Cystoid Macular Edema
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Cystoid macular edema (CME) is caused by fluid accumulation in retinal spaces when normal homeostatic mechanisms are disrupted. Arising from a variety of sources, CME manifests with decreased visual acuity, metamorphopsia, and central scotoma and is a leading cause of central vision loss. Diagnosis involves clinical examination and imaging, and treatment options include anti-inflammatory medications, anti-VEGF agents, or surgery, depending on the underlying cause. While most cases resolve within 3-4 months, chronic CME may cause permanent damage.
Disease Entity
International Classification of Disease (ICD)
2014 ICD-9-CM 362.53 Cystoid Macular Degeneration
Disease
Cystoid macular edema (CME) refers to retinal thickening of the macula due to a disruption of the normal blood–retinal barrier, which causes leakage from the perifoveal retinal capillaries and accumulation of fluid within the intracellular spaces of the retina, primarily in the outer plexiform layer.[1] Visual loss occurs from retinal thickening and fluid collection that distorts the architecture of the photoreceptors. Cystoid macular edema is a leading cause of central vision loss in the developed world.[2]
Pathophysiology
The vitreous, retina, retinal pigment epithelium (RPE), and choroid receive their circulation through retinal and choroidal vasculature and exist in a delicate balance of homeostatic mechanisms. When in balance, the osmotic force, hydrostatic force, capillary permeability, and tissue compliance within the vasculature result in a capillary filtration rate that equals the rate of fluid removal from extracellular retinal tissue (such as glial and RPE cells).[3][4] Disruption of these normal interactions affects the retinal environment, causing an imbalance and fluid accumulation in cystoid spaces within the inner layers of the retina, most commonly the outer plexiform layer (OPL). Because the OPL exists in a watershed area between the retinal and choroidal circulation systems, it is prone to fluid accumulation—particularly in the central retina, due to its anatomical avascular zone.[5] Accumulation of fluid commonly occurs in the Henle’s fiber layer, causing a classic petaloid pattern.
Vitreomacular traction is a common cause for CME. Stress and tractional forces at the Muller cell end-feet contributes to the release of inflammatory factors (e.g., basic fibroblastic grown factor, vascular endothelial growth factor (VEGF), platelet-derived growth factor) and results in blood–retinal barrier breakdown due to separation of the retina and RPE, lysis of Muller cells, leakage, and edema.[6][7][8][9]
Diagnosis
Signs
Clinically significant foveal edema (retinal thickening more than 300 μm) can be seen as a loss of foveal reflex on slit lamp exam or direct/indirect ophthalmoscopy; visualization can be enhanced using green light to outline the cystic spaces. In contrast, subclinical foveal edema (edema less than 300 μm) is best visualized through retinal imaging.[10] Vitritis and optic nerve head swelling can also be seen with CME.
Symptoms
Symptoms include a decrease in visual acuity associated with retinal edema, loss of contrast sensitivity and color vision, metamorphopsia (demonstrated on an Amsler grid), micropsia, and central scotoma. Leakage on fluorescein angiography does not seem to correlate with a decrease in visual acuity.[11]
Risk Factors
Risk factors for CME that results in leakage on FA are described in Table 1. These can be remembered using the mnemonic DEPRIVENS: diabetes, epinephrine, pars planitis, retinitis pigmentosa (RP), Irvine-Gass, vein occlusion, nicotinic acid/niacin, and surgery.[12][13][14][15][16]
| Diabetes |
Diabetic macular edema (DME) is associated with leakage from microaneurysms and retinal capillaries causing circinate rings of hard exudates or lipoprotein deposits. The Early Treatment Diabetic Retinopathy Study (ETDRS) defines clinically significant macular edema as:
|
|---|---|
| Epinephrine |
In one study, 28% of aphakic eyes treated with epinephrine drops vs 13% of untreated aphakic eyes developed CME.[18] Cystoid macular edema resolved with cessation of epinephrine drops. |
| Pars Planitis/Uveitis |
Pars planitis involves accumulation of T-cell inflammatory mediators (e.g., interferon-gamma, interleukin-2, interleukin-10, tumor necrosis factor-α) that have been associated with CME.[19] |
| Retinitis Pigmentosa | Cystoid macular edema associated with retinitis pigmentosa (RP) may or may not involve leakage. Usually, this type of CME responds to topical dorzolamide or systemic acetazolamide. In rare cases of CME due to RP with intermediate uveitis, good response is generally seen with posterior sub-Tenon triamcinolone acetonide or oral steroid treatment.[20][21] |
| Irvine-Gass |
Irvine-Gass is an inflammatory process occurring in up to 20% of cataract extractions with IOL. Clinically significant decreases in visual acuity have been reported in 1–20% of patients, especially in more complicated surgeries, such as those involving violation of the posterior capsule. Cystoid macular edema from Irvine-Gass usually occurs 6–10 weeks postoperatively; 95% of cases resolve spontaneously within 6 months.[22][23][24][25] |
| Vein Occlusion | Both central retinal vein occlusion (CRVO) and branch retinal vein occlusion may cause CME, which usually responds well to anti-VEGF agents. |
| Nicotinic acid and Niacin | Blurry vision associated with CME has been reported in doses greater than 1.5 g/day.[26] |
| Surgery |
Various types of surgery can induce inflammation and alter the retinal blood flow. Treatment for CME prior to undergoing surgeries such as pars plana vitrectomy (PPV), laser photocoagulation, cryopexy, or glaucoma procedures can prevent acceleration or persistence of pre-existing edema.[27] |
Other forms of CME do not demonstrate leakage on FA, including CME associated with juvenile retinoschisis, Goldmann-Favre disease, certain types of RP, nicotinic acid maculopathy, phototoxicity, antimicrotubule agents, and certain other medications.
Despite being first-line agents for glaucoma, prostaglandin analogs (PGAs) continue to carry the stigma of potentially causing CME. Though this concern has led many clinicians to avoid PGAs in patients with other CME risk factors, current evidence fails to support this association. A large database study of 39,948 patients comparing the incidence of CME among patients newly prescribed common glaucoma medications found the lowest CME incidence among patients prescribed PGAs (0.13%) compared to beta-blockers (0.65%), alpha agonists (0.55%), or carbonic anhydrase inhibitors (1.76%). After adjusting for sociodemographic factors, all three other medication classes were associated with significantly higher odds of developing CME than PGAs.[28]
The persistence of this myth highlights how case reports and theoretical mechanisms can sometimes carry more weight in daily practice than larger, more rigorous studies that fail to confirm our suspicions, and the impact extends to clinical trial design and drug development. Many studies still exclude patients on PGAs from CME-risk populations, potentially limiting understanding of these medications' true safety profile. However, recent large-scale studies have shown no increased incidence of CME in patients using PGAs, even in high-risk populations, such as patients with recent intraocular surgery or a history of uveitis.[29]
Imaging
Color Fundus Photography (CFP) depicts intraretinal cysts within the foveal region of the macula in Henle's layer in a honeycomb pattern (Image 1).
Fluorescein Angiography (FA) visualizes circulation of the retina and choroid. In the early phase of FA, capillary dilation in the perifoveal region is appreciated (Image 2). In the late phase, which may take 5–15 minutes, leakage into the cystoid spaces is distributed radially in Henle’s layer forming a classic petaloid leakage pattern or expansile dot appearance (Image 3). Fundus fluorescein angiography can also show late staining of the optic disc (especially in intermediate uveitis, Irvine-Gass syndrome, or birdshot chorioretinopathy). If leakage is seen elsewhere (e.g., peripheral retina, near the optic nerve), the appearance is more honeycomb-like.[10][30][31]
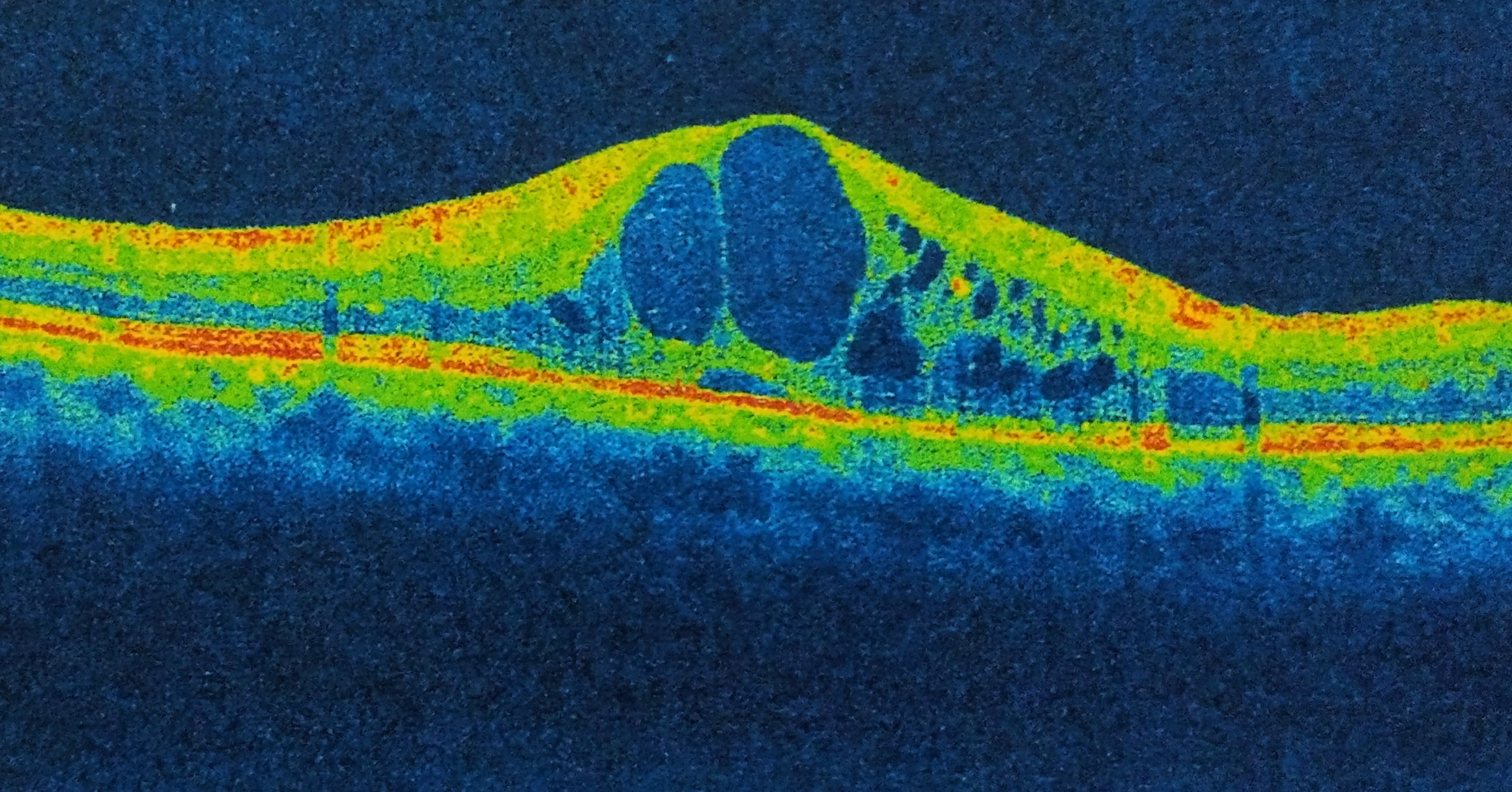
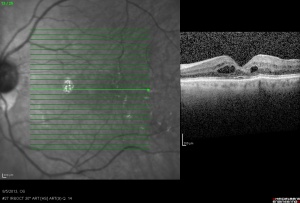
Optical Coherence Tomography (OCT) objectively obtains cross-sectional, high-resolution images of the retina. In CME, OCT can be diagnostic through measurement of retinal thickening with depiction of the intraretinal cystic areas of low reflectivity in OPL (Image 4)[32][33] and depiction of the mechanical forces induced by vitreomacular interface abnormalities (such as VMT or epiretinal membrane (ERM)) via a hyperreflective band on the inner surface of the retina.[34][35] In severe CME, subfoveal fluid may be evident on OCT.
Autofluorescence (AF) depicts the health of the RPE. Intraretinal cysts appear hyperautofluorescent.
Retinal Thickness Analyzer (RTA) generates a wide 3D map of the retina.
Management
General treatment
Therapeutic approaches, whether medical or surgical, are dependent on the underlying etiology. Most cases are self-limiting and resolve within 3–4 months. If CME persists, then medical or surgical therapy is warranted.
Medical Therapy[24]
NSAIDS — Topical or systemic indomethacin inhibits cyclooxygenase enzyme, decreasing the production of prostaglandins. Ketorolac tromethamine (0.5%), indomethacin (1%), nepafenac (0.1%), bromfenac (0.07%), and diclofenac (1%) have been used postoperatively for aphakic or pseudophakic CME.[36][37]
Corticosteroids — Topical, periocular, systemic, intravitreal, or implanted corticosteroids are used to inhibit phospholipase A2, consequently inhibiting prostaglandin and leukotriene production, and are particularly helpful in managing uveitic macular edema. Triamcinolone acetonide can be administered through various routes (intravitreal, sub-Tenon, peribulbar/trans-septal/orbital floor). Intravitreal triamcinolone reduces fluid accumulation by stimulating endogenous adenosine signaling in Muller cells and decreasing VEGF production.[38][39] Several corticosteroid-based intravitreal options are available, including a biodegradable dexamethasone implant, a helical triamcinolone acetonide implant, a fluocinolone acetonide implant, and injectable fluocinolone acetonide. Well-known side effects of steroid injection include glaucoma and cataract formation.[40]
Carbonic Anhydrase Inhibitors — Carbonic anhydrase inhibitors (CAIs) alter the polarity of the ionic transport systems in the RPE, moving fluid away from the intracellular spaces.[41] These agents are helpful in paclitaxel-, docetaxel-, and RP-induced CME.[42][43][44]
Anti-VEGF Agents — Pegaptanib (anti-VEGF 165 RNA aptamer), ranibizumab (antibody fragment), and bevacizumab (full antibody) act by decreasing vascular permeability from disrupted endothelial cells. Marked reduction in retinal thickness and fluid accumulation has been noted with anti-VEGF agent use, with significant improvement in visual acuity and minimal side effects.[45][46][47][48][49]
Pharmacologic Vitreolysis Agents — Chondroitinase, dispase, hyaluronidase, plasmin, and microplasmin can induce posterior vitreous detachment to relieve CME due to vitreomacular traction (VMT).[50][51][52][53] Ocriplasmin (formerly microplasmin) was an initially promising proteolytic enzyme used for pharmacologic vitreolysis, with phase 2/3 trials indicating that it could induce VMT release in 58% of patients within 1 month of injection and resolve VMT in some cases when injected 7 days prior to vitrectomy while also providing subjective visual dysfunction improvement.[54][55][56][57][58]
Surgery
Pars plana vitrectomy (PPV) can help to relieve macula edema due to tractional or nontractional components, especially when refractory to medical therapy.
Tractional components can be addressed by releasing the posterior hyaloid in VMT or by conducting an internal limiting membrane (ILM) peel of an epiretinal membrane. Pars plana vitrectomy to relieve the VMT that contributes to CME secondary to diabetes has been shown to improve macular edema in 80–92% of patients.[59][60] The Vitrectomy-Aphakic-Cystoid Macular Edema Study, involving patients with chronic aphakic CME, showed statistically significant improvement in visual outcomes following vitrectomy, and Harbour et al. demonstrated that vitrectomy performed to address vitreous incarceration in the anterior segment and pseudophakic CME led to improvement in visual acuity in all patients.[61][62] While ILM peeling has resulted in anatomic improvement for CME secondary to diabetes, central retinal vein occlusion, uveitic macular edema, and RP, corresponding visual acuity results are inconclusive.[63][64][65][66][67][68][69][70][71][72][73][74] Neodymium yttrium aluminum garnet (Nd:YAG) laser surgery can also help to relieve tractional components, such as vitreous adhesions to iris.
Nontractional components are theoretically addressed by clearing the inflammatory factors during PPV.[75][76] According to one study, high vitreous levels of VEGF in patients with central retinal vein occlusion correlated with decreased improvement in visual acuity after vitrectomy, suggesting that high VEGF levels may be associated with ischemia and permanent photoreceptor damage.[77] However, another study found that an increase in VEGF levels in patients with branch retinal vein occlusion correlated with an improved post-vitrectomy visual outcomes.[78] Furthermore, studies have shown that oxygen in the posterior segment and the rate of oxygen exchange in the vitreal cavity increase after PPV.[79][80][81][82][83][84] Overall, PPV performed for nontractional components of CME (e.g., secondary to diabetes and uveitic macular edema) has shown inconclusive results regarding improvement in visual acuity.[85][86][87][88][89][90] Pendergast et al. demonstrated that vitrectomy resulted in improved visual acuity for pseudophakic CME without any tractional component.[91]
Side effects of vitrectomy include cataract, retinal detachment, vitreous hemorrhage, and elevated IOP.
Prognosis
Cystoid macular edema is usually self-limiting and spontaneously resolves within 3-4 months. Depending on the etiology, medical or surgical options may help with resolution. If the edema is chronic (more than 6–9 months), permanent damage to the photoreceptors with retinal thinning and fibrosis can occur.[92][93][94][95]
Additional Resources
- Porter D, Vemulakonda GA. "What Is Macular Edema?" American Academy of Ophthalmology. EyeSmart/Eye Health. https://www.aao.org/eye-health/diseases/what-is-macular-edema. Accessed March 17, 2023.
References
- ↑ Gass JD, Norton EW. Follow-up study of cystoid macular edema following cataract extraction.Trans Am Acad Ophthalmol Otolaryngol. 1969;73:665-682.
- ↑ Hogan P et al. American Diabetes Association: Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917-932.
- ↑ Scholl S et al. Pathophysiology of macular edema. Ophthalmologica. 2010;224:8-15.
- ↑ Bringmann A et al. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36:241-249.
- ↑ Scholl S et al. General pathophysiology of macular edema. Eur J Ophthalmol. 2010;21:10-19.
- ↑ Jampol LM. Niacin maculopathy. Ophthalmology. 1988;95:1704-1705.
- ↑ Lindqvist N et al. Retinal glial (Muller) cells: sensing and responding to tissue stretch. Invest Ophthalmol Vis Sci. 2010;51:1683-1690.
- ↑ Augustin A et al. Macular edema. General pathophysiology. Dev Ophthalmol. 2010;47:10-26.
- ↑ Praidou A et al. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009;34:152-161.
- ↑ 10.0 10.1 Staurenghi G et al. Macular edema. Diagnosis and detection. Dev Ophthalmol. 2010;47:27-48.
- ↑ Nussenblatt RB et al. Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology. 1987;94:1134-1139.
- ↑ Kearns TP, Hollenhurst RW. Venous-stasis retinopathy of occlusive disease of the carotid artery. Mayo Clin Proc. 1963;38:304-312.
- ↑ Sturrock GD, Mueller HR. Chronic ocular ischaemia. Br J Ophthalmol. 1990;13:187-191.
- ↑ Sharma S, Brown GC. Ocular ischemic syndrome. In: Ryan SJ, ed. Retina. 4th ed. St Louis, MO: Mosby;2005:1483-1502.
- ↑ Sivalingam A et al. The ocular ischemic syndrome. II. Mortality and systemic morbidity. Int Ophthalmol.1989;13:187-191.
- ↑ Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. 2011;31(1):4-12. Review.
- ↑ Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report no 1. Arch Ophthalmol. 1985;103:1796–1806.
- ↑ Thomas JV et al. Correlation of epinephrine use and macular edema in aphakic glaucomatous eyes. Arch Ophthalmol. 1978;96:625-628.
- ↑ Wakefield D, Lloyd A. The role of cytokines in the pathogenesis of inflammatory eye disease. Cytokine. 1992;4:1–5.
- ↑ Tripathy, Koushik, Rohan Chawla, Pradeep Venkatesh, Rajpal Vohra, Yog Raj Sharma, Varun Gogia, Shreyans Jain, and Alkananda Behera. “Ultra-Wide Field Fluorescein Angiography in Retinitis Pigmentosa with Intermediate Uveitis.” Journal of Ophthalmic & Vision Research 11, no. 2 (June 2016): 237–39. https://doi.org/10.4103/2008-322X.183929.
- ↑ Tripathy, Koushik. “Cystoid Macular Edema in Retinitis Pigmentosa with Intermediate Uveitis Responded Well to Oral and Posterior Subtenon Steroid.” Seminars in Ophthalmology, March 29, 2017, 1–2. https://doi.org/10.1080/08820538.2017.1303521.
- ↑ Wright PL et al. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol. 1988;106:740-744.
- ↑ Ursell PG et al. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999;25:1492-1497.
- ↑ 24.0 24.1 Ray S, D’Amico DJ. Pseudophakic cystoid macular edema. Semin Ophthalmol. 2002;17:167-180.
- ↑ Bradford JD et al. Cystoid macular edema following extracapsular cataract extraction and posterior chamber intraocular lens implantation. Retina. 1988;8:161–164.
- ↑ Fraunfelder FW et al. Adverse ocular effects associated with niacin therapy. Br J Ophthalmol. 1995;79:54-56.
- ↑ Dowler JG et al. The natural history of macular edema after cataract surgery in diabetes. Ophthalmology. 1999;106:663–668.
- ↑ Zhou Y et al. SOURCE Consortium. Incidence of Acute Cystoid Macular Edema after Starting a Prostaglandin Analog Compared with Other Classes of Glaucoma Medications. Ophthalmol Glaucoma. Published online August 8, 2024. doi:10.1016/j.ogla.2024.07.010
- ↑ Niyadurupola N et al. Topical prostaglandin analogue use and cystoid macular oedema following uneventful cataract surgery: a randomised control trial. Br J Ophthalmol. 2022;106(12):1662-1666. doi:10.1136/bjophthalmol-2021-319149
- ↑ Shetty N. Cystoid macular edema. Atlas of fundus fluorescein angiography. New York, Informa Healthcare. 2004;130–4.
- ↑ Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142:405-412.
- ↑ Mavrofrides EC, Cruz-Villegas V, Puliafito CA. Miscellaneous retinal diseases. In: Schuman JS, Puliafito CA, Fujimoto JG, eds. Optical coherence tomography of ocular diseases. Thorofare, NJ: SLACK, Inc. 2004:457-482.
- ↑ Kim SJ, Bressler NM. Optical coherence tomography and cataract surgery. Curr Opin Ophthalmol. 2009;20(1):46-51. Review.
- ↑ Baskin DE. Optical coherence tomography in diabetic macular edema. Curr Opin Ophthalmol. 2010;21:172-177.
- ↑ Williamson TH, Grewal J, Gupta B, et al. Measurement of PO2 during vitrectomy for central retinal vein occlusion, a pilot study. Graefes Arch Clin Exp Ophthalmol. 2009;247:1019-1023.
- ↑ Flach AJ et al. Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. Am J Ophthalmol. 1991;112:514-519.
- ↑ Burnett J et al. Double-masked trial of fenoprofen sodium: treatment of chronic aphakic cystoid macular edema. Ophthalmic Surg. 1983;14:150–152.
- ↑ Reichenbach A et al. Muller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:627-636.
- ↑ Zhang X et al. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57:1026-1033.
- ↑ Loewenstein A, Goldstein M. Intravitreal triamcinolone acetonide for diabetic macula edema. Isr Med Assoc J. 2006;8:426–427.
- ↑ Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106:1190-1195.
- ↑ Telender DG, Sarraf D. Cystoid macular edema with Docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22:151-153.
- ↑ Joshi MM, Garretson B. Paclitaxel retinopathy. Arch Ophthalmol. 2007;125:709-710.
- ↑ Fishman GA et al. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol. 1989;107:1445–1452.
- ↑ Iturralde D et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279–284.
- ↑ Moschos MM, Moschos MN. Intraocular bevacizumab for macular edema due to CRVO. A multlifocal-ERG and OCT study. Doc Ophthalmol. 2008;116:147–152.
- ↑ Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol. 2008;2(4):919-930.
- ↑ Falavarjani KG et al. Intravitreal bevacizumab for pseudophakic cystoid macular edema: A systematic review. J Ophthalmic Vis Res. 2012;7(3):235-239.
- ↑ Waheed NK, Duker JS. OCT in the management of diabetic macular edema. Curr Ophthalmol Rep. 2013;1:128-133.
- ↑ Gandorfer A et al. Plasmin-assisted vitrectomy eliminates cortical vitreous remnants. Eye (Lond). 2002;16:95-97.
- ↑ Sakuma T et al. Intravitreal injection of autologous plasmin enzyme for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2010;150:876-882.
- ↑ Udaondo P et al. Intravitreal plasmin without vitrectomy for macular edema secondary to branch retinal vein occlusion. Arch Ophthalmol. 2011;129:283-287.
- ↑ Sakuma T et al. Use of autologous plasmin during vitrectomy for diabetic maculopathy. Eur J Ophthalmol. 2006;16:138-140.
- ↑ Rheaume MA, Vavvas D. Pharmacologic vitreolysis. Semin Ophthalmol. 2010;25:295-302.
- ↑ de Smet MD, Gandorfer A, Stalmans P, et al. Microplasmin intravitreal administration in patients with vitreomacular traction scheduled for vitrectomy: the MIVI I trial. Ophthalmology. 2009;116:1349-1355.
- ↑ Stalmans P, Delaey C, de Smet MD, et al. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham-controlled phase II trial (the MIVI-IIT trial). Retina. 2010;30:1122-1127.
- ↑ Benz MS, Packo KH, Gonzalez V, et al. A placebo-controlled trial of microplasmin intravitreous injection to facilitate posterior vitreous detachment before vitrectomy. Ophthalmology. 2010;117:791-797.
- ↑ Dugel PU, Group M-TS. A single injection of microplasmic for the treatment of symptomatic vitreomacular adhesion (sVMA): results of the Phase III MIVI-TRUST Program [abstract]. Invest Ophthalmol Vis Sci (suppl.). 2011;52:6628.
- ↑ Lewis H et al. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753-759.
- ↑ Pendergast SD et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178-186.
- ↑ Fung WE. Vitrectomy for chronic aphakic cystoid macular edema. Results of a national, collaborative, prospective, randomized investigation. Ophthalmology. 1985;92:1102–1111.
- ↑ Harbour JW et al. Pars plana vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol. 1995;120:302-307.
- ↑ Stolba U et al. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140:295-301.
- ↑ Stefaniotou M et al. Vitrectomy results for diffuse diabetic macular edema with and without inner limiting membrane removal. Eur J Ophthalmol. 2004;14:137-143.
- ↑ Bahadir M et al. Visual acuity comparison of vitrectomy with and without internal limiting membrane removal in the treatment of diabetic macular edema. Int Ophthalmol. 2005;26:3-8.
- ↑ Patel JI et al. Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina. 2006;26:5-13.
- ↑ Kumar A et al. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:360-368.
- ↑ Gentile RC et al. Taut internal limiting membrane causing diffuse diabetic macular edema after vitrectomy: clinicopathological correlation. Ophthalmologica. 2011;226:64-70.
- ↑ Hoerauf H et al. Pars plana vitrectomy for diabetic macular edema. Internal limiting membrane delamination vs posterior hyaloid removal. A prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2011;249:997-1008.
- ↑ DeCroos FC et al. Pars plana vitrectomy, internal limiting membrane peeling, and panretinal endophotocoagulation for macular edema secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147:627-633.
- ↑ Schaal S et al. Surgical intervention in refractory CME – role of posterior hyaloid separation and internal limiting membrane peeling. Ocul Immunol Inflamm. 2008;16:209-210.
- ↑ Gutfleisch M et al. Pars plana vitrectomy with intravitreal triamcinolone: effect on uveitic cystoid macular oedema and treatment limitations. Br J Ophthalmol. 2007;91:345-348.
- ↑ Garcia-Arumi J et al. Vitreoretinal surgery for cystoid macular edema associated with retinitis pigmentosa. Ophthalmology. 2003;110:1164-1169.
- ↑ Hagiwara A et al. Macular abnormalities in patients with retinitis pigmentosa: prevalence on OCT examination and outcomes of vitreoretinal surgery. Acta Ophthalmol. 2011;89:122-125.
- ↑ Noma H et al. Pigment epithelium-derived factor and vascular endothelial growth factor in branch retinal vein occlusion with macular edema. Graefes Arch Clin Exp Ophthalmol. 2010;248:1559-1565.
- ↑ Matsunaga N et al. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy. Ophthalmology. 2008;115:1916-1922.
- ↑ Noma H et al. Visual acuity and foveal thickness after vitrectomy for macular edema. Ophthalmologica. 2010;224:367-373.
- ↑ Yamasaki M et al. Changes in foveal thickness after vitrectomy for macular edema with branch retinal vein occlusion and intravitreal vascular endothelial growth factor. Int Ophthalmol. 2009;29:161-167.
- ↑ Williamson TH et al. Measurement of PO2 during vitrectomy for central retinal vein occlusion, a pilot study. Graefes Arch Clin Exp Ophthalmol. 2009;247:1019-1023.
- ↑ Stefansson E et al. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31:284-289.
- ↑ Holekamp NM et al. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302-310.
- ↑ Siegfried CJ et al. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51:5731-5738.
- ↑ Giblin FJ et al. Enzyme-induced posterior vitreous detachment in the rat produces increased lens nuclear pO2 levels. Exp Eye Res. 2009;88:286-292.
- ↑ Quiram PA et al. Microplasmin-induced posterior vitreous detachment affects vitreous oxygen levels. Retina. 2007;27:1090-1096.
- ↑ Massin P et al. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169-177.
- ↑ Bandello F et al. Diabetic macular edema. Dev Ophthalmol. 2010;47:73-110.
- ↑ Haller JA et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087-1093.
- ↑ Kiryu J et al. Pars plana vitrectomy for cystoid macular edema secondary to sarcoid uveitis. Ophthalmology. 2001;108:1140-1144.
- ↑ Wiechens B et al. Pars plana vitrectomy in cystoid macular edema of different forms of chronic uveitis. Ophthalmology. 2003;100:33-43.
- ↑ Stavrou P et al. Pars plana vitrectomy in patients with intermediate uveitis. Ocul Immunol Inflamm. 2001;9:141-151.
- ↑ Pendergast SD et al. Vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol. 1999;128:317-323.
- ↑ Guex-Crosier Y. The pathogenesis and clinical presentation of macular edema in inflammatory diseases. Doc Ophthalmol. 1999;97:297–309.
- ↑ Henderly DE et al. Pars planitis. Trans Ophthalmol Soc UK. 1986;105:227–232.
- ↑ Henderly DE et al. The significance of the pars plana exudates in pars planitis. Am J Ophthalmol. 1987;103:669–671.
- ↑ Agarwal A. Gass’ Atlas of Macular Diseases. 5th ed. Waltham, MA: Elsevier Inc;2012:500-508.