Radiation Retinopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Radiation retinopathy is a predictable and often radiation dose-dependent complication following exposure to any type of radiation. The diagnosis is made when there is a history of radiation exposure and a physical exam revealing the characteristic funduscopic features. The diagnosis can be further supported with fluorescein angiography or optical coherence tomography. Treatment options are limited but the mainstays include laser treatment (panretinal photocoaguation and/or focal macular laser) and injections of anti-VEGF medications or triamcinolone.
Disease Entity
Disease
Radiation retinopathy is a predictable complication following exposure to any source of radiation. It was first described in 1933 by Stallard and appears clinically as microaneurysms, telangiectases, neovascularization, vitreous hemorrhage, hard exudates, cotton wool spots and macular edema.
Etiology
Radiation retinopathy is caused by exposure to any radiation source including external beam and plaque brachytherapy.
Specifically, external beam radiation as treatment for nasopharyngeal, paranasal sinus or orbital tumors, where there is limited protection for the eye, often can lead to clinically significant radiation retinopathy.[1] Plaque brachytherapy in the treatment of intraocular tumors can also cause severe damage to the immediate retina and choroid.
Risk Factors
Higher total radiation dose has been shown to increase the risk of radiation retinopathy. The fractionation, field design, type and rate of administration of radiation should also be accounted for. While there are no definite thresholds, estimates for external beam radiation range from 15 to 60 Gy, with the incidence of retinopathy increasing steadily at doses greater than 45 Gy.[2] Hyperfractionation has been associated with a decreased incidence of radiation retinopathy.[3] Patients who receive less than 25 Gy in fractions of 2 Gy or less are unlikely to develop significant retinopathy.
With plaque brachytherapy, the risk of radiation retinopathy is related to total radiation dose. In the treatment of uveal melanoma, therapeutic apical doses range from 80 to100 Gy. Factors that affect total radiation dose such as tumor height and location also increase the risk of retinopathy. Tumor thickness greater than 4 mm has been associated with a greater risk for radiation maculopathy.[4] A decreased distance to the fovea has also been associated with decreased time to maculopathy.[5]
Comorbidities such as diabetes, hypertension, simultaneous chemotherapy and pregnancy are associated with an increased risk of radiation retinopathy.[6] Risk factors for developing proliferative radiation retinopathy include younger age, pre-existing diabetes mellitus and shorter tumor distance to the optic disc.[7]
Pathophysiology
Exposure of radiation is thought to cause preferential loss of vascular endothelial cells with relative sparing of the pericytes.[8] It is hypothesized that differential sensitivity between endothelial cells and pericytes is the result of direct exposure of the endothelial cells to high ambient oxygen and iron found in the blood which generates free radicals and leads to cell membrane damage.[9] This damage leads to occlusion of capillary beds and microaneurysm formation. The retinal ischemia from areas of retinal non-perfusion ultimately leads to macular edema, neovascularization, vitreous hemorrhage, and tractional retinal detachment. In one report, the mean time to development of proliferative radiation retinopathy following plaque brachytherapy was 32 months and using Kaplan-Meier analysis, was found to develop in 7% of patients at 10 and 15 years post-plaque.[7]
Primary Prevention
Appropriate shielding of the ocular structures during external beam radiation can minimize radiation exposure. As previously mentioned, hyperfractionation of external beam radiation has been associated with a decreased incidence of radiation retinopathy.
With respect to plaque brachytherapy, various strategies to decrease the radiation dose to the macula have been attempted. These include the use of collimating plaques as well as eccentric positioning of the plaques.[10],[11] A study with vitreous substitutes including silicone oil, heavy oil and perfluorocarbon demonstrated attenuation of the radiation dose from iodine-125 plaque radiation in cadaveric eyes.[12] It remains to be seen if any of these strategies ultimately reduce the incidence of retinopathy.
A randomized controlled trial assessed the efficacy of periocular triamcinolone (40 mg) given at the time of plaque application in preventing radiation maculopathy.[13] At 18-month follow-up, eyes treated with periocular triamcinolone demonstrated less macular edema on optical coherence tomography and had less moderate and severe vision loss compared to the control group. The study reported similar rates of elevated intraocular pressure and cataract progression between the two groups.
Another study examined the use of laser photocoagulation in the prevention of radiation retinopathy and maculopathy.[14] With plaque brachytherapy, a predictable area of ischemia develops in and immediately surrounding the location of the plaque. Prophylatically treating this area with scatter laser photocoagulation may theoretically prevent retinopathy. Of 16 patients who were treated prohylactically with scatter laser photocoagulation, none lost more than 3 lines of vision at final follow-up.[14]
Diagnosis
History
Patients will have a history of radiation exposure. Radiation retinopathy has been reported to develop any where from 1 month[15] to 15 years[16] but most commonly occurs between 6 months and 3 years.[6]
Physical Examination
Evaluation for radiation retinopathy includes a complete ophthalmologic exam, including a dilated funduscopic exam to look for pathologic features described below.
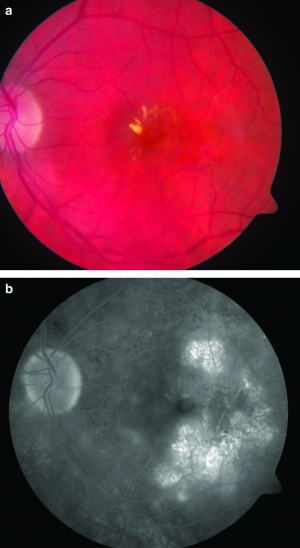
[The figure demonstrates macular edema, exudates, microaneurysms, and vessel telangiectasias (figure a) and cystoid macular edema on fluorescein angiography (figure b).[17]]
Signs [1]Dilated funduscopic exam reveals the following features:
- Retinal microaneurysms
- Retinal hemorrhages
- Retinal telangiectatic vessels
- Retinal hard exudates
- Macular edema
- Cotton-wool spots
- Retinal neovascularization
- Vitreous hemorrhage
- Tractional retinal detachment
Other associated features may include:
- Optic disc edema
- Neovascularization of the iris
- Neovascularization of the angle or neovascular glaucoma
- Cataract
Symptoms
Patients with early or mild retinopathy may be asymptomatic. Other patients with more advanced disease can present with decreased vision or floaters.
Clinical Diagnosis
Clinical diagnosis is based on the history and physical examination.
Diagnostic procedures
A fluorescein angiogram can be helpful in highlighting the microvascular features of radiation retinopathy. Indocyanine green angiography can reveal precapillary arteriolar occlusion and areas of choroidal hypoperfusion.[1]Optical coherence tomography (OCT) can be helpful in evaluating macular edema. A study by Horgan et al. found that OCT was able to detect evidence of macular edema approximately 5 months earlier than clinically detectable radiation maculopathy.[18]
Differential diagnosis [1]
- Diabetic retinopathy
- Branch retinal vein occlusion
- Central retinal vein occlusion
- Hypertensive retinopathy
- Coats Disease
- Perifoveal telangiectasia
Management
Medical therapy
Vascular endothelial growth factor (VEGF) is a secreted protein that promotes vascular leakage and angiogenesis.[19] Intravitreal anti-VEGF drugs are currently used to treat numerous ophthalmologic disorders including diabetic retinopathy, retinal vein occlusion and age-related macular degeneration. Several studies have examined the use of intravitreal bevacizumab, Ranibizumab and aflibercept in the treatment of radiation retinopathy and maculopathy.[20][21][22][23] Many of the studies demonstrated an improvement in macular edema following intravitreal anti-VEGF injections though a sustained decrease often required multiple injections. However, visual acuity was not found to improve significantly in most of the studies.
Triamcinolone acetonide is thought to downregulate various cytokines and regulate capillary permeability. It is used to treat macular edema secondary to various retinal pathologies and has similarly been used to treat radiation maculopathy. While the data is limited, some studies suggest that intravitreal triamcinolone acetonide (4 mg/0.1 ml) does transiently reduce macular edema and improve visual acuity.[24][25] Further studies are needed to determine whether these results are sustainable and whether the benefit of frequent injections outweigh the known risks of glaucoma, cataract, and endophthalmitis.
Grid macular laser photocoagulation has been used to treat radiation maculopathy with variable success. Studies by Kinyoun et al. and Hykin et al. demonstrated a benefit in visual acuity following treatment. [26][27] However, the effect was not sustained with longer follow-up in the study by Hykin et al.
Sector scatter and pan-retinal laser photocoagulation has also been used to treat non-proliferative and proliferative radiation retinopathy. In a study by Finger et al., patients received sector scatter laser photocoagulation at the first sign of retinopathy, proliferative or non-proliferative.[14] Retinopathy regressed in 64% of their treated patients. Similarly, in a study by Bianciotto et al., pan-retinal photocoagulation was found to cause regression of neovascularization in 66% of eyes with proliferative radiation retinopathy.[7]
There are also case reports of successful treatment of radiation retinopathy using photodynamic therapy, hyperbaric oxygen and oral pentoxifylline.[28][29][30]
Surgery
Advanced proliferative radiation retinopathy complicated by vitreous hemorrhage and/or tractional retinal detachment may require pars plana vitrectomy.
Complications
As previously mentioned, advanced radiation retinopathy can be complicated by neovascularization, vitreous hemorrhage and/or tractional retinal detachment. Proliferative radiation retinopathy has been reported to develop in 3 to 25% of eyes treated with plaque brachytherapy.[7],[31] Neovascular glaucoma can also develop and remains a difficult and devastating complication that can lead to enucleation. In patients who received I-125 plaque brachytherapy, enucleation secondary to neovascular glaucoma are reported to occur in 1-12% of treated eyes.[32]
Prognosis
Mild non-proliferative radiation retinopathy, characterized by subtle capillary dropout and occasional microaneurysms may not significantly affect vision and may be stable for years. However, many studies report a significant decline in visual function following plaque brachytherapy. In the Collaborative Ocular Melanoma Study (COMS), patients were followed for 3 years after receiving plaque brachytherapy and life table estimates predicted that 43% of patients would have a visual acuity of 20/200 or less.[33] In patients with large tumors (defined as tumor height >7.5 mm), the percentage of patients retaining a visual acuity of 20/200 or better has been reported to be as low as 5.5%.[34]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Yanoff M, Duker JS, Augsburger JJ. Ophthalmology. 2nd ed. St. Louis, MO: Mosby; 2004.
- ↑ Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. Nov 15 1994;30(4):765-773.
- ↑ Monroe AT, Bhandare N, Morris CG, Mendenhall WM. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol Biol Phys. Mar 1 2005;61(3):856-864.
- ↑ Stack R, Elder M, Abdelaal A, Hidajat R, Clemett R. New Zealand experience of I125 brachytherapy for choroidal melanoma. Clin Experiment Ophthalmol. Oct 2005;33(5):490-494.
- ↑ Puusaari I, Heikkonen J, Kivela T. Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology. Sep 2004;111(9):1768-1777.
- ↑ Jump up to: 6.0 6.1 Durkin SR, Roos D, Higgs B, Casson RJ, Selva D. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol Scand. May 2007;85(3):240-250.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Bianciotto C, Shields CL, Pirondini C, Mashayekhi A, Furuta M, Shields JA. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. May;117(5):1005-1012.
- ↑ Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy--clinical, histopathological, ultrastructural and experimental correlations. Eye. 1991;5 ( Pt 2):239-251.
- ↑ Archer DB, Gardiner TA. Ionizing radiation and the retina. Curr Opin Ophthalmol. Jun 1994;5(3):59-65.
- ↑ Damato B, Patel I, Campbell IR, Mayles HM, Errington RD. Visual acuity after Ruthenium(106) brachytherapy of choroidal melanomas. Int J Radiat Oncol Biol Phys. Oct 1 2005;63(2):392-400.
- ↑ Puusaari I, Heikkonen J, Kivela T. Effect of radiation dose on ocular complications after iodine brachytherapy for large uveal melanoma: empirical data and simulation of collimating plaques. Invest Ophthalmol Vis Sci. Oct 2004;45(10):3425-3434.
- ↑ Oliver SC, Leu MY, DeMarco JJ, Chow PE, Lee SP, McCannel TA. Attenuation of iodine 125 radiation with vitreous substitutes in the treatment of uveal melanoma. Arch Ophthalmol. Jul;128(7):888-893.
- ↑ Horgan N, Shields CL, Mashayekhi A, et al. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: a randomized controlled trial. Ophthalmology. Jul 2009;116(7):1383-1390.
- ↑ Jump up to: 14.0 14.1 14.2 Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. Jun 2005;89(6):730-738.
- ↑ Char DH, Lonn LI, Margolis LW. Complications of cobalt plaque therapy of choroidal malanomas. Am J Ophthalmol. Oct 1977;84(4):536-541.
- ↑ Stallard HB. Radiotherapy for malignant melanoma of the choroid. Br J Ophthalmol. Mar 1966;50(3):147-155.
- ↑ Wen JC, Oliver SC, McCannel TA. Ocular complications following I-125 brachytherapy for choroidal melanoma. Eye (Lond). Jun 2009;23(6):1254-1268.
- ↑ Horgan N, Shields CL, Mashayekhi A, Teixeira LF, Materin MA, Shields JA. Early macular morphological changes following plaque radiotherapy for uveal melanoma. Retina. Feb 2008;28(2):263-273.
- ↑ Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15-35; discussion 35-16.
- ↑ Mason JO, 3rd, Albert MA, Jr., Persaud TO, Vail RS. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina. Sep 2007;27(7):903-907.
- ↑ Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (avastin) for radiation retinopathy. Arch Ophthalmol. Jun 2007;125(6):751-756.
- ↑ Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys. Mar 15 2008;70(4):974-977.
- ↑ Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retina. Jul-Aug 2008;28(7):964-968.
- ↑ Shields CL, Demirci H, Dai V, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. Oct-Nov 2005;25(7):868-874.
- ↑ Sutter FK, Gillies MC. Intravitreal triamcinolone for radiation-induced macular edema. Arch Ophthalmol. Oct 2003;121(10):1491-1493.
- ↑ Kinyoun JL, Zamber RW, Lawrence BS, Barlow WE, Arnold AM. Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol. Feb 1995;79(2):144-149.
- ↑ Hykin PG, Shields CL, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. Aug 1998;105(8):1425-1429.
- ↑ Bakri SJ, Nickel J, Yoganathan P, Beer PM. Photodynamic therapy for choroidal neovascularization associated with submacular hemorrhage in age-related macular degeneration. Ophthalmic Surg Lasers Imaging. Jul-Aug 2006;37(4):278-283.
- ↑ Gall N, Leiba H, Handzel R, Pe'er J. Severe radiation retinopathy and optic neuropathy after brachytherapy for choroidal melanoma, treated by hyperbaric oxygen. Eye. Jul 2007;21(7):1010-1012.
- ↑ Gupta P, Meisenberg B, Amin P, Pomeranz HD. Radiation retinopathy: the role of pentoxifylline. Retina. 2001;21(5):545-547.
- ↑ Gunduz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. May 1999;117(5):609-614.
- ↑ Wen JC, Oliver SC, McCannel TA. Ocular complications following I-125 brachytherapy for choroidal melanoma. Eye (Lond). Jun 2009;23(6):1254-1268.
- ↑ Melia BM, Abramson DH, Albert DM, et al. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology. Feb 2001;108(2):348-366.
- ↑ Bechrakis NE, Bornfeld N, Zoller I, Foerster MH. Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology. Oct 2002;109(10):1855-1861.