Indocyanine Green Angiography
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Indocyanine green angiography (ICGA) provides improved imaging of choroidal vasculature compared to fluorescein angiography. Because of the excitation and emission properties of indocyanine green (excitation at 790 nm and emission at 835 nm), pathologies involving retinal and choroidal vascular systems can be imaged even in the presence of overlying melanin, serosanguinous fluid, xanthophyll pigment, or lipid exudations.
History
Indocyanine green (ICG) was developed in Kodack laboratories, and its physical and physiological properties were first described by Fox and Wood in 1960.[1] Kogure and others first used ICG to visualize the fundus of an owl monkey in the 1970s.[2] However, it was not routinely used in humans until 1990s because of technological limitations of the fundus cameras. There was an uptick in ICGA administration in the 1990s due to improvements in digital video angiography, scanning laser ophthalmoscopy, and optical systems of fundus cameras.[3][4] In addition, the advent of ICGA allowed for better detection of occult choroidal neovascularization (CNV), which in turn led to an increase in the number of eyes that were eligible for photocoagulation which, at the time, was the only treatment option for CNV.[5] Since the 2000s, in the era of anti-vascular endothelial growth factor (anti-VEGF) therapy which does not require accurate localization of the CNVs, ICGA has been mostly limited to the indications summarized below.[6] However, ICGA, combined with FA, OCT, and OCTA, is still extremely useful in clinical practice.
Properties
Two properties make ICG an effective dye for visualizing choroidal vasculature. First, indocyanine green’s affinity to circulating proteins is high (95%), limiting its leakage from vessel walls. Comparatively, more heavily leaking fluorescein (only 80% bound) from the retinal and choriodal vessels can obscure the details of adjacent retinal and choroidal vasculature. Another drawback of fluorescein dye in visualizing the choroid is that fluorescein molecule absorbs and emits shorter wavelength photons. Since the retinal pigment epithelium (RPE) also absorbs and emits photons around this wavelength, the resulting scatter from RPE can obscure the choroidal vessels. Indocyanine, however, absorbs and emits photons in the infrared spectrum, allowing the viewer to see the choroid through the retinal pigment epithelium or disease processes such as overlying hemorrhage.[7]
See “Dyes in Ophthalmology” for more information.
Uses
For a thorough review of the uses of indocyanine green, see Cohen et al.’s “Is indocyanine green still relevant?” editorial in Retina 2011.[8]
Age-Related Macular Degeneration (ARMD)
In the era of anti-VEGF treatments, the localization of CNV is not absolutely necessary for managing exudative ARMD as it was in the past. However, ICGA is utilized by many clinicians in combination with other retinal imaging modalities to identify the subtype of CNV in AMD (type 1: sub-retinal pigment epithelium, type 2: subretinal, and type 3: intraretinal) and to differentiate ARMD lesions from simulating lesions.[8]
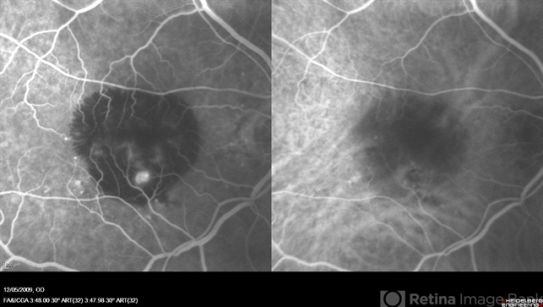
Type 1 macular neovascularization (MNV), with CNV network located under the RPE, is the most common form of exudative AMD. Type 1 CNV corresponds to “occult CNV” based on the Macular Photocoagulation Study and is identified as fibrovascular pigment epithelium detachment with a late stippled fluorescence or a late leakage from an undetermined source (LLUS). Often, clinical examination, OCT, and FA are sufficient for the diagnosis and follow-up; however, ICGA may be required when the lesion is covered with hemorrhage or if there is a question about the presence of accompanying type 3 MNV (retinal angiomatous proliferation – RAP) that is reported in about one fourth of the eyes with type 1 CNV. The presence of a RAP lesion has at least two clinical significances. First, RAP is shown to have a more aggressive clinical course that needs frequent and long-term injection of anti-VEGF agents. Second, it has been shown that RAP lesions respond better to treatment with anti-VEGF agents combined with photodynamic therapy (PDT). ICGA may delineate the presence of type 1 CNV usually around the perimeter of pigment epithelial detachment or show the feeder and draining vessels. RAP lesions are similarly seen as an interconnected retinal arteriole and venule branch.
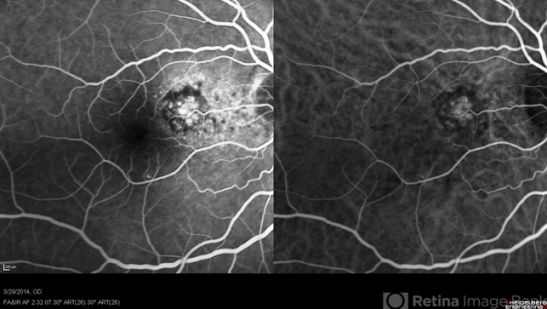
Type 2 CNV known as “classic CNV” is located under the retina and above the RPE and is visualized nicely with ICGA as the network of abnormal vessels in the setting of improved delineation of retinal and choroidal circulations. This improved spatial and temporal visualization with ICGA allows identification of the feeding choroidal vessels into the type 2 CNV lesion.
Polypoidal Choroidal Vasculopathy (PCV)
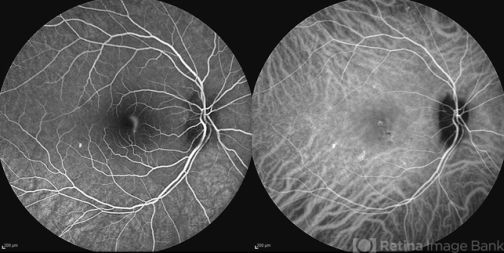
Aneurysmal type 1 MNV or polypoidal choroidal vasculopathy (PCV) is characterized by a growth of cavernous thin-walled vessels between the RPE and the Bruch’s membrane and is often associated with multiple, recurrent, serosanguineous detachments of the RPE and neurosensory retina secondary to leakage and bleeding from the polypoid lesions. ICGA delineates the polypoid or “bulging” capillaries through the overlying RPE and serosanguinous fluid with higher sensitivity and specificity and provides a better insight into the possible clinical course and prognosis. PCV polyps (that may be located in the peripapillary area or at the macula) start to fill before retinal vessels and continue to fill long after retinal vessels are filled. Thus, polyps are usually seen as early small hypercyanescence that leak slowly as the surrounding hypocyanescence become increasingly hypercyanescent. In later phases, dye gradually and uniformly “washes out” from the bulging polypoidal lesions.
PCV can be misdiagnosed or confused with chronic central serous chorioretinopathy or with classic or occult CNV in the setting of AMD. Indeed, in some patients, a transitional phase between these two pathologies can contain PCV patholgies. PCV lesions are usually responsive to more frequent anti-VEGF treatment with or without photodynamic therapy. In this setting, ICGA can guide management primarily by:
1. Revealing the polypoidal lesions and the branching vascular networks.
2. Identify active PCV for selective treatment with photodynamic therapy (PDT)
3. Identify recurrences after PDT (seen as late geographic hypercyanescence).[9][10][11]
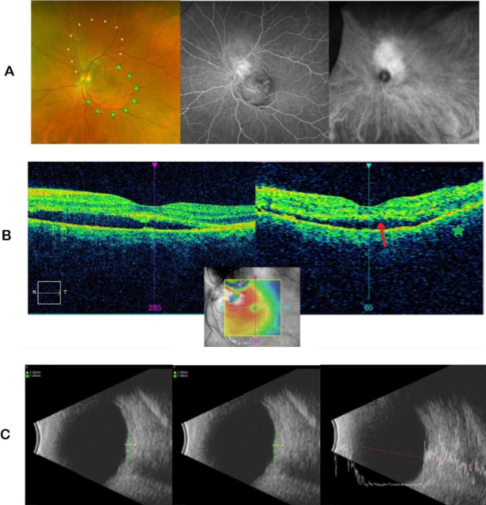
Choroidal tumors
ICGA and ultrasonography help identify choroidal hemangiomas and differentiate them from simulating tumors such as choroidal melanoma or metastasis. Choroidal hemangioma has a distinctive filling pattern on ICGA: progressive filling of abnormal choroidal vessels in the early phase, followed by an intense hypercyanescence at 2-4 minutes, with decreased cyanescence of the tumor at later frames compared to the rest of the choroid, known as the washout phenomenon.[12][13][14][15][16]
Although other choroid tumors do not have a characteristic ICGA feature, ICGA may still be used to differentiate them from simulating nontumor lesions such as peripheral exudative hemorrhagic chorioretinopathy (PEHCR). [8]
Central Serous Chorioretinopathy (CSCR)
Choroidal hypervascularity in the pachychoroid spectrum of diseases such as central serous chorioretinopathy (CSCR) may be better delineated with ICGA. ICGA may show an area of choroidal hypervascularity larger than the leakage spot seen in FA and is especially helpful in guiding photodynamic therapy to include entire hypervascular and leaking areas.[17][18] In addition, chronic CSCR complicated with CNV with or without leakage and hemorrhage may be better delineated with ICGA.
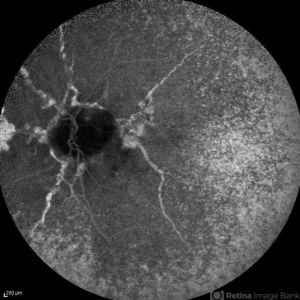
Pathologic Myopia
ICGA may provide a clear view of the complications associated with pathologic myopia. CNV lesions associated with lacquer cracks (such as lesions underneath subretinal hemorrhages related to newly formed lacquer cracks) are better visualized with ICGA.[19]
Angioid Streaks
Angioid streaks are typically visualized with ICGA more clearly, extensively, and prominently as compared to FA or fundus exam.[20]
Inflammatory
Multiple evanescent white dot syndrome (MEWDS)
ICGA is helpful in the diagnosis of white dot syndromes, particularly if the fundus lesions are of an atypical appearance or have already faded away.[21] In MEWDS, ICGA shows numerous hypercyanescent dots scattered throughout the posterior pole and a hypocyanescent area surrounding the optic nerve. The hypocyanescent areas on ICGA disappear over time as the inflammation resolves. [22][23][24]
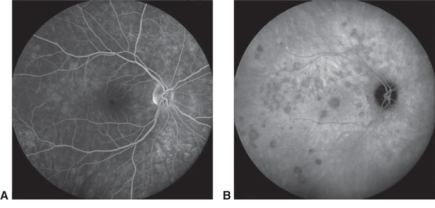
Acute Posterior Multifocal Placoid Pigment Epitheliopathy
Acute posterior multifocal placoid pigment epitheliopathy is a multifocal inflammation involving the choriocapillaris, and the resulting delayed and defective choroidal filling may manifest as a unique pattern of multifocal hypocyanescence in areas seen in the retinal examination as white spots.[25]
Vogt-Koyanagi-Harada disease
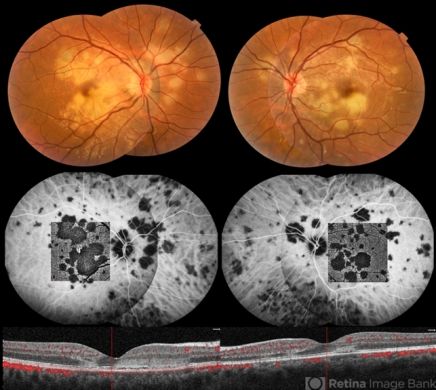
Although ICGA is not crucial for diagnosing VKH, active disease can be differentiated from completely treated and inactive VKH by the ICGA pattern. ICGA signs of acute VKH include early choroidal vessel hypercyanescence, intermediate to late phase fuzziness of choroidal stromal vessels, disc hypercyanescence, and hypocyanescent dark dots (HDDs). ICGA is an excellent modality to detect subtle choroidal inflammation in subclinical VKH reactivation, thus helping to monitor adequate response to corticosteroid treatment.[26][27]
Birdshot Chorioretinopathy
Deep, cream-colored lesions diffusely scattered throughout the fundus in Birdshot chorioretinopathy (BS) appear as round or oval hypocyanascent spots with a similar size spread across the fundus, particularly affecting the nasal quadrants. FA does not show these lesions well, and ICGA often shows more spots than clinical examination. Other ICGA features include an alteration of the vascular pattern of the choroid, with choroidal vessels appearing fuzzy and indistinct in the intermediate phases of the angiogram and a late diffuse hypercyanescence resulting from the ICG dye leaking through the inflamed choroidal vessels. In the chronic phase of the disease, the hypocyanescent dots persist in the late phases of the angiogram and correspond to chorioretinal scars. [28]
Choroidal Granulomas
Choroidal granulomatous diseases such as sarcoidosis and tuberculosis may manifest as hypocyanascent lesions that reflect filling defects in the areas occupied by a collection of granulomatous cells. [29][30]
Serpiginous Choroidopathy
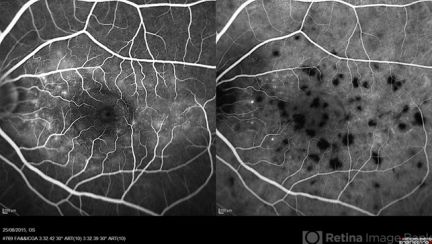
ICGA can help to differentiate between active, inactive, and healed serpiginous choroidopathy (SC) lesions.[31][32][33] In subclinical and clinically active lesions, ICGA will typically show one of the two patterns: 1) early and late hypocyanescence with ill-defined margins, or 2) early hypocyanescence with increased cyanescence toward late-phase of ICGA. ICGA in healed SC lesions will show early and late-phase hypocyanescence with well-defined margins.
Ocular Infections
While posterior inflammation involving the choroid could be seen with ICGA in many infectious processes, ICGA often does not offer additional information to help with the diagnosis or management of ocular infectious conditions.[8]
Trauma
ICGA can reveal choroidal involvement as with traumatic choroidopathies such as choroidal rupture and traumatic ocular hypotony. However, since ICGA adds little diagnostic or prognostic information, it is rarely ordered in ocular trauma settings.[34][35] [36][37]
Procedure
The standard dosing for ICG with scanning laser ophthalmoscopy camera systems is 25 mg of ICG dissolved in 3 ml of saline and 1 ml of the solution is injected. For combined FA and ICGA, saline is replaced with fluorescein sodium solution at 10, 20, or 25% concentrations. There are three temporal phases for ICGA imaging, similar to fluorescein angiography:[7][38]
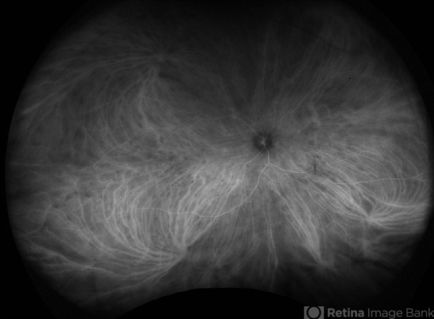
Early phase - within the first minute after injecting the eye, when larger choroidal arteries and veins are highlighted but retinal arteries are not filled.[38] Choroidal vessels start to fill from larger outer Haller’s layer vessels followed by intermediate Sattler’s layer. The choriocapillaris layer fills last but camera resolutions are not high enough to identify individual choriocapillaris. Thus, filling of choriocapillaris is usually seen as indistinct general haze, more evident in the posterior pole.
Middle phase - up to 5 to 10-15 minutes after injection. Retinal arteries and veins as well as the choroidal vasculature are filled. Choroidal cyanescence will become more diffuse and less distinct in this phase as the choriocapillaris is filled.[38]
Late or recirculation phase - more than 10-15 minutes after the dye is injected. During this phase, a pathologic hypercyanescent lesion is more visible against the slowly fading background.[38]
Safety
ICGA is a safe and well-tolerated imaging study with extremely rare toxicity and allergic reactions such as nausea, vomiting, sneezing, and pruritus in 0.15% of the procedures, moderate allergic reactions such as urticaria and pyrexia in 0.2% of the patients, and severe reactions such as bronchospasm and anaphylactic reaction in 0.05% of the patients.[39] An editorial described four reports of patients who have experienced an adverse reaction out of 240,000 indocyanine intravenous injections, one patient had urticaria and three patients had anaphylactic reactions, with one resulting in death.[7][40]
Absolute contraindications
1. Prior allergic reaction to ICG
2. Iodine/Iodide allergy: Although prior allergy to contrast dyes containing iodine or allergic reaction to shellfish are traditionally mentioned as contraindications for ICGA, such “allergic reactions” against indocyanine green are not biologically plausible. Unlike x-ray contrast media, iodine containing disinfectant solutions, or iodine containing medications such as amiodarone where iodide is bound to the main large molecule and can be identified by immune system as antigen, the ICG molecule does not contain the ion iodide and small sodium iodide molecule that is added to stabilize ICG in saline solution, are not detectable by immune system as antigens. Nevertheless, it is a common agreement to avoid ICGA if there is a history of allergy to iodine. In addition, since up to 840μg of iodide can be administered with ICGA imaging, there is a risk of thyroid storm in individuals with uncontrolled hyperthyroidism. In these situations, ICG should be used with caution or not used at all. Infracyanine green is an iodine free preparation of ICG available for those with an absolute contraindication to ICG and can be given with some modifications in the administration techniques. [38]
Relative contraindications
1. End stage renal disease
2. Liver disease
3. Pregnancy (Category C: adequate safety studies have not been conducted)[7]
Summary
ICGA is a safe and important imaging modality that offers a better view of the choroidal vasculature and related pathologies. Although it is not used as often as before in managing choroidal neovascular lesions, ICGA is still helpful in guiding the treatment of PCV and CSC and diagnosing choroidal hemangiomas and ocular inflammations primarily affecting the choroid.
References
- ↑ Fox IJ, Wood EH. Indocyanine green: physical and physiologic properties. Proc Mayo Clin 1960; 35: 732-44.
- ↑ Kogure K, David NJ, Yamanouchi U, Chromokos E. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol 1970; 83: 209-14.
- ↑ Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol 1986; 9: 37-41.
- ↑ Scheider A, Schroedel C. High resolution indocyanine green angiography with a scanning laser ophthalmoscope. Am J Ophthalmol 1989; 108: 458-9.
- ↑ Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina 1992; 12: 191-223.
- ↑ Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Owens SL. Indocyanine green angiography. Br J Ophthalmol. 1996;80(3):263-266. doi:10.1136/bjo.80.3.263
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Cohen SY, Dubois L, Quentel G, Gaudric A. Is indocyanine green angiography still relevant?. Retina. 2011;31(2):209-221. doi:10.1097/IAE.0b013e31820a69db
- ↑ Rosen RB, Hathaway M, Rogers J, et al. Simultaneous OCT/SLO/ICG imaging. Invest Ophthalmol Vis Sci 2009;50: 851–860.
- ↑ Eandi CM, Ober MD, Freund KB, Slakter JS, Yannuzzi LA. Selective photodynamic therapy for neovascular age-related macular degeneration with polypoidal choroidal neovascularization. Retina 2007;27:825–831.
- ↑ Yamashiro K, Tsujikawa A, Nishida A, Mandai M, Kurimoto Y. Recurrence of polypoidal choroidal vasculopathy after photodynamic therapy. Jpn J Ophthalmol 2008;52:457–462.
- ↑ Shields CL, Shields JA, De Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol 1995;79:237–245.
- ↑ Arevalo JF, Shields CL, Shields JA, Hykin PG, De Potter P. Circumscribed choroidal hemangioma: characteristic features with indocyanine green videoangiography. Ophthalmology 2000;107:344–350.
- ↑ Schalenbourg A, Piguet B, Zografos L. Indocyanine green angiographic findings in choroidal hemangiomas: a study of 75 cases. Ophthalmologica 2000;214:246–252.
- ↑ Piccolino FC, Borgia L, Zinicola E. Indocyanine green angiography of circumscribed choroidal hemangiomas. Retina 1996;16:19–28.
- ↑ Wen F, Wu D. Indocyanine green angiographic findings in diffuse choroidal hemangioma associated with Sturge-Weber syndrome. Graefes Arch Clin Exp Ophthalmol 2000;238: 625–627.
- ↑ Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2003;23:288–298.
- ↑ Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina 1994;14:231–242.
- ↑ Axer-Siegel R, Cotlear D, Priel E, Rosenblatt I, Snir M, Weinberger D. Indocyanine green angiography in high myopia. Ophthalmic Surg Lasers Imaging 2004;35:139–145.
- ↑ Quaranta M, Cohen SY, Krott R, Sterkers M, Soubrane G, Coscas GJ. Indocyanine green videoangiography of angioid streaks. Am J Ophthalmol 1995;119:136–142.
- ↑ Obana A, Kusumi M, Miki T. Indocyanine green angiographic aspects of multiple evanescent white dot syndrome. Retina 1996;16:97–104.
- ↑ Ie D, Glaser BM, Murphy RP, Gordon LW, Sjaarda RN, Thompson JT. Indocyanine green angiography in multiple evanescent white-dot syndrome. Am J Ophthalmol 1994;117:7–12.
- ↑ Pece A, Sadun F, Trabucchi G, Brancato R. Indocyanine green angiography in enlarged blind spot syndrome. Am J Ophthalmol 1998;126:604–607.
- ↑ Martinet V, Ducos de Lahitte G, Terrada C, Simon C, LeHoang P, Bodaghi B. Multiple evanescent white dot syndrome and acute idiopathic blind spot enlargement: angiographic and electrophysiologic findings [French]. J Fr Ophtalmol 2008;31:265–272.
- ↑ Howe LJ, Woon H, Graham EM, Fitzke F, Bhandari A, Marshall J. Choroidal hypoperfusion in acute posterior multifocal placoid pigment epitheliopathy. An indocyanine green angiography study. Ophthalmology 1995;102:790–798.
- ↑ Bouchenaki N, Herbort CP. The contribution of indocyanine green angiography to the appraisal and management of Vogt-Koyanagi-Harada disease. Ophthalmology 2001;108:54–64.
- ↑ Kawaguchi T, Horie S, Bouchenaki N, Ohno-Matsui K, Mochizuki M, Herbort CP. Suboptimal therapy controls clinically apparent disease but not subclinical progression of Vogt-Koyanagi-Harada disease. Int Ophthalmol 2010;30:41–50.
- ↑ Fardeau C, Herbort CP, Kullmann N, Quentel G, LeHoang P. Indocyanine green angiography in birdshot chorioretinopathy. Ophthalmology. 1999;106(10):1928-1934. doi:10.1016/S0161-6420(99)90403-7
- ↑ Akova YA, Kadayifcilar S, Aydin P. Assessment of choroidal involvement in sarcoidosis with indocyanine green angiography. Eye 1999;13:601–603.
- ↑ Wolfensberger TJ, Herbort CP. Indocyanine green angiographic features in ocular sarcoidosis. Ophthalmology 1999; 106:285–289
- ↑ Giovannini A, Mariotti C, Ripa E, Scassellati-Sforzolini B. Indocyanine green angiographic findings in serpiginous choroidopathy. Br J Ophthalmol 1996;80:536–540.
- ↑ Giovannini A, Ripa E, Scassellati-Sforzolini B, Ciardella A, Tom D, Yannuzzi L. Indocyanine green angiography in serpiginous choroidopathy. Eur J Ophthalmol 1996;6: 299–306.
- ↑ Nazari Khanamiri H, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58(3):203-232. doi:10.1016/j.survophthal.2012.08.008
- ↑ Kohno T, Miki T, Hayashi K. Choroidopathy after blunt trauma to the eye: a fluorescein and indocyanine green angiographic study. Am J Ophthalmol 1998;126:248–260.
- ↑ Masaoka N, Sawada K, Komatsu T, Fukushima A, Ueno H. Indocyanine green angiographic findings in 3 patients with traumatic hypotony maculopathy. Jpn J Ophthalmol 2000;44:283–289.
- ↑ Kohno T, Miki T, Shiraki K, Kano K, Hirabayashi-Matsushita M. Indocyanine green angiographic features of choroidal rupture and choroidal vascular injury after contusion ocular injury. Am J Ophthalmol 2000;129:38–46.
- ↑ Baltatzis S, Ladas ID, Panagiotidis D, Theodossiadis GP. Multiple post-traumatic choroidal ruptures obscured by hemorrhage: imaging with indocyanine green angiography. Retina 1997;17:352–354.
- ↑ Jump up to: 38.0 38.1 38.2 38.3 38.4 Gologorsky D Rosen RB. Principles of Ocular Imaging : A Comprehensive Guide for the Eye Specialist. Thorofare NJ: SLACK Incorporated; 2021. http://public.eblib.com/choice/PublicFullRecord.aspx?p=6647261. Accessed July 21 2023.
- ↑ Hope-Ross M, Yannuzzi L, Gragoudas ES, Guyer DR, Slakter FS, Sorenson JA, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994; 101: 529-33.
- ↑ Carski TR, Staller BJ, Hepner G, Banka V, Finney RA. Adverse reactions after administration of indocyanine green. JAMA 1978; 240: 635.

