients required 1.9 fewer IOP lowering medications at 1 year post-operative. Treatment failure occurred in 8/85 patients (9%) due to the need for subsequent glaucoma surgery. The most common complication was a transient hyphema reported in 30% of patients at 1 week post-operative follow-up, which resolved by one month. Since the original publication, Grover and colleagues have reported the successful use of the GATT technique in primary congenital glaucoma, juvenile open angle glaucoma, and even eyes with prior incisional glaucoma surgery.[20] [21] These early results represent a promising, conjunctival sparing technique that can be used in conjunction with, or independent of cataract surgery.
TRAB 360/OMNI
Device Design
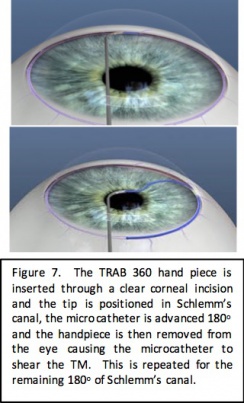
The TRAB 360 system (Sight Sciences, Menlo Park, California) is a single-use hand piece that allows the surgeon to perform trabeculotomy through a clear corneal incision. The tip of the hand piece is used to pierce the trabecular meshwork and the microcatheter is advanced from the tip of the device and threaded through 180 degrees of Schlemm’s canal. With the microcatheter still in Schlemm’s canal, the device is retracted from the eye tearing the microcatheter through the trabecular meshwork for 180 degrees. The device is then rotated and reinserted and the procedure is repeated for the remaining 180 degrees of the angle to accomplish a full 360 degree treatment.
Indication
TRAB 360 can be performed either in adult or pediatric patients with open angle glaucoma and a clear cornea. Patients can be phakic, pseudophakic or aphakic. It can be performed as a stand-alone procedure or can be performed in conjunction with cataract surgery.
Clinical Outcomes
A study by Sarkisian et al. evaluated the safety and efficacy of TRAB360 in patients with refractory primary open angle glaucoma. There were 81 eyes of 57 patients with various types of glaucoma (angle closure, congenital, inflammatory, neovascular, steroid-induced and open angle glaucoma) that underwent TRAB360 as a stand-alone procedure. Outcome measures included IOP reduction after surgery, proportion of eyes achieving ≥20% reduction in IOP at 12 months and number of IOP lowering eye drops required. At 1 year post-op, only 41 eyes (51%) were available for analysis. The mean IOP reduction was 7.3±6.7 mmHg and 59% of eyes experienced a ≥20% reduction in IOP. The number of IOP lowering medications decreased from 1.7±1.3 eye drops pre-operatively to 1.1±1.0 eyes drops at 12 months. Furthermore,37% of eyes did not require IOP lowering medications at 1 year.[22]
Enhancing aqueous outflow through Schlemm’s canal
In addition to the above-mentioned techniques for bypassing resistance at the level of the trabecular meshwork, other MIGS procedures aim to improve the conventional outflow pathway at the level of Schlemm’s canal and its downstream collector channels. These procedures work through cannulation and dilation of Schlemm’s canal and the distal outflow system to reduce outflow resistance. These are mentioned below.
Visco 360/OMNI
Device Design
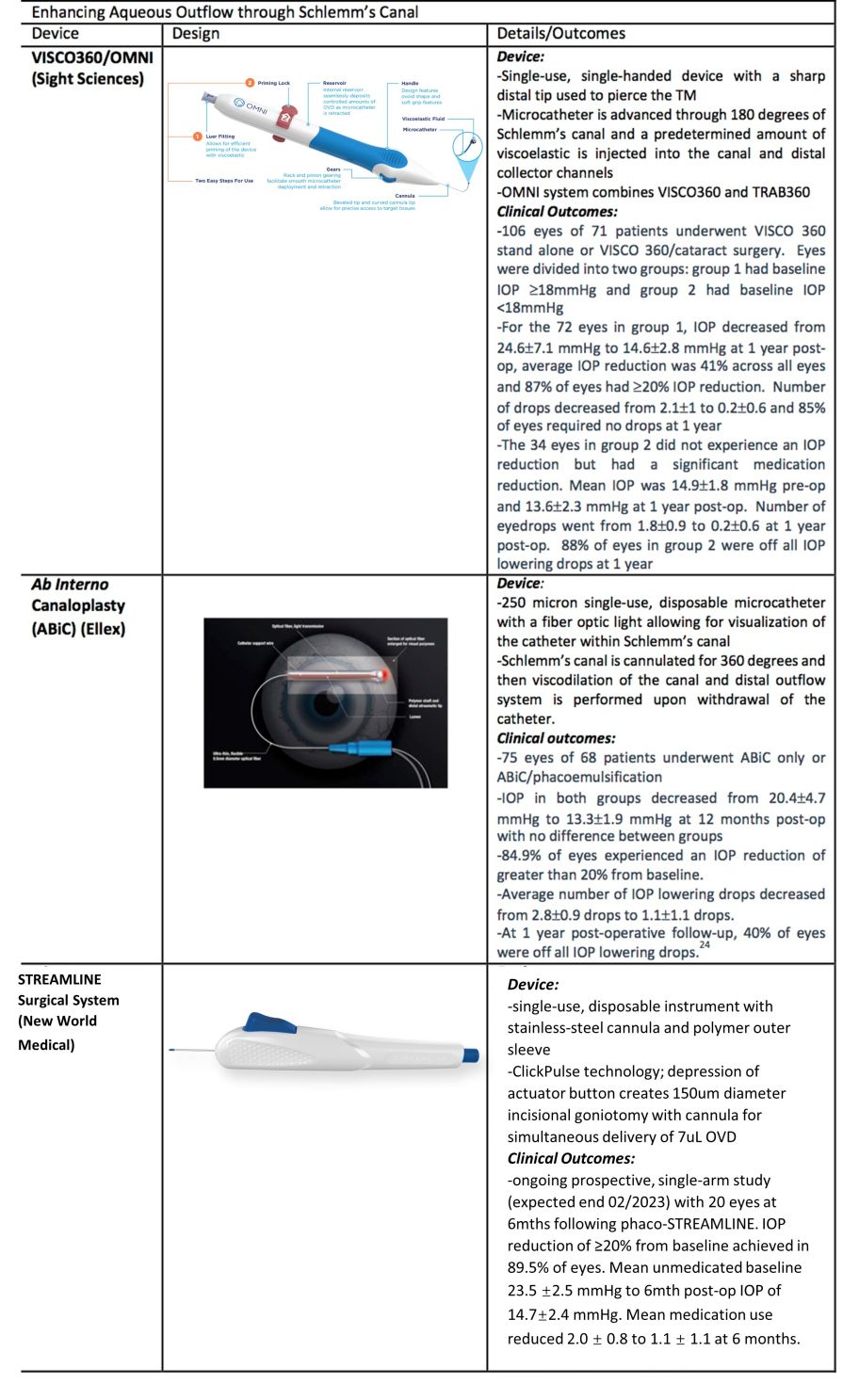
The VISCO360 device (Sight Sciences, Menlo Park, California) allows for catheterization and viscodilation of Schlemm’s canal. Similar to the TRAB360 device, it is a single-use, single-handed device with a sharp distal tip that is used to pierce the trabecular meshwork and a microcatheter is then advanced through 180 degrees of Schlemm’s canal. A predetermined amount of viscoelastic fluid is then injected into Schlemm’s canal and the distal collector channels. The procedure is then repeated for the remaining 180 degree of Schlemm’s canal. The procedure allows for dilation without tissue destruction of the natural drainage system.
The OMNI System (Sight Science, Menlo Park, California) is the same hand piece used for VISCO360 and combines the VISCO360 and TRAB360 procedures. After the surgeon performs viscodilation, the microcatheter is again introduced into Schlemm’s canal and a trabeculotomy is performed to achieve both viscodilation and trabeculotomy in one procedure.
Indication
The Visco 360/OMNI System is indicated for the treatment of open angle glaucoma and ocular hypertension. It can be performed as a stand-alone procedure or in combination with cataract surgery. It can be performed in patients who are phakic, pseudophakic or aphakic.
Clinical Outcomes
A study by Ondrejka and Korber evaluated the effectiveness of the VISCO360 viscosurgical system for the treatment of mild to moderate primary open angle glaucoma.[23] The study included 106 eyes from 71 patients and divided patients into two groups based on baseline IOP: eyes in group 1 had baseline IOP ≥18 mmHg and group 2 eyes had baseline IOP<18 mmHg. Both groups underwent canal viscodilation with or without cataract extraction. Effectiveness was measured by both amount of IOP reduction as well as reduction in IOP-lowering topical medications at 12 months post-operatively. Of the 72 eyes in group 1, 11 underwent VISCO360 alone and 61 underwent VISCO360 with cataract extraction. At 12 months post-operatively, there was a significant reduction in IOP from a baseline of 24.6±7.1 mmHg to 14.6±2.8 mmHg with an average reduction in IOP of 41% across all eyes. Additionally, 87% of eyes in group 1 achieved ≥20% reduction in IOP from baseline. There was also a significant reduction in IOP lowering drops in group 1 eyes from 2.1±1 to 0.2±0.6 and 85% of eyes required no medications to maintain pre-operative IOP or lower. Of the 34 eyes in group 2, 33 underwent VISCO360 with cataract extraction and only 1 underwent stand-alone VISCO360. Eyes in groups 2 had a mean baseline IOP of 14.9±1.8 mmHg and a post-operative mean IOP of 13.6±2.3 mmHg with no significant difference between the two time points. There was a significant reduction in medications at 12 months from 1.8±0.9 to 0.2±0.6 post-operatively. Further, 88% of eyes in groups 2 were off all IOP lowering drops at 12 months with IOP at baseline. In both groups, there was an excellent safety profile with only 14 eyes experiencing a hyphema, most of which were sub-millimeter in size. There were no subsequent surgical interventions related to the VISCO360.
Ab Interno Canaloplasty (ABiC)
Surgical Approach
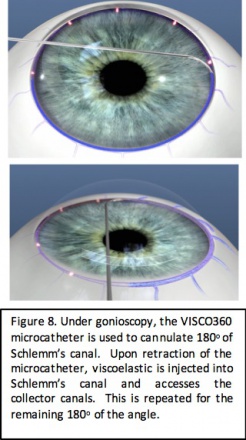
ABiC is performed using the iTrack microcatheter system (Ellex iScience, Fremont, CA) which is a 250 micron microcatheter with a fiber optic light allowing for visualization of the catheter while in Schlemm’s canal. After making a nasal goniotomy, the catheter is advanced 360 degrees into Schlemm’s canal and slowly withdrawn while performing viscodilation of the canal and the distal outflow system. This approach allows for enhancement of the natural outflow system without tissue destruction. Furthermore, it is believed to decrease herniation of the inner wall of Schlemm’s canal into the distal collector channels to improve outflow.
Indication
ABiC can be performed either in conjunction with cataract extraction or as a stand-alone procedure. It is indicated for the treatment of mild-moderate glaucoma in addition to more advanced and refractory glaucoma.
Clinical Outcomes
Gallardo et al. evaluated the efficacy of ABiC in a single-site 12 month study. 75 eyes of 68 patients with POAG underwent ABiC: 41 eyes underwent stand-alone ABiC and 34 eyes underwent combined ABiC/phacoemulsification. Average pre-operative IOP in both groups was 20.4±4.7 mmHg which decreased to 13.3±1.9 mmHg at 12 months post-operatively. There was no significant difference between IOP reduction in eyes that underwent stand-alone ABiC versus eyes that underwent combined ABiC/phacoemulsification. Average IOP reduction at 12 months post-op was 32.3% in both groups; 32.8% in the stand-alone ABiC group and 31.7% in the ABiC/phacoemulsification group. 84.9% of eyes experienced an IOP reduction of greater than 20% from baseline. Furthermore, the average number of IOP lowering drops decreased from 2.8±0.9 drops to 1.1±1.1 drops, which represents a 60.0% reduction on average. At 1 year post-operative follow-up, 40% of eyes were off all IOP lowering drops.[24]
STREAMLINE Surgical System
Device Design
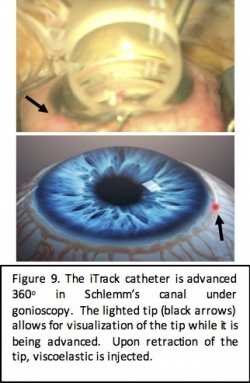
The STREAMLINE Surgical System (New World Medical, Rancho Cucamonga, CA) received FDA approval October 8, 2021. The device is single-use and disposable, consisting of a stainless-steel cutting inner cannula with a polymer outer sleeve. When the actuator button on the handset is fully depressed, the outer cannula retracts from the inner cannula tip which pierces the TM to create a 150uM diameter goniotomy while simultaneously delivering approximately 7uL of viscoelastic for dilation of Schlemm’s canal and the distal collector channels. The device is primed and loaded with enough OVD to perform the procedure approximately eight times. The precision goniotomies can be extended to create larger goniotomies over several clock hours as needed.
Indication
STREAMLINE can be used to treat ocular hypertension and open-angle glaucoma. The procedure can be performed with cataract surgery or as a standalone procedure, and can thus be used in both phakic and pseudophakic eyes.
Clinical Outcomes
There is an ongoing clinical trial evaluating the safety and efficacy of the STREAMLINE system. Started in March 2021 and expected to continue through February 2023 for a total of 60 participants, an interim analysis was published in April 2022. The study is a prospective, non-randomized trial that has thus far reported on 20 eyes in 20 subjects with mild to severe POAG who received phaco-STREAMLINE following washout of all IOP-lowering medications. Baseline unmedicated IOP was 23.5± 2.5 mmHg. At 6 months, post-treatment mean IOP was significantly lower at 14.7 ± 2.4 mmHg. IOP reduction ≥20% reduction was achieved in 89.5% of eyes at 6 months. Lastly, mean medication use went from 2.0 ± 0.8 to 1.1 ± 1.1. Thus far, three eyes had transient IOP spikes that were treated with topical medications.[25]
Enhancing aqueous outflow through the suprachoroidal space
While the above MIGS approaches augment the conventional outflow pathway, another MIGS approach is to target outflow through the unconventional, uveoscleral pathway. The CyPass microstent was the only FDA-approved device that targeted this mechanism of IOP reduction. However, as mentioned below, the device has been withdrawn from the market.
CyPass Micro-Stent
Device
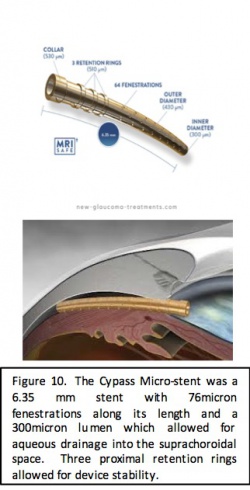
The CyPass Micro-Stent (Alcon, Fort Worth, Texas) is a polymide, supraciliary device for ab-interno implantation. The goal of the device is to create a controlled cyclodialysis cleft with stented outflow to the suprachoroidal space. The stent is 6.35mm long with an outer diameter of 430 microns, an inner diameter of 300 microns and with 76 micron fenestrations along the length of the device. During surgery, the implant is loaded onto a retractable guidewire and because of the flexibility of the stent, it is able to assume the curvature of the guidewire allowing the stent to conform to the scleral curvature. The device is inserted through the initial phacoemulsification incision, and advanced toward the sclera spur. The guide wire is used to perform blunt dissection of the ciliary body in order to allow passage into the supraciliary space where the stent can be placed. The proximal end of the stent has three retention rings to allow for improved stability of the device in the angle.
Indications
CyPass received FDA approval in July 2016 for use in conjunction with cataract surgery in patients with mild to moderate primary open angle glaucoma. The device was withdrawn from the market in August 2018 based on results of the 5-year COMPASS-XT trial which demonstrated a significant decrease in corneal endothelial cell counts in patients undergoing CyPass with phacoemulsification compared to patients undergoing phacoemulsification alone.[26]
Clinical Outcomes
The COMPASS trial reported on the two year safety and efficacy outcomes for Cypass.[27] The study enrolled 505 subjects randomized to phacoemulsification alone (131 patients) or phacoemulsification with Cypass (374 patients). Baseline IOP of patients in the phacoemulsification/Cypass group was 24.2±2.8 mmHg with an average of 1.4±0.9 medications. Baseline IOP in patients in the phacoemulsification only group was 24.5±3.0 mmHg with an average of 1.3±1.0 medications. There was significant reduction in IOP in both groups at 2 year follow-up, however IOP reduction was significantly greater in the Cypass group compared to phacoemulsification alone; IOP decreased 7.4 mmHg from baseline in the stent group compared to a decrease of 5.4 mmHg in the phacoemulsification only group. Additionally, 77% of patients in the stent group had ≥20% reduction in IOP compared to 60% in the phacoemulsification only group. Average number of medications decreased from 1.3±1.0 at baseline to 0.6±0.8 at 2 years in the phacoemulsification only group, and from 1.4±0.9 drops at baseline to 0.2±0.6 at 2 years in the Cypass group. Further, 85% of eyes in the Cypass group were drop free at 2 years.[27]
Shunting aqueous outflow into the subconjunctival space
Similar to traditional filtering surgery, another MIGS surgical approach to decreasing IOP is to shunt aqueous from the anterior chamber to the subconjunctival space. The Xen gel stent implant is currently the only FDA-approved device which utilizes this approach. Because this approach results in the formation of a filtering bleb, there is debate as to whether this approach can truly be classified in the MIGS category.
XEN Glaucoma Implant
Device
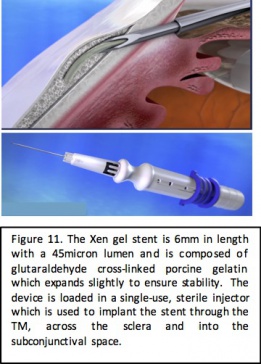
The XEN gel stent (Allergan, Dublin, Ireland) is a 6mm device composed of porcine-derived gelatin cross linked with glutaraldehyde. The inner lumen of the tube is 45 microns in diameter and the outer diameter of the gel stent is 150 microns. After implantation, the gelatin in the stent hydrates slightly causing expansion of the device to ensure improved stability. The gel stent is loaded in a single-use, single-handed disposable injector with a 27-gauge needle. The injector is inserted into the anterior chamber through a clear corneal incision and the tip of the injector is used to pierce through TM and the sclera into the subconjunctival space. The slider of the injector is advanced to deploy the stent into place and create an outflow from the anterior chamber to the subconjunctival space. Mitomycin C is typically injected subconjunctivally before or after stent placement to limit bleb fibrosis.
Indications
The Xen gel stent is FDA approved for use in the treatment of patients with refractory glaucoma in whom maximal medical therapy is insufficient or in patients who have failed previous glaucoma treatments. It can be performed in phakic or pseudophakic patients.
Clinical Outcomes
The initial study with the Xen gel stent included 65 patients with refractory glaucoma, which was defined as poorly controlled IOP on maximally tolerated medical therapy or patients who had failed previous filtering surgery or cilioablative procedure. Surgical technique utilized in this study included conjunctival cut-down, pre-treatment with mitomycin C soaked sponges followed by ab interno implantation of the Xen gel stent and then conjunctival closure. At 12 months post-operatively, mean IOP decreased from baseline by 9.1 mmHg and 75.4% of patient had ≥20% reduction in IOP. Average number of IOP lowering mediations decreased from 3.5 drops at baseline to 1.7 at 1 year post-operative. Most adverse events were mild and the most common included the need for needling, transient decrease in visual acuity and transient hypotony.[28]
Reducing aqueous production by ciliary body ablation
Another mechanism to achieve IOP reduction in MIGS is the decrease of aqueous production through ablation of the ciliary body. This approach is employed in endocyclophotocoagulation (ECP) during which a laser endoscopic probe is inserted through a clear corneal incision and used to directly visualize and ablate the ciliary body.
Endocyclophotocoagulation (ECP)
Surgical Approach
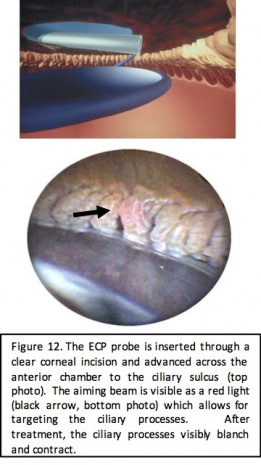
ECP is performed with an endoscopic probe attached to a laser unit (BVI, Little Silver, NJ) which includes a diode laser (810nm), a Xenon light source, a helium-neon aiming beam, and fiber optic imaging. The connected video monitor provides for endoscopic viewing by the surgeon and a foot pedal allows for surgeon control of the laser. The probe comes in a 19, 20, or 23 gauge size that is either straight or curved and is inserted into the anterior chamber through a clear corneal incision. Laser setting start at 0.2-0.25 Watts, continuous cycle and power is titrated to achieve both blanching and contraction of the ciliary processes. Treatment typically consists of 200-360 degrees of the angle.
Indications
ECP can be used to treat patients with a wide range of glaucoma severity from mild to severe or refractory as well as most glaucoma subtypes. ECP is most commonly used in open angle glaucoma but also has a particularly advantageous role in the treatment of angle closure or plateau iris where it can function to deepen the angle. It can be performed in conjunction with phacoemulsification or as a stand-alone procedure in phakic or pseudophakic patients. ECP can also be performed in conjunction with other angle based procedures above to provide additional IOP lowering.
Clinical Outcomes
The technique of ECP was first described by Uram in 1995.[29] In 1997, Chen et al. evaluated the safety and efficacy of ECP in refractory glaucoma of multiple subtypes. The study included 68 patients with refractory glaucoma who had either failed maximal medical therapy, had previous failed filtration surgery or transscleral cyclodestructive procedure. ECP treatment was performed on 180-360 degrees of the ciliary body. In these patients, mean IOP decreased from 27.7±10.3 mmHg pre-operatively to 17.0±6.7 mmHg at final post-operative time point (average 12.9 months). 90% of eyes achieved an IOP of £21 mmHg by final follow-up. Furthermore, mean number of medications decreased from 3.0±1.3 to 2.0±1.3 drops post-operatively. There were no cases of hypotony or phthisis and only four eyes lost 2 or more lines of Snellen visual acuity.[30] ECP is commonly combined with phacoemulsification and the addition of ECP offers both a significant IOP and medication reduction compared to phacoemulsification alone.[31] Francis et al. (2014) compared IOP reduction with phacoemulsification alone to ECP with phacoemulsification. The study included 80 patients that underwent phacoemulsification (control group) and 80 eyes that underwent phacoemulsification with ECP (study group); baseline IOP was 18.1±3.0 mmHg in both groups. At 2 years post-op follow-up, IOP in the study group had decreased to 16.0±3.3 mmHg compared to 17.3±3.2 mmHg in the control group with a significantly greater decrease in the study group. The mean number of glaucoma medications decreased from 1.5±0.8 to 0.4±0.7 drops at 2 years in the study group and from 2.4±1.0 to 2.2±1.1 in the control group. This represents a significantly greater reduction in number of glaucoma medications at two years with the addition of ECP at the time of cataract surgery. [31]
MIGS in Combination
There are a few studies published of outcomes of phacoemulsification combined with two different MIGS procedures.
Phaco + iStent + VISCO360
Heersink and Dovich reported 6-month outcomes in POAG eyes receiving phaco-iStent-VISCO360 (n = 86) compared to phaco-iStent (n = 100). A greater proportion of the former group achieved ≥20% reduction in IOP and an IOP <18mmHg on the same or few medications. Specifically, the mean IOP reduction was 2.9±3.6 mmHg and 46% achieved IOP <18mmHg on same or fewer medications in the phaco-iStent-VISCO360 cohort, compared to 1.7±3.1 mmHg and 35% achieving IOP <18 on same or fewer medications in the phaco-iStent cohort.[32]
ICE (iStent + cataract extraction + endocyclophotocoagulation)
A retrospective study published in 2017 compared 12-month outcomes of ICE (study group) compared to phaco + iStent (control group) in OAG. Included patients ranged from mild to severe OAG. Mean preoperative IOP was 21.49 ± 9.56 mmHg in the study group (51 eyes) and 20.66 ± 3.23 mmHg in the control group (50 eyes). At 12 months, the study group had both a greater mean IOP reduction (7.14 vs. 4.48mmHg) as well as medication reduction (63% vs. 38%).[33]
PEcK (phacoemulsification + endoscopic cyclophotocoagulation + KDB)
A retrospective case series compared outcomes between PEcK (53 eyes) and first generation iStent + cataract extraction + ECP, referred to as ICE-1 (23 eyes). Of note, none of the ICE-1 cohort had moderate-to-severe or severe glaucoma compared to the PEcK cohort. Baseline IOP for PEcK was 18.3± 5.9 and 14.7 ± 4.3 mmHg for ICE-1. At 12 months, the mean PEcK IOP reduction was 5.1 ± 4.4 and the mean ICE-1 reduction was 2.3 ± 4.0. Mean medication reduction at 12 months was 1.6± 1.5 for PEcK and 0.97±0.66 for ICE-1.[34]
Stand-alone Surgery
Implant-based MIGS
Salient results of iStent Inject and Hydrus microstent studies are shown in the chart below. For more information on device design and clinical indications, please refer to the "MIGS Devices and Outcomes" section earlier in the article. * Indicates IOP after wash-out.
| Device | Study Design | IOP Reduction | Medication Reduction | Post-op Adverse Events > 1% or additional glaucoma surgery |
|---|---|---|---|---|
| iStent inject (Glaukos) | iStent inject (N=94) vs. latanoprost/timolol (N=98) (Fea et al., 2014)[35]
Randomized controlled trial |
|
|
|
| iStent inject only (N=66) (Voskanyan et al., 2014)[36]
Prospective cohort |
|
|
| |
| iStent inject on 2 medications (N=53)
(Berdahl et al., 2017)[37] Prospective cohort |
|
|
None | |
| iStent inject on 1 medication (N=57) (Lindstrom et al. 2016, 2020)[38][39]
Prospective cohort |
|
|
| |
| iStent inject only (N=33) (Hengerer et al., 2019)[40]
Prospective cohort |
|
|
| |
| iStent inject only in POAG (N=17), PEX (N=15), and PG (N=3) (Klamann et al., 2015)[41]
Retrospective case series |
|
|
| |
| iStent inject only in previously failed trabeculectomy (N=22) (Davids et al., 2018)[42]
Retrospective case series |
|
|
| |
| iStent inject after at least 1 failed filtration surgery (N=42) (Macher et al., 2018)[43]
Retrospective case series |
|
|
| |
| iStent infinite (Glaukos) | iStent infinite after failed glaucoma surgery or maximum tolerated medical treatment (MTMT) (N=71) (Sarkisian et al., 2023)[44]
|
|
|
|
| Hydrus microstent (Alcon) | 2 iStents (N=75) vs. Hydrus microstent (N=73) (Ahmed et al., 2020)[45]
|
|
|
|
| Hydrus (N=31) vs. selective laser trabeculoplasty (N=25) (SLT) (Fea et al., 2017)[46]
|
|
|
| |
| Hydrus only (N=406) (Agar et al., 2022)[47]
Retrospective case series |
|
|
| |
| Hydrus (N=21) vs. Canaloplasty (N=24) (Gandolfi et al., 2016)[48]
|
|
|
|
Non-implant-based MIGS
The chart below summarizes salient results (from standalone procedures only) from studies on Kahook Dual Blade Goniotomy (KDB), Trabectome Trabeculotomy, iTrack catheter ab-interno canaloplasty (Abic), OMNI combined canaloplasty and trabeculectomy, and gonioscopy assisted transluminal trabeculectomy (GATT). * Indicates IOP after washout.
| Non-implant MIGS | Premise and Study Type | IOP Reduction | Medication Reduction | Post-op adverse events > 1% or additional glaucoma surgery |
| Kahook Dual Blade (KDB) Goniotomy (New World Medical) | KDB only (N=53) (Berdahl et al., 2018)[49]
Retrospective case series |
|
|
|
| KDB only (N=42) (ElMallah et al., 2020)[50]
Retrospective case series |
|
|
| |
| KDB only (N=53) (Salinas et al., 2018)[51]
|
|
|
| |
| KDB only (N=40) (Bravetti et al., 2023)[52]
|
|
|
| |
| Trabectome Goniotomy (MicroSurgical Technology) | Standalone Trabectome (N=21) vs. combined with phacoemulsification (N=28) (Akil et al., 2017)[53]
|
|
|
|
| Standalone Trabectome (N=235) vs. combined with phacoemulsification (N=352)(Neiweem et al., 2016)[54]
|
|
|
| |
| Ab-interno canaloplasty with iTrack catheter (Nova Eye Medical) | Standalone iTrack (N=11) canaloplasty vs. combined with phacoemulsification (N=34) (Khaimi, 2021)[55]
|
|
|
|
| Standalone iTrack canaloplasty (N=4) vs. combined with phacoemulsification (N=23) (Koerber & Ondrejka, 2022)[56]
|
|
|
| |
| Standalone iTrack (N=21) canaloplasty vs. combined with phacoemulsification (N=23) (Gallardo 2021, 2022) [57][58]
|
|
|
| |
| Ab-interno canaloplasty and trabeculotomy with OMNI surgical system (Sight Sciences) | Standalone OMNI (N=9) vs. combined with phacoemulsification
(Grabska-Liberek, et al., 2022)[59] Prospective cohort |
|
|
|
| Standalone OMNI (canaloplasty only) (N=50) vs. Combined with phacoemulsification outcomes (Toneatto et al., 2022)[60]
|
|
|
| |
| OMNI only; split into groups > 18 (N=24) or ≤ 18 mmHg (N=24) at baseline (Vold et al., 2021)[61]
Retrospective case series |
|
|
| |
| OMNI only (N=38) (Klabe & Kaymak, 2021)[62]
|
|
|
| |
| Ab-interno trabeculotomy with TRAB360 (Sight Sciences) | TRAB360 only (N=81) (Sarkisian et al., 2019)[63]
Retrospective case series |
|
|
|
| Gonioscopy assisted transluminal trabeculotomy (GATT) | GATT only in failed glaucoma surgery (N=44) (Wang et al., 2023)[64]
|
|
|
|
| GATT only in failed trabeculectomy (N=26) (Cubuk et al., 2020)[65]
|
|
|
| |
| Standalone GATT (N=40) vs. combined with phacoemulsification (N=68) in POAG and PEX (Bozkurt et al., 2021)[66]
|
|
|
|
Conclusion
The advent of microinvasive glaucoma surgery has allowed for improved management of patients with mild to moderate glaucoma. As surgeon experience expands and the number of MIGS approaches grows, these procedures may even be utilized in more severe cases and across a wide range of clinical scenarios. MIGS procedures are an especially useful treatment option in patients with poor medication tolerance, poor compliance and patients who need more IOP-lowering than drops or laser trabeculoplasty can provide. MIGS can be easily incorporated into routine phacoemulsification surgery and can be used in patients who are well-controlled on drops but who desire drop independence. The field of MIGS has expanded rapidly in the last decade adding to the glaucoma surgeons’ armamentarium and their ability to tailor surgical approaches to each specific patient. Future devices and surgical approaches will continue to be developed in the coming years.
References
- ↑ Resnikoff S, Pascolini D, Etyaale D et al. (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
- ↑ Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090
- ↑ Rein DB, Zhang P, Wirth KE, et al. The Economic Burden of Major Adult Visual Disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–1760. Doi:10.1001/archopht.124.12.1754
- ↑ Samples JR, Singh K, Lin SC, et al. Laser trabeculoplasty for open-angle glaucoma: a report by the American academy of ophthalmology. Ophthalmology. 2011; 118(7):2296-2302.
- ↑ Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology 2009;116:191-9.
- ↑ Ahmed I. A Brief History of MIGS. Aug 15, 2017. The Ophthalmologist
- ↑ Saheb H and Ahmed I. Micro-invasive glaucoma surgery: current perspectives and future directions. Current Opinons Ophthalmol. 2012; 23(2) 96-104.
- ↑ Resnikoff S, Pascolini D, Etyaale D et al. (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
- ↑ 9.0 9.1 Brian A. Francis, Kuldev Singh, Shan C. Lin, Elizabeth Hodapp, Henry D. Jampel, John R. Samples, Scott D. Smith, Novel Glaucoma Procedures: A Report by the American Academy of Ophthalmology. Ophthalmology, Volume 118, Issue 7, July 2011, Pages 1466-1480.
- ↑ Samuelson T, Katz LJ, Wells JM, Duh Y, Giamporcaro J. Randomized Evaluation of the Trabecular Micro-Bypass Stent with Phacoemulsification in Patients with Glaucoma and Cataract. Ophthalmology 2011; 118: 459-467.
- ↑ 11.0 11.1 Samuelson T, Sarkisian S, Lubeck D, Stiles M, Duh Y, Romo E, Giamporcaro J, Hornbeak D, Katz LJ, iStent inject Study Group. Prospective Randomized, Controlled Pivotal Trial of an Ab Interno Implanted Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract: Two-Year Results. Ophthalmology Jun 2019; 126(6): 811-821.
- ↑ 12.0 12.1 12.2 Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma. 2013;22(3):205-8.
- ↑ Greenwood M, Seibold L, Radcliffe N, Dorairaj S, Aref A, RomanJ, Lazcano-Gomez G, Darlington J, Abdullah S, Jasek M, Bahjri K, Berdahl J. Goniotomy with a single-use dual blade: Short term results. J Cataract Refract Surg 2017; 43: 1197-1201.
- ↑ Dorairaj S, Seibold LK, Radcliffe N, Aref A, Jimenez-Roman J, Lazcano-Gomex G, Darlington J, Mansouri K, Berdahl J. 12-Month Outcomes of Goniotomy Perfored Using the Kahook Daul Blade Combined with Cataract Surgery in Eyes with Medically Treated Glaucoma. Adv Ther 2018; 35:1460-1469.
- ↑ Sieck EG, Epstein RS, Kennedy JB, SooHoo JR, Pantcheva MB, Patnaik JL, Wagner BD, Lynch AM, Kahook MY, Seibold LK. Outcomes of Kahook Dual Blade Goniotomy with and without Phacoemulsification Cataract Extraction. Ophthalmol Glaucoma. 2018 Jul-Aug;1(1):75-81. Doi: 10.1016/j.ogla.2018.06.006. Epub 2018 Jul 6. PMID: 32672636.
- ↑ Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: Initial results. J Cataract Refract Surg; 34(7). 1096-1103.
- ↑ GATT Procedure Gonioscopy-Assisted Transluminal Trabeculotomy http://glaucomaassociates.com/gonioscopy-assisted-transluminal-trabeculotomy/ Accessed June 02, 2020.
- ↑ Grover, D. GATT with 5-0 Prolene Suture https://www.youtube.com/watch?v=1laohyDLi2A Accessed June 02,2020.
- ↑ Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy. Ophthalmology 2014; 121: 855-61.
- ↑ Grover DS, Smith O, Fellman R, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmology 2015; 99: 1092-96.
- ↑ Grover DS, Godfrey DG, Smith O, et al. Outcomes of Gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior incisional glaucoma surgery. J Glaucoma 2017; 26: 41-45.
- ↑ Sarkisian S, Mathews B, Ding K, Patel A, Nicek Z. 360o ab-interno trabeculotomy in refractory open-angle glaucoma. Clinical Ophthalmology 2019; 13: 161-168.
- ↑ Ondrejka S and Korber N. 360o ab-interno Schlemm’s canal viscodilation in primary open angle glaucoma. Clinical Ophthalmology 2019; 13: 1235-1246.
- ↑ Gallardo M, Supnet R, Ahmed II. Viscodilation of Schlemm’s Canal for the Reduction of IOP Via an Ab-interno Approach. Clinical Ophthalmology 2018; 12: 2149-2155.
- ↑ Lazcano-Gomez G, Garg SJ, Yeu E, Kahook MY. Interim Analysis of STREAMLINE® Surgical System Clinical Outcomes in Eyes with Glaucoma. Clin Ophthalmol. 2022 Apr 27;16:1313-1320. Doi: 10.2147/OPTH.S358871. PMID: 35510271; PMCID: PMC9058234.
- ↑ Reiss G, Clifford B, Vold S, He J, Hamilton C, Dickerson J, Lane S. Safety and Effectiveness of CyPass Supraciliary Micro-stent in Primary Open-Angle Glaucoma: 5-Yaer Results from the COMPASS XT Study. Am J Ophthalmol 2019; 208: 219-225.
- ↑ 27.0 27.1 Vold S, Ahmed II, Craven R, Mattox C, Stamper R, Packer M, Brown R, Ianchulev T. Two-Year COMPASS Trial Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucom and Cataracts. Ophthlamology 2016; 123: 2103-2112.
- ↑ Grover DS, Flynn WJ, Bashford KP, Lewis RA, Duh YJ, Nangi RS, Niksch B. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Am J Ophthalmolo 2017; 183: 25-36.
- ↑ Uram M. Endoscopic Cyclophotocoagulation in Glaucoma Management. Current Opin Ophthalmol 1995; 6(2): 19-29.
- ↑ Chen J, Cohn R, Lin S, Cortes A, Alvarado J. Endoscopic Photocoagulation of the Ciliary Body for Treatment of Refractory Glaucomas. Am J Ophthalmology 1997; 124: 787-796.
- ↑ 31.0 31.1 Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg 2014; 40: 1313-1321.
- ↑ Heersink M, Dovich JA. Ab interno canaloplasty combined with trabecular bypass stenting in eyes with primary open-angle glaucoma. Clin Ophthalmol. 2019 Aug 12;13:1533-1542. Doi: 10.2147/OPTH.S215667. PMID: 31496645; PMCID: PMC6697664.
- ↑ Ferguson TJ, Swan R, Sudhagoni R, Berdahl JP. Microbypass stent implantation with cataract extraction and endocyclophotocoagulation versus microbypass stent with cataract extraction for glaucoma. J Cataract Refract Surg. 2017;43:377–82.
- ↑ Klug E, Chachanidze M, Nirappel A. et al. Outcomes of phacoemulsification and endoscopic cyclophotocoagulation performed with dual blade ab interno trabeculectomy or trabecular micro-bypass stent insertion. Eye 36, 424–432 (2022).
- ↑ Fea, A. M., et al. (2014). "Prospective unmasked randomized evaluation of the iStent inject ((R)) versus two ocular hypotensive agents in patients with primary open-angle glaucoma." Clin Ophthalmol 8: 875-882.
- ↑ Voskanyan L, Garcia-Feijoo J, Belda JI, et al. Prospective, unmasked evaluation of the iStent(R) inject system for open-angle glaucoma: synergy trial. Adv Ther. Feb 2014;31(2):189-201. doi:10.1007/s12325-014-0095-y
- ↑ Berdahl J, Voskanyan L, Myers JS, Hornbeak DM, Giamporcaro JE, Katz LJ, et al. Implantation of two second-generation trabecular micro-bypass stents and topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 18-month follow-up. Clin Exp Ophthalmol. 2017;45(8):797-802.
- ↑ Lindstrom R, Lewis R, Hornbeak DM, Voskanyan L, Giamporcaro JE, Hovanesian J, et al. Outcomes Following Implantation of Two Second-Generation Trabecular Micro-Bypass Stents in Patients with Open-Angle Glaucoma on One Medication: 18-Month Follow-Up. Adv Ther. 2016;33(11):2082-90.
- ↑ Lindstrom R, Sarkisian SR, Lewis R, Hovanesian J, Voskanyan L. Four-Year Outcomes of Two Second-Generation Trabecular Micro-Bypass Stents in Patients with Open-Angle Glaucoma on One Medication. Clin Ophthalmol. 2020;14:71-80.
- ↑ Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Second-Generation Trabecular Micro-Bypass Stents as Standalone Treatment for Glaucoma: A 36-Month Prospective Study. Adv Ther. 2019;36(7):1606-17.
- ↑ Klamann MK, Gonnermann J, Pahlitzsch M, Maier AK, Joussen AM, Torun N, et al. iStent inject in phakic open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):941-7.
- ↑ Davids AM, Pahlitzsch M, Boeker A, Torun N, Bertelmann E, Maier-Wenzel AK, et al. iStent inject as a reasonable alternative procedure following failed trabeculectomy? Eur J Ophthalmol. 2018;28(6):735-40.
- ↑ Macher T, Haberle H, Wachter J, Thannhauser C, Aurich H, Pham DT. Trabecular microbypass stents as minimally invasive approach after conventional glaucoma filtration surgery. J Cataract Refract Surg. 2018;44(1):50-5.
- ↑ Sarkisian SR, Jr., Grover DS, Gallardo MJ, Brubaker JW, Giamporcaro JE, Hornbeak DM, et al. Effectiveness and Safety of iStent Infinite Trabecular Micro-Bypass for Uncontrolled Glaucoma. J Glaucoma. 2023;32(1):9-18.
- ↑ Ahmed IIK, Fea A, Au L, Ang RE, Harasymowycz P, Jampel HD, et al. A Prospective Randomized Trial Comparing Hydrus and iStent Microinvasive Glaucoma Surgery Implants for Standalone Treatment of Open-Angle Glaucoma: The COMPARE Study. Ophthalmology. 2020;127(1):52-61.
- ↑ Fea AM, Ahmed, II, Lavia C, Mittica P, Consolandi G, Motolese I, et al. Hydrus microstent compared to selective laser trabeculoplasty in primary open angle glaucoma: one year results. Clin Exp Ophthalmol. 2017;45(2):120-7.
- ↑ Agar A, Healey P, Lee G. Long-term outcomes of standalone Schlemm’s canal hydrus microstent implantation for primary open-angle glaucoma in a real-world setting: Findings from the SPECTRUM registry. The Royal Australian and New Zealand College of Ophthalmologists 2022; Brisbane2022.
- ↑ Gandolfi SA, Ungaro N, Ghirardini S, Tardini MG, Mora P. Comparison of Surgical Outcomes between Canaloplasty and Schlemm's Canal Scaffold at 24 Months' Follow-Up. J Ophthalmol. 2016;2016:3410469.
- ↑ Berdahl JP, Gallardo MJ, ElMallah MK, Williamson BK, Kahook MY, Mahootchi A, et al. Six-Month Outcomes of Goniotomy Performed with the Kahook Dual Blade as a Stand-Alone Glaucoma Procedure. Adv Ther. 2018;35(11):2093-102.
- ↑ ElMallah MK, Berdahl JP, Williamson BK, Dorairaj SK, Kahook MY, Gallardo MJ, et al. Twelve-Month Outcomes of Stand-Alone Excisional Goniotomy in Mild to Severe Glaucoma. Clin Ophthalmol. 2020;14:1891-7.
- ↑ Salinas L, Chaudhary A, Berdahl JP, Lazcano-Gomez GS, Williamson BK, Dorairaj SK, et al. Goniotomy Using the Kahook Dual Blade in Severe and Refractory Glaucoma: 6-Month Outcomes. J Glaucoma. 2018;27(10):849-55.
- ↑ Bravetti GE, Gillmann K, Salinas L, Berdahl JP, Lazcano-Gomez GS, Williamson BK, et al. Surgical outcomes of excisional goniotomy using the kahook dual blade in severe and refractory glaucoma: 12-month results. Eye (Lond). 2023;37(8):1608-13.
- ↑ Akil H, Chopra V, Huang AS, Swamy R, Francis BA. Short-Term Clinical Results of Ab Interno Trabeculotomy Using the Trabectome with or without Cataract Surgery for Open-Angle Glaucoma Patients of High Intraocular Pressure. J Ophthalmol. 2017;2017:8248710.
- ↑ Neiweem AE, Bussel, II, Schuman JS, Brown EN, Loewen NA. Glaucoma Surgery Calculator: Limited Additive Effect of Phacoemulsification on Intraocular Pressure in Ab Interno Trabeculectomy. PLoS One. 2016;11(4):e0153585.
- ↑ Khaimi MA. Long-term medication reduction in controlled glaucoma with iTrack ab-interno canaloplasty as a standalone procedure and combined with cataract surgery. Ther Adv Ophthalmol. 2021;13:25158414211045751.
- ↑ Koerber N, Ondrejka S. Four-Year Efficacy and Safety of iTrack Ab-interno Canaloplasty as a Standalone Procedure and Combined with Cataract Surgery in Open-Angle Glaucoma. Klin Monbl Augenheilkd. 2022.
- ↑ Gallardo MJ. 24-Month Efficacy of Viscodilation of Schlemm's Canal and the Distal Outflow System with iTrack Ab-Interno Canaloplasty for the Treatment of Primary Open-Angle Glaucoma. Clin Ophthalmol. 2021;15:1591-9.
- ↑ Gallardo MJ. 36-Month Effectiveness of Ab-Interno Canaloplasty Standalone versus Combined with Cataract Surgery for the Treatment of Open-Angle Glaucoma. Ophthalmol Glaucoma. 2022;5(5):476-82.
- ↑ Grabska-Liberek I, Duda P, Rogowska M, Majszyk-Ionescu J, Skowyra A, Koziorowska A, et al. 12-month interim results of a prospective study of patients with mild to moderate open-angle glaucoma undergoing combined viscodilation of Schlemm's canal and collector channels and 360 degrees trabeculotomy as a standalone procedure or combined with cataract surgery. Eur J Ophthalmol. 2022;32(1):309-15.
- ↑ Toneatto G, Zeppieri M, Papa V, Rizzi L, Salati C, Gabai A, et al. 360 degrees Ab-Interno Schlemm's Canal Viscodilation with OMNI Viscosurgical Systems for Open-Angle Glaucoma-Midterm Results. J Clin Med. 2022;11(1).
- ↑ Vold SD, Williamson BK, Hirsch L, Aminlari AE, Cho AS, Nelson C, et al. Canaloplasty and Trabeculotomy with the OMNI System in Pseudophakic Patients with Open-Angle Glaucoma: The ROMEO Study. Ophthalmol Glaucoma. 2021;4(2):173-81.
- ↑ Klabe K, Kaymak H. Standalone Trabeculotomy and Viscodilation of Schlemm's Canal and Collector Channels in Open-Angle Glaucoma Using the OMNI Surgical System: 24-Month Outcomes. Clin Ophthalmol. 2021;15:3121-9.
- ↑ Sarkisian SR, Mathews B, Ding K, Patel A, Nicek Z. 360 degrees ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol. 2019;13:161-8.
- ↑ Wang Y, Zhang W, Xin C, Sang J, Sun Y, Wang H. Gonioscopy-assisted transluminal trabeculotomy for open-angle glaucoma with failed incisional glaucoma surgery: two-year results. BMC Ophthalmol. 2023;23(1):89.
- ↑ Cubuk MO, Ucgul AY, Unsal E. Gonioscopy-assisted transluminal trabeculotomy as an option after failed trabeculectomy. Int Ophthalmol. 2020;40(8):1923-30.
- ↑ Bozkurt E, Yenihayat F, Olgun A, Yazici AT, Sahbaz I. The efficacy of gonioscopy-assisted transluminal trabeculectomy combined with phacoemulsification. Int Ophthalmol. 2021;41(1):35-43.