Aflibercept
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Aflibercept, a humanized recombinant fusion protein, is a soluble decoy receptor that binds vascular endothelial growth factor-A (VEGF-A), VEGF-B, and placental growth factor (PIGF)[1] with a greater affinity than the body’s native receptors. It is called a decoy receptor since VEGF does not bind to its original receptors and mistakenly binds with aflibercept, thereby reducing VEGF activity.
VEGF-A is a biochemical signal protein that promotes angiogenesis throughout the body and in the eye. By decreasing VEGF-A activation of its native receptors, aflibercept reduces subsequent growth of new blood vessels.[2] [3]
Overview of VEGF and its Role in Retinal Disease
VEGF is a member of the platelet-derived growth factor (PDGF) family. The VEGF gene family consists of VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF, located on chromosome 6p12.[4] The binding of VEGF with its receptors leads to endothelial cell proliferation and new blood vessel growth and, therefore, plays a key role in angiogenesis. New blood vessel growth and development are extremely complex and coordinated processes that require a cascade of receptor activations. In this cascade, VEGF represents an initial and critical rate-limiting step in physiological angiogenesis. [5][6] [7] The critical role of VEGF in angiogenesis can be seen in the fact that a loss of a single VEGF allele can result in defective vascularization.[8]
There are nine VEGF-A isoforms: VEGF121, VEGF145, VEGF148, VEGF162, VEGF165, VEGF165b, VEGF183, VEGF189, and VEGF206. [9] The most abundant isoform found in the eye is VEGF165. [10] [11] VEGF165 is a secreted heparin-binding homodimeric 45-kDa glycoprotein with a significant fraction bound to the cell surface. [12] VEGF activates endothelial cells by binding VEGFR-1 (Flt-1) and VEGFR-2 (KDR) endothelial cell receptors, which in turn activate intracellular signal transduction cascades. [12] VEGFR-2 is thought to be principally responsible for VEGF signaling in angiogenesis.[12]
VEGF-A levels have been found to be elevated in the vitreous of patients with neovascular (wet) age-related macular degeneration (AMD),[13]diabetic macular edema, retinal vein occlusion, and myopic choroidal neovascularization. Choroidal neovascularization (CNV) in AMD may be instigated by several events, such as accumulation of lipid metabolic byproducts, oxidative stress, reduction in choriocapillaris blood flow, and alterations in Bruch’s membrane. [14] [15] [16] Hypoxia has been shown to induce VEGF gene transcription. As a response to metabolic distress, the retinal pigment epithelium (RPE) and retinal tissue produce various factors, particularly VEGF, which induce CNV proliferation. VEGF has been shown to be a chemo-attractant for endothelial cell precursors, causing CNV in mouse models. [17] VEGF also prevents endothelial cell apoptosis. [18] Additionally, VEGF promotes metalloproteinase production by endothelial cells, causing tissue degradation that facilitates invasion by new vessels. [19] [20]
VEGF is a powerful agonist of vascular permeability, which causes vascular leakage and macular edema. [21] PIGF may work synergistically with VEGF, contributing to vascular inflammation and leukocyte infiltration.[2] [3] VEGF is thought to cause increased vascular permeability by formation of fenestrations in microvascular endothelium. [22] [23] Furthermore, VEGF was shown to up-regulate leukocyte adhesion to ICAM-1 in mice, thereby promoting vascular permeability and capillary non-perfusion. [24] On this basis, inhibition of VEGF activity is central to the treatment of macular edema and prevention of progressive capillary non-perfusion, especially in diabetic retinopathy and retinal vein occlusion.
Mechanism of Action
Aflibercept is a 115 kDa fusion protein. It consists of an IgG backbone fused to extracellular VEGF receptor sequences of the human VEGFR1 and VEGFR2.[2] [3] As a soluble decoy receptor, it binds VEGF-A with a greater affinity than its natural receptors. In an experimental model, aflibercept’s equilibrium disassociation constant (Kd, inversely related to binding affinity) for VEGF-A165 was 0.49 pM, compared with 9.33 pM and 88.8 pM for experimental native VEGFR1 and VEGFR2, respectively.[2] Aflibercept’s high affinity for VEGF prevents the subsequent binding and activation of native VEGF receptors. Reduced VEGF activity leads to decreased angiogenesis and vascular permeability. [2] [3] Inhibition of PIGF and VEGF-B may also aid the treatment of angiogenic conditions.[2] PIGF has been associated with angiogenesis and can be elevated in multiple conditions, such as wet AMD.[2][25] [26] [27] [28] VEGF-B overexpression has been connected with breakdown of the blood-retinal barrier and retinal angiogenesis.[29]Thus, inhibition of VEGF-A, VEGF-B, and PIGF may all contribute to the efficacy of aflibercept.
Aflibercept has a unique binding action and binds to both sides of the VEGF dimer, forming an inert 1:1 complex, also termed a VEGF trap. Additionally, aflibercept is the only drug in its class to bind to PIGF-2.[2] [3]
Indications for Use
Ophthalmology
Intravitreal aflibercept injection (EYLEA®; Regeneron Pharmaceuticals, Inc) was approved by the FDA in 2011 for the treatment of neovascular (wet) age-related macular degeneration (AMD) following two large clinical trials.[30] [31] Since then, it has also been approved for macular edema following retinal vein occlusion (RVO), diabetic macular edema (DME), diabetic retinopathy (DR) in patients with DME, and, most recently, for retinopathy of prematurity (ROP).[30][32]
Oncology
Ziv-aflibercept (Zaltrap®, Sanofi, developed in collaboration with Regeneron Pharmaceuticals, Inc.) in combination with 5-fluorouracil, leucovorin, irinotecan-(FOLFIRI), is indicated for patients with metastatic colorectal cancer (mCRC) that is resistant to or has progressed following an oxaliplatin‑containing regimen. Ziv-aflibercept contains the same protein (active drug) as aflibercept, but is specifically formulated for injection as an intravenous infusion. Ziv-aflibercept is not intended for ophthalmic use, as the osmolarity of the ziv-aflibercept preparation is significantly higher than that of intravitreal aflibercept injection.[33] However, intravitreal ziv-aflibercept has been used with success for multiple ocular conditions with acceptable safety profile[34].
Intravitreal Aflibercept Injection: Dosing, Administration, and Preparation
The approved dose of intravitreal aflibercept injection (IAI) is 2.0mg in 0.05ml. Recommended dosing regimen and frequency vary according to disease indication:[30]
- Neovascular (Wet) Age-Related Macular Degeneration (AMD) - The recommended dose for aflibercept is 2 mg (0.05 mL or 50 microliters) administered by intravitreal injection every 4 weeks (monthly) for the first 12 weeks (3 months), followed by 2 mg (0.05 mL) via intravitreal injection once every 8 weeks (2 months).
- Macular Edema Following Retinal Vein Occlusion (RVO) - The recommended dose for aflibercept is 2 mg (0.05 mL or 50 microliters) administered by intravitreal injection once every 4 weeks (monthly)
- Diabetic Macular Edema (DME) and Diabetic Retinopathy (DR) in Patients with DME- The recommended dose for aflibercept is 2 mg (0.05 mL or 50 microliters) administered by intravitreal injection every 4 weeks (monthly) for the first 5 injections, followed by 2 mg (0.05 mL) via intravitreal injection once every 8 weeks (2 months)
- Myopic choroidal neovascularization (CNV) - The recommended dose for aflibercept is 2 mg (0.05 mL or 50 microliters) administered by intravitreal injection at baseline and then repeat once every 4 weeks (monthly) if persistence or recurrence occurs.[35]
- Retinopathy of Prematurity (ROP) - The recommended dose for aflibercept is is 0.4 mg (0.01 mL or 10 microliters of 40 mg/mL solution) administered by intravitreal injection. The treatment interval between doses injected into the same eye should be at least 10 days should the injections need to be repeated.
Aflibercept is typically given by transconjunctival intravitreal injections into the posterior segment via the pars plana.
Aflibercept is supplied as one single-use, 3-mL, sterile, glass vial designed to deliver 0.05 mL of 40 mg/mL and as a 0.05ml prefilled syringe (PFS). It should be refrigerated at 2-8 degrees C. It should not be frozen or used beyond the date on the carton and container label.[30] Injection forces have been shown to be consistently greater with aflibercept PFS than with other syringes, including tuberculin syringes[36]. Thus, ophthalmologists should limit injection speed, especially with PFS, as injection speed is associated with higher injection forces for all syringe types[37].
High dose aflibercept (EYLEA® HD), administered as an intravitreal injection of 8.0mg aflibercept in 0.07 mL (114.3 mg/mL), was approved in 2023 for wet AMD, DME, and DR.[38] For all three indications, the recommended dose for aflibercept is 8 mg (0.07 mL or 70 microliters) administered by intravitreal injection every 4 weeks (monthly) for the first 12 weeks (3 months), followed by 8 mg (0.07 mL) via intravitreal injection once every 8 to 16 weeks (2 to 4 months) for wet AMD and DME or every 8 to 12 weeks (2 to 3 months) for DR.[38] High dose aflibercept is available in a single-dose vial.
Review of Pivotal Trial Data in Ophthalmology
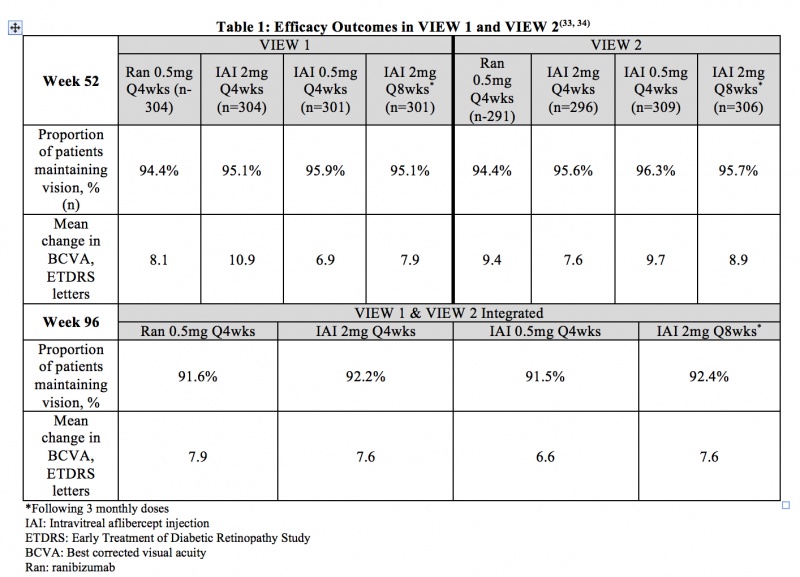
Age-related Macular Degeneration (neovascular with CNV) - VIEW 1 and VIEW 2 were two prospective, multicenter, double-masked, randomized, parallel-group, active-controlled phase 3 studies that evaluated the efficacy and safety of various doses and dosing regimens of IAI compared with ranibizumab in a non-inferiority paradigm in wet AMD. In total, 2,457 patients were randomized in the two trials. The primary endpoint was the proportion of patients who maintained vision (defined as loss of less than 15 ETDRS letters) from baseline at 52 weeks. Patients were followed for 96 weeks. Table 1 summarizes key efficacy results from VIEW 1 and VIEW 2 studies.[39] [40]
Adverse Events
Through week 96 of the VIEW 1 and VIEW 2 studies, the most frequent ocular adverse events (>10% of patients for the overall study population) were conjunctival hemorrhage, eye pain, retinal hemorrhage, and reduced visual acuity. The most frequent serious non-ocular adverse events (>1% of patients for the overall study population) were falls, pneumonia, myocardial infarction, and atrial fibrillation. The incidence of arterial thromboembolic events (ATEs) as defined by the Antiplatelet Trialists’ Collaboration (APTC) criteria was 3.2% for patients receiving ranibizumab and 3.3% for patients receiving IAI. Overall, adverse events were infrequent and occurred with similar rates across all treatment groups.[39] [40]
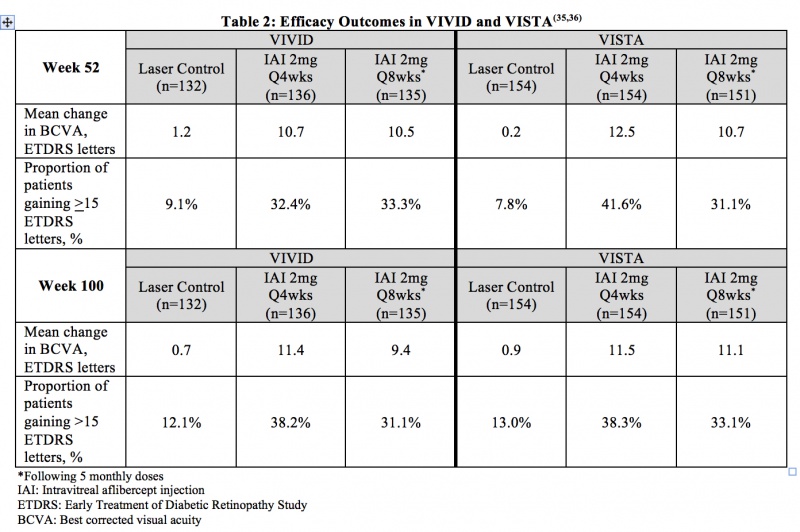
Diabetic Macular Edema (DME) – VIVID and VISTA were two prospective, multicenter, double-masked, randomized, laser-controlled phase 3 studies that evaluated the efficacy and safety of IAI compared with laser in patients with DME. In total, 872 patients were randomized in the two trials. The primary endpoint of VIVID and VISTA was the mean change from baseline of BCVA at week 52 as measured by ETDRS letter score. Patients were followed for a total of 148 weeks. Table 2 summarizes key efficacy results of the two studies.[41] [42]
Adverse Events
The most common adverse reactions, occuring in > 10% of patients receiving IAI through week 100, were conjunctival hemorrhage, cataract, and eye pain. The incidence of non-ocular SAEs was slightly higher for some events in the combined IAI group (e.g. anemia and cerebrovascular accident), and for others in the laser group (e.g. acute myocardial infarction and acute cardiac failure) with no apparent general trend. Arterial thromboembolic events as defined by APTC criteria occurred at similar rates across the IAI treatment groups and the laser control group.[41] [42]
Diabetic Retinopathy (DR) in Patients with DME – In the VIVID and VISTA trials, patients’ Diabetic Retinopathy Severity Score (DRSS) were assessed at baseline and approximately every 6 months thereafter for the duration of the study. The proportion of patients who experienced a > 2 step improvement in DRSS at 100 weeks was included as a secondary endpoint of the studies. In VIVID, 29.3% of patients improved at least 2 steps on ETDRS-DRSS from baseline with IAI 2mg q4 weeks administration and 32.6% with IAI 2 mg q8 weeks administration, compared to 8.2% in the laser control group. In VISTA, 37.1% of patients improved at least 2 steps on ETDRS-DRSS from baseline with Q4 weeks administration and 37.1% with Q8 weeks administration, compared to 15.6% in the laser control group.[42]
Macular Edema due Retinal Vein Occlusion (RVO)
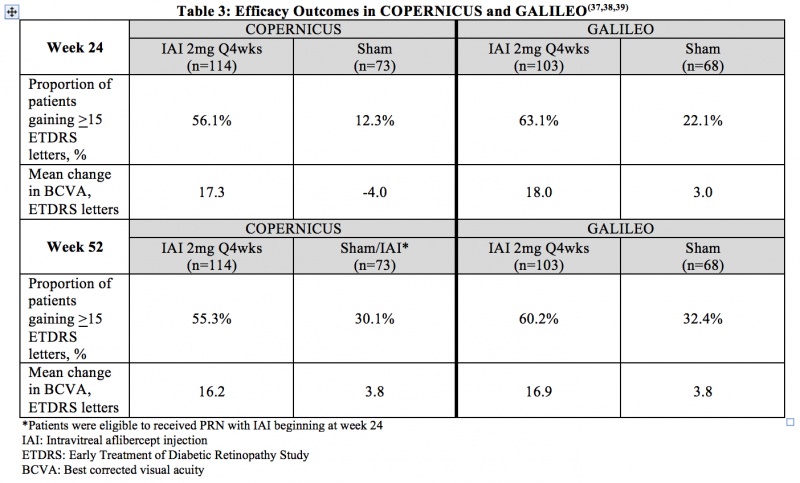
Macular edema due to Central RVO (CRVO) - COPERNICUS and GALILEO were two prospective, multicenter, double-masked, randomized, sham-controlled phase 3 studies that evaluated the efficacy and safety of monthly (2mg q4 weeks) IAI compared with sham in patients with CRVO. In total, 361 patients were randomized in the two trials. The primary endpoint was the proportion of patients who gained at least 15 letters in BCVA at week 24. Table 3 summarizes key efficacy outcomes from the two trials.[43] [44] [45]
Adverse Events
Through week 52 in COPERNICUS, the most common ocular adverse events in the IAI arm were conjunctival hemorrhage, eye pain, and maculopathy. The most common ocular adverse event in the sham/IAI arm through week 52 was increased intraocular pressure. Through week 24 in GALILEO, the most common ocular adverse events reported for IAI-treated eyes were eye pain, increased IOP, and conjunctival hemorrhage. From week 24 to week 52 in GALILEO, worsening of macular edema, increased IOP, and reduced visual acuity were the most common ocular adverse events. At week 52 in COPERNICUS, hypertension and upper respiratory tract infection were the most common non-ocular adverse events in both study arms, while in GALILEO, nasopharyngitis was the most common non-ocular adverse event at week 52 in both study arms. APTC-defined arteriothromboembolic events were rare and occurred with similar frequencies in the IAI and sham arms through week 52 in both COPERNICUS and GALILEO.[43] [44] [45]
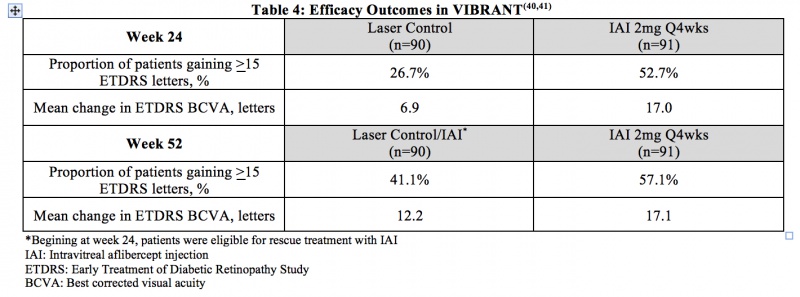
Macular Edema due to Branch RVO (BRVO) – VIBRANT was a prospective, multicenter, double-masked, randomized, laser-controlled phase 3 study that evaluated the efficacy and safety of monthly (2 mg q4 week) IAI compared with laser in patients with BRVO. In total, 183 patients were randomized. The primary endpoint of VIBRANT was the proportion of patients who gained at least 15 ETDRS letters in BCVA at week 24. Table 4 summarizes key efficacy results at week 24 and week 52 from the VIBRANT trial.[46] [47]
Adverse Events
Through week 52, the most common ocular adverse event in the IAI and laser treatment groups was conjunctival hemorrhage. The rate of non-ocular SAEs was higher in the IAI group compared to the laser control group. Pneumonia, anemia, and dehydration were the only serious adverse events that occurred in two or more patients in either study arm. In the laser control group, 2 APTC-defined arteriothromboembolic events (ATEs) occurred, and no ATEs occurred in the IAI group.[46] [47]
Myopic CNV – The MYRROR Study, was an international, multicenter, randomized, double-masked, sham controlled, phase 3 study that evaluated the efficacy of (2mg) IAI compared with sham in patients with Myopic CNV. In total, 122 patients were randomized to IAI (n=91) or sham (n=31). The primary endpoint was the mean change in BCVA from baseline to week 24 in patients with myopic CNV, where IAI patients gained 12.1 letters while sham lost 2 letters. By week 48, IAI and sham/IAI patients groups gained 13.5 and 3.9 letters.[35]
Adverse Events
The incidence of ocular adverse effects was similar in the IAI and sham/IAI groups (37.4 % vs 38.7, respectively), and most were of mild intensity and resolved within the study period. The most common adverse event was conjunctival hemorrhage (11.0 %). [35]
Retinopathy of Prematurity (ROP) - The FIREFLEYE randomized, phase 3 clinical trial was conducted across 27 countries. Infants were randomized 2:1 to receive a 0.4-mg dose of intravitreal aflibercept (n = 75) or laser photocoagulation (n = 43) at baseline for treatment requiring ROP. Aflibercept did not meet criteria for noninferiority when comparing proportion of infants achieving treatment success at week 24[48].
Adverse Events
In FIREFLEYE, the SAE rates were 13.3% (ocular) and 24.0% (systemic) in the aflibercept group compared with 7.9% and 36.8%, respectively, in the laser group. Three deaths, which occurred 4 to 9 weeks after aflibercept treatment, were considered unrelated to aflibercept[49]. No endophthalmitis or any other intraocular inflammatory events were reported. In the nonrandomized 2-year follow-up of the FIREFLEYE clinical trial, disease control was stable and visual function was appropriate in children through 2 years of age. No adverse effects on safety, including growth and neurodevelopment, were identified[50].
Warning, Safety, and Precautions in Ophthalmic Use
Three major warnings and precautions have been noted: 1) Endophthalmitis and retinal detachments have been reported, on rare occasions, after intravitreal injection, including after intravitreal aflibercept injection 2) Acute intraocular pressure elevations have been reported within 60 minutes of intravitreal injection, including with intravitreal aflibercept injection 3) Arterial thromboembolic events have been reported, on rare occasions, after intravitreal administration of VEGF inhibitors, including with intravitreal aflibercept injection. The most commonly reported adverse reactions (>5%) include conjunctival hemorrhage, eye pain, cataract, vitreous floaters, intraocular pressure increased, and vitreous detachment.[30]
Bacterial endophthalmitis has been reported following the use of all intravitreal anti-VEGF medications. Large retrospective reviews show the rate of infection with intravitreal anti-VEGF medications to be between 0.022% and 0.16%.[51]
Regarding the rates of ocular inflammation with the different vehicles of aflibercept as PFS or in a vial form, one study reported 0.3 vs 1.2 events per 10,000 units in the PFS vs vial form, respectively. Specifically, endophthalmitis was 0.1 vs. 0.6 per 10,000 units in the PFS vs. vial, respectively. Adverse events reports are rare in both presentations, being much lower in the PFS presentation. [52]
Considerations and Comparisons
Intravitreal aflibercept, bevacizumab, and ranibizumab are all commonly used to treat a variety of retinal diseases. Some key areas of comparison are listed here.
As previously noted, aflibercept is a 115 kDa soluble decoy receptor that binds VEGF-A and PIGF with a greater affinity than their natural receptors, inhibiting the subsequent binding and activation of VEGF receptors. Bevacizumab and ranibizumab, on the other hand, are a monoclonal antibody and an antibody fragment, respectively, that bind to soluble VEGF and inhibit its binding to receptors on the surface of endothelial cells.[2] [3]
Aflibercept has a unique binding action compared to bevacizumab and ranibizumab. It binds to both sides of the VEGF dimer, forming an inert 1:1 complex. Ranibizumab also binds to a single VEGF molecule, but only on one side. Bevacizumab, on the other hand, can bind to multiple VEGF molecules. Aflibercept is unique in its ability to bind to multiple VEGF-A isoforms. An in-vitro comparison of these three VEGF inhibitors showed aflibercept to have the highest affinity, by a significant margin (94 fold), for VEGF-A165. Additionally, aflibercept was the only drug to bind to PIGF-2. [2] [3]
Estimated half life based on mathematical modeling showed aflibercept to have an intravitreal half-life of 7.13 days as compared to the intravitreal half-life of ranibizumab of 4.75 days and bevacizumab of 8.25 days.[53] [54] [55]
The incidence of serious systemic adverse effects is rare with all 3 medications.[56]Specifically, the incidence of arteriothrombotic events and death have been found to be similar for all 3 medications.[57]
Aflibercept was approved for ocular use in 2011. Ranibizumab was approved in June 2006. Off-label use of the intravenous formulation of bevacizumab was compounded for the intravitreal route in the treatment of ocular disorders around mid-2005.[58] [59]
Protocol T: Comparative Effectiveness Study of Intravitreal Aflibercept, Bevacizumab, or Ranibizumab for DME[60]
Diabetic Retinopathy Clinical Research (DRCR) Network Protocol T is a two-year, randomized, multi-center clinical trial at 89 United States sites evaluating the safety and efficacy of intravitreal aflibercept, bevacizumab and ranibizumab in central-involved DME patients with visual acuity (VA) of 20/32 to 20/320. This independent study was supported by the National Institutes of Health (NIH), and was the first randomized clinical trial comparing these three anti-VEGF medications in any indication. The study enrolled 660 patients. The primary endpoint was the mean change in visual acuity from baseline at one year.[60]
The results showed that all three medications were safe and effective. At year 1 for the primary endpoint, intravitreal aflibercept demonstrated a significantly greater improvement in visual acuity compared to both bevacizumab and ranibizumab, especially in patients presenting with worse visual acuity. In this study, sub-groups based on visual acuity were prospectively delineated to be 20/40 or better and 20/50 or worse (pre-defined sub-groups). In the group presenting with 20/40 or better vision, no statistically significant difference was found between the three drugs. In the group presenting with 20/50 or worse vision, intravitreal aflibercept showed statistically significant superior visual acuity outcomes, with monthly injections. Fewer injections (one less injection over one year) and laser photocoagulation treatments were reported in the intravitreal aflibercept group. Ocular and systemic adverse events were reported to have similar frequencies for all 3 groups (no statistical significant differences in key safety metrics).[60]
Cost Comparison
A single dose of intravitreal aflibercept (~$1,850/dose) is comparable in cost to ranibizumab (~$1,950/dose for 0.5mg dose), although notably more expensive than compounded bevacizumab (~$50/dose).[61] [62][63]However, the FDA approved treatment schedule of intravitreal aflibercept in DME and AMD indicates fewer injections over time, as compared to FDA approved treatment schedules for ranibizumab.
All three medications are considered safe and effective for treatment of neovascular (wet) age-related macular degeneration (AMD), macular edema following retinal vein occlusion (RVO), diabetic macular edema (DME), and, most recently, diabetic retinopathy (DR) in patients with DME. However, aflibercept is currently the only FDA-approved agent for ROP. All three medications are widely used in the USA and throughout the globe.
The price of treatment is a constant concern for both the patient and the health care systems.. A cost-effectiveness study of aflibercept monotherapy vs bevacizumab first followed by aflibercept if needed for diabetic macular edema, using cost-effectiveness ratio and cost per quality-adjusted life-year, found that with over 2 years of treatment, the bevacizumab first group may confer substantial cost savings without sacrificing visual acuity gains.[64] Additionally, the use of biosimilars in the future could be considered as alternatives to help improve costs for the healthcare system.[65]
Acknowledgements
Original Author: Christian Swinney B.A. and Peter A Karth, M.D., M.B.A.
Additional Resources
- Turbert D, Vemulakonda GA. Anti-VEGF Treatments. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/drugs/anti-vegf-treatments-list. Accessed March 07, 2019.
- Turbert D, Vemulakonda GA. Eylea. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/drugs/eylea-list. Accessed March 07, 2019.
References
- ↑ Sharma, Yog Raj, Koushik Tripathy, Pradeep Venkatesh, and Varun Gogia. “Aflibercept – How Does It Compare with Other Anti-VEGF Drugs?” Austin J Clin Ophthalmol 1, no. 3 (2014): 1016.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171-185.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF-Trap. Br J Ophthalmol. 2008;92:667-668.
- ↑ . Pasqualetti G, Danesi R, Del Tacca M, Bocci G. Vascular endothelial growth factor pharmacogenetics: a new perspective for anti-angiogenic therapy. Pharmacogenomics. 2007;8(1):49-66.
- ↑ Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242-8.
- ↑ Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653-60.
- ↑ Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685-93.
- ↑ Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581-611.
- ↑ Bhisitkul RB. Vascular endothelial growth factor biology: clinical implications for ocular treatments. Br J Ophthalmol. 2006;90(12):1542-7.
- ↑ Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735-45.
- ↑ Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R et al. The carboxyl-terminal domain (111-165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271(13):7788-95.
- ↑ Jump up to: 12.0 12.1 12.2 Ng EWM, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40(3):352-68.
- ↑ Frank RN. Growth factors in age-related macular degeneration: pathogenic and therapeutic implications. Ophthalmic Res. 1997;29(5):341-53.
- ↑ Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(3):Suppl:1-139.
- ↑ Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100(10):1519-35.
- ↑ Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;5:27.
- ↑ Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964-72.
- ↑ Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1(10):1024-8.
- ↑ Lamoreaux WJ, Fitzgerald ME, Reiner A, Hasty KA, Charles ST. Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res. 1998;55(1):29-42.
- ↑ Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2(4):289-300.
- ↑ Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983-5.
- ↑ Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108 ( Pt 6):2369-79.
- ↑ Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57(4):765-72.
- ↑ Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol. 2000;156(5):1733-9.
- ↑ Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:rel.
- ↑ Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583.
- ↑ Witmer AN, Vrensen GF, Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29.
- ↑ Miyamoto N, Kozak Y, Jeanny JC, Glotin A, Mascarelli F, Massin P, BenEzra D, Behar-Cohen F. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–470.
- ↑ Zhong X, Huang H, Shen J, Zacchigna S, Zentilin L, Giacca M, Vinores SA. Vascular endothelial growth factor-B gene transfer exacerbates retinal and choroidal neovascularization and vasopermeability without promoting inflammation. Mol Vis. 2011;17:492–507.
- ↑ Jump up to: 30.0 30.1 30.2 30.3 30.4 EYLEA® aflibercept Injection full U.S. prescribing information. Regeneron Pharmaceuticals, Inc. December 2023.
- ↑ Semeraro F, Morescalchi F, Duse S, Parmeggiani F, Gambicorti E, Costagliola C. Aflibercept in wet AMD: specific role and optimal use. Drug Des Devel Ther. 2013;7:711-22.
- ↑ Cabrera López F. Management of aflibercept in routine clinical practice. Arch Soc Esp Oftalmol. 2015;90 Suppl 1:29-34.
- ↑ Zaltrap® (ziv-aflibercept) full U.S. prescribing information. Sanofi-aventis U.S., LLC. September 2014.
- ↑ Singh, Sumit Randhir, Michael W Stewart, Goura Chattannavar, Mohammed Ashraf, Ahmed Souka, Mazen ElDardeery, Neeraj Wadhwa, et al. “Safety of 5914 Intravitreal Ziv-Aflibercept Injections.” British Journal of Ophthalmology, August 11, 2018, bjophthalmol-2018-312453. https://doi.org/10.1136/bjophthalmol-2018-312453.
- ↑ Jump up to: 35.0 35.1 35.2 Ikuno Y, Ohno-Matsui K, Wong TY, Korobelnik JF, Vitti R, Li T, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: The MYRROR study. Ophthalmology. 2015 Jun 1;122(6):1220–7.
- ↑ Lee DJ, Scruggs BA, Sánchez E, Thomas M, Faridi A. Transient Vision Loss Associated with Prefilled Aflibercept Syringes: A Case Series and Analysis of Injection Force. Ophthalmol Sci. 2022 Jun;2(2):100115. doi: 10.1016/j.xops.2022.100115. Epub 2022 Jan 21. PMID: 36211641; PMCID: PMC9541561.
- ↑ Lee DJ, Scruggs BA, Sánchez E, Thomas M, Faridi A. Transient Vision Loss Associated with Prefilled Aflibercept Syringes: A Case Series and Analysis of Injection Force. Ophthalmol Sci. 2022 Jun;2(2):100115. doi: 10.1016/j.xops.2022.100115. Epub 2022 Jan 21. PMID: 36211641; PMCID: PMC9541561.
- ↑ Jump up to: 38.0 38.1 EYLEA® HD (aflibercept) Injection full U.S. prescribing information. Regeneron Pharmaceuticals, Inc. December 2023.
- ↑ Jump up to: 39.0 39.1 Heier JS et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-Related Macular Degeneration. Ophthalmology. 2012;119(12):2537-2548.
- ↑ Jump up to: 40.0 40.1 Schmidt-Erfurth, U. et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-Related Macular Degeneration: Ninety-six week results of the VIEW studies. Ophthalmology. 2014 121, 193–201.
- ↑ Jump up to: 41.0 41.1 Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology. 2014 Nov;121(11):2247-2254.
- ↑ Jump up to: 42.0 42.1 42.2 Brown DM, Schmidt-Erfurth U, Do DV et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100 week results from the VISTA and VIVID studies. Ophthalmology. In press. http://dx.doi.org/10.1016/j.ophtha.2015.06.017.
- ↑ Jump up to: 43.0 43.1 Boyer D et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119(5):1024-1032.
- ↑ Jump up to: 44.0 44.1 Korobelnik JF et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology. 2014;121(1):202-208.
- ↑ Jump up to: 45.0 45.1 Brown, D.M.; Heier, J.S.; Clark, W.L.; Boyer, D.S.; Vitti, R.; Berliner, A.J.; Zeitz, O.; Sandbrink, R.; Zhu, X.; Haller, J.A. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am. J. Ophthalmol. 2013, 155, 429–437.
- ↑ Jump up to: 46.0 46.1 Campochiaro PA, Clark WL, Boyer DS, Heier JS, Brown DM, Vitti R, Kazmi H, Berliner AJ, Erickson K, Chu KW, Soo Y, Cheng Y, Haller JA. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015 Mar;122(3):538-44.
- ↑ Jump up to: 47.0 47.1 Data on file. Regeneron Pharmaceuticals, Inc.
- ↑ Stahl A, Sukgen EA, Wu WC, Lepore D, Nakanishi H, Mazela J, Moshfeghi DM, Vitti R, Athanikar A, Chu K, Iveli P, Zhao F, Schmelter T, Leal S, Köfüncü E, Azuma N; FIREFLEYE Study Group. Effect of Intravitreal Aflibercept vs Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity: The FIREFLEYE Randomized Clinical Trial. JAMA. 2022 Jul 26;328(4):348-359. doi: 10.1001/jama.2022.10564. PMID: 35881122; PMCID: PMC9327573.
- ↑ Stahl A, Sukgen EA, Wu WC, Lepore D, Nakanishi H, Mazela J, Moshfeghi DM, Vitti R, Athanikar A, Chu K, Iveli P, Zhao F, Schmelter T, Leal S, Köfüncü E, Azuma N; FIREFLEYE Study Group. Effect of Intravitreal Aflibercept vs Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity: The FIREFLEYE Randomized Clinical Trial. JAMA. 2022 Jul 26;328(4):348-359. doi: 10.1001/jama.2022.10564. PMID: 35881122; PMCID: PMC9327573.
- ↑ Stahl A, Nakanishi H, Lepore D, Wu WC, Azuma N, Jacas C, Vitti R, Athanikar A, Chu K, Iveli P, Zhao F, Leal S, Schlief S, Schmelter T, Miller T, Köfüncü E, Fielder A; FIREFLEYE next Study Group. Intravitreal Aflibercept vs Laser Therapy for Retinopathy of Prematurity: Two-Year Efficacy and Safety Outcomes in the Nonrandomized Controlled Trial FIREFLEYE next. JAMA Netw Open. 2024 Apr 1;7(4):e248383. doi: 10.1001/jamanetworkopen.2024.8383. PMID: 38687481; PMCID: PMC11061767.
- ↑ Fintak DR, Shah GK, Blinder KJ, Regillo CD, Pollack J, Heier JS et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28(10):1395-9.
- ↑ Schmidt-Ott U, Fitzpatrick S, Hasanbasic Z, Leal S, Morgan-Warren P, Zhang X, et al. Reported Rates of Intraocular Inflammation with Intravitreal Aflibercept Administered via Pre-Filled Syringe or from Vials in Clinical Practice Between 2012 and 2022. Clinical Ophthalmology. 2023;17:385–90.
- ↑ Thomas M, Mousa S, and Mousa S. Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2013; 7: 495–501.
- ↑ Stewart MW. Clinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on aflibercept. Clin Ophthalmol. 2012;6:1175–1186.
- ↑ Stewart MW, Grippon S, Kirkpatrick P. Aflibercept. Nat Rev Drug Discov. 2012;11(4):269–270.
- ↑ Johnson D, Sharma S. Ocular and systemic safety of bevacizumab and ranibizumab in patients with neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2013;24(3):205-12.
- ↑ Scott LJ, Chakravarthy U, Reeves BC, Rogers CA. Systemic safety of anti-VEGF drugs: a commentary. Expert Opin Drug Saf. 2015;14(3):379-88.
- ↑ Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother. 2007;41(4):614-25.
- ↑ Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331-5.
- ↑ Jump up to: 60.0 60.1 60.2 Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-203.
- ↑ StewartM, GripponS, Kirkpatrick P. Aflibercept. Nature Reviews Drug Discovery. 2012;11:269-270.
- ↑ Martin, D. F. et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364:1897–1908.
- ↑ Kumar, Atul, Koushik Tripathy, and Rohan Chawla. “Intraocular Use of Bevacizumab in India: An Issue Resolved?” The National Medical Journal of India 30, no. 6 (December 2017): 345–47. https://doi.org/10.4103/0970-258X.239079.
- ↑ Hutton DW, Glassman Adam R, Liu D, Sun JK. Cost-effectiveness of Aflibercept Monotherapy vs Bevacizumab First Followed by Aflibercept If Needed for Diabetic Macular Edema. JAMA Ophthalmol. 2023;141(3):268–74.
- ↑ Zhang C, Friedman S, Mruthyunjaya P, Parikh R. The Biosimilar Paradox: How Anti-VEGF Biosimilars will Increase Patient and Overall Healthcare Costs. Ophthalmology. 2023