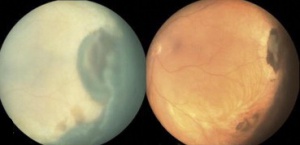
Peripheral Exudative Hemorrhagic Chorioretinopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Peripheral exudative hemorrhagic chorioretinopathy (PEHCR) is an uncommon degenerative process of the retina with sub-retinal or sub-pigment epithelium hemorrhage and exudative mass outside of the macular region. PEHCR can mimic choroidal mass or uveal melanoma. Of all "pseudomelanomas" (entities that mimic melanoma), this little-known retinal disease is in fact, the second most common only after choroidal nevus[1] or after vasoproliferative tumor[2], depending on which case series is considered. Awareness of PEHCR is important because the management and prognosis of PEHCR is vastly different from that of melanoma and other retinal disorders that may be on the differential.
Disease and History
The first published description of PEHCR was by Reese and Jones in 1962[3] in which hemorrhagic choroidal lesions were seen peripheral to the macula in elderly patients thought to have age-related macular degeneration. With a number of additional case series that followed, including that of Annesley in 1980 with 27 patients[4] and of Shields with 146 patients[5], it has become clear that PEHCR is a retinal degeneration seen largely Caucasian females with bilateral occurrence in about 30% of those affected. According to Mirshahi et al[6], Mantel et al[7], and Shields et al[5]., the mean age of the patients who present with PEHCR ranges between 77 and 83. Ninety-nine percent of the patient population consisted of Caucasians in the largest case series published (by JA Shields and CL Shields) with 146 patients[5], although PEHCR has been reported in Asian and Black patients as well[8][4]. The incidence PEHCR is unknown but is presumed to be low. The low rate of incidence is thought to be at least partially due to the non-standardized naming of this little known disease entity. Terms considered to refer to PEHCR include massive spontaneous retinal hemorrhage[9], eccentric disciform retinal degeneration, extramacular disciform degeneration[10], and peripheral age-related retinal degeneration. The far-peripheral location of these lesions and the absence of symptoms in many of the affected patients also contribute to the under-diagnosis of this entity.
Risk Factors and Associations
Age
With the mean age of the patients presenting with PEHCR ranging between 77-83, age is considered to be a risk factor. One author even suggests exclusion of PEHCR from the differential for patients under the age of 60[7].
Hypertension
One systemic disease found consistently among many of the patients with PEHCR is hypertension. Shields et al reported the rate of systemic HTN at 51% of their 146 patients with PEHCR[5]; 55% of 40 patients in Mantel's 2009 case series[7], 44.4% out of 27 patients in the case series by Annesley, 100% of 8 patients by Seibel et al.[11], and 3 out of 4 patients in the Korean case series[8] to name a few of published case series to date. To place this in context, hypertension is seen in about 1 out of 3 adults in the US[12].
Anti-coagulation and Anti-Platelet
The use of systemic anticoagulation or anti-platelet agents is also prevalent in the PEHCR population. Of the affected patients, use of these pharmacologic agents ranges between 61% (Mantel 2012[13]) and 73% (Shields[5]), although in the 1980 case series by Annesley, only 7.4% of the 27 patients were on anticoagulation dicumarol (no longer in use) . One may conjecture that PEHCR arises not from the use of anticoagulant or anti-platelet agents but in association with the indication for use of these pharmacologic agents. Recommendation for the use of anti-platelet and anti-coagulant agents has been modified over the past several decades for both primary and secondary prevention of cardiac and cerebral events.
ARMD/PCV
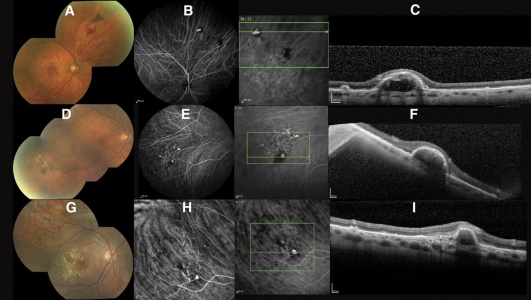
The relationship between PEHCR and age-related macular degeneration(AMRD)/polypoidal choroidal vasculopathy(PCV) is controversial and unclear but gradually being elucidated. The co-existence of PEHCR with ARMD/PCV had been observed since early on though with significant range of prevalence. Annesley reports in his 1980 case series of 27 patients that two patients (7.4%) had pre-existing visual impairment due to macular degeneration. In the largest case series by Shields et al[5], macular drusen, macular RPE alterations, and macular CNV was seen in 17%, 23%, and 8% of the ipsilateral eyes and, with similar proportions, in the contralateral eyes. In contrast, Mantel in his 2009 case series[7] reports the prevalence of ARMD among his cohort of 40 patients to be close to 70%. It is without argument that PEHCR shares risk factors/associations in common with ARMD (age of affected patients, prevalence of HTN among patients, and female predominance), and authors like Tsui et al[15] may claim that PEHCR is a peripheral counterpart of ARMD, many retina specialists make arguments against such relationship. For example, ARMD-like macular changes are absent in many eyes with PEHCR; Drusen--hallmark of ARMD--are absent around the lesions of PEHCR, and PEHCR lesions tend to resolve rather than progress like ARMD.
The argument that PEHCR may be a variant of PCV was first supported extensively in Mantel's 2012 case series of 40 patients. The series was followed by a series of 8 patients by Sarraf et al who report a causative relationship between PCV and a subtype PEHCR in which peripheral polyps are observed in OCT, FA, and ICG-A[14]. In a 2013 case of PEHCR by Mashayekshi and Shields[16], the authors report a large hemorrhagic lesion that grew out of PEDs associated with polyps and took on the typical appearance of PEHCR. They conclude that PCV may underlie the development of PEHCR in some cases, similar to what was suggested by Sarraf et al[14] [17].
At this point there is no histologic evidence to support the causative relationship between PCV and ARMD, but the hypothetical relationship is evidenced by multiple case reports and series that utilize high-definition imaging modalities as mentioned above.
Clinical Features
Symptoms
Although patients often present without symptoms, the most common symptoms are, in order of decreasing frequency, visual loss/decrease, floaters, photopsia, metamorphopsia, and rarely pain. Pain is thought to be due to a rare complication involving angle-closure that arises from massive peripheral hemorrhage under the RPE[9]. Between 48% and 51% of the patients in the series by Mantel (2009)[7] and Shields[5] had BCVA between 20/20-20/40, although about 20% of the patients in each series had 20/200 or worse due to vitreous hemorrhage, subretinal hemorrhage, subretinal fluid, or lipid exudation.
Exam findings
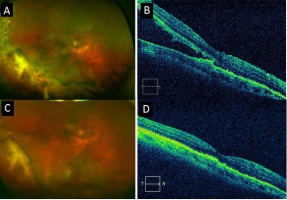
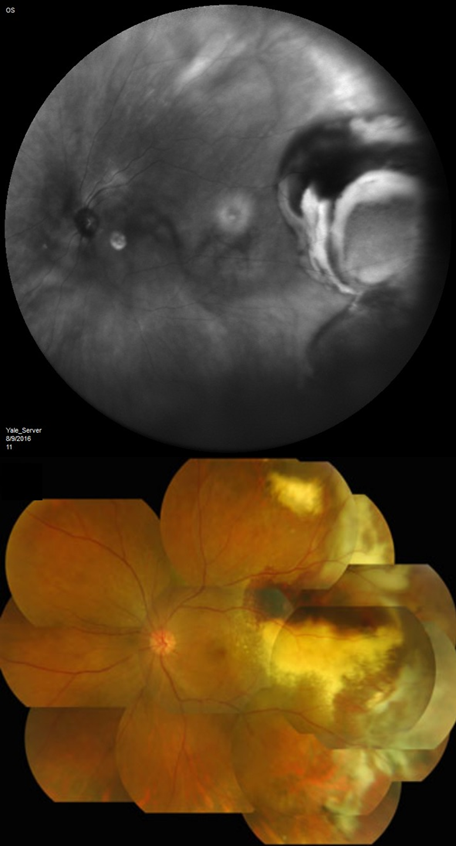
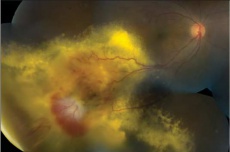
Mean basal dimension of PEHCR lesions average 10.1mm. In all case series published to date, the lesions are found most commonly in the temporal quadrant (in greater than 75% of cases)[4][5][13], specifically in the infero-temporal quadrant and between the equator and the ora serrata. Nasal lesions are associated with bilateral involvement and almost always co-exist with temporal lesions either as an extension of a large temporal lesion or as distinct lesions. The common location of the PEHCR lesions is in contrast to the common location of choroidal melanoma lesions, which in more than 80% of cases, are located at the macula or between the macula and the equator according to a case series of more than 8,000 uveal melanomas by Shields et al[19].
PEHCR fundus findings include subretinal hemorrhage, sub-RPE hemorrhage or fluid, RPE detachment or RPE tear, subretinal fibrosis, lipid exudation, and chronic changes such as RPE hyperplasia/atrophy which may suggest prior, undocumented, asymptomatic lesion that has since resolved. Exudation and vitreous hemorrhage can lead to decrease in visual acuity.
OCT
Optical coherence tomography (OCT) is useful not necessarily to assess the PEHCR lesion itself, which is often too far in the periphery to be captured on OCT, but to assess the extent of exudation to the fovea. Progressive choroidal thickness may be observed towards the temporal periphery. [20]When a PEHCR patient without associated vitreous hemorrhage presents with decreased visual acuity, OCT may reveal the presence of subretinal fluid that may explain the vision[18]. Some practitioners advocate for the use of anti-VEGF with or without laser photocoagulation or cryotherapy based on the OCT findings. See management for more details.
Ultrasound
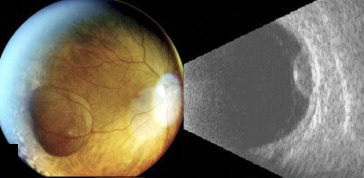
B- and A-scans provide important clinical information about the lesions both for identification and for exclusion of differentials. Ultrasonographic form and internal features should be noted.
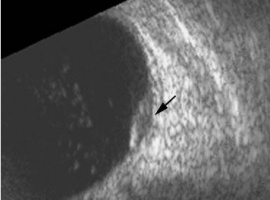
Ultrasonographic Form
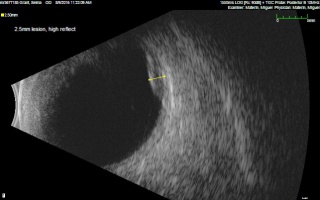
In the case series of 56 eyes with PEHCR by Mantel et al.[7], about half of the lesions were dome-shaped and the other half multi-lobar on the B scan. "Plateau-shaped elevation lesions" were described by Shields et al[5], and the term may describe lesions lesions with a large base diameter. Highly irregular lesions were also observed in a small minority (3.6%) of the patients per Mantel et al although in none of the eyes reviewed by Shields et al.
The average thickness of the lesions is between 2mm and 3mm in the series by Mantel et al and Shields et al, ranging from 1.0 to 9.0mm. The basal dimension ranged from 10mm to 25mm with average of 10.1mm in the series by Shields et al[5] with no evidence of choroidal excavation. This is a subtle diagnostic feature that separates PEHCR from choroidal melanoma.
Serial measurement of the basal diameter and elevation of the lesion is useful in confirming regression or stability of the lesion.
Ultrasonographic Features
The features of the lesions range between hollow, solid, intermediate, and irregular in acoustic quality, which may correspond to the clinical and/or angiographic evidence of hemorrhagic PED vs. serous pigment epithelium detachment. Shields et al[5] commented that 100% of the eyes in their series of 173 eyes lacked intrinsic vascular pulsation, which is important in differentiating the lesion from choroidal melanoma. Another distinguishing feature of PEHCR is the presence of clot "retraction cleft" that separates the sub-retinal or sub-RPE clot from the underlying choroid visible on ultrasonography. Retraction cleft is not seen with melanomas.
Internal Reflectivity
There is no classic feature of PEHCR on A-scan. Of the 130 eyes that had A-scan data from the series by Shields et al[5]., internal reflectivity of the lesions ranged between low, intermediate, and high (41%, 36, and 19% respectively) depending on the chronicity and density of hemorrhage. Note that melanomas classically have low to medium internal reflectivity on A-scan[1].
Angiography
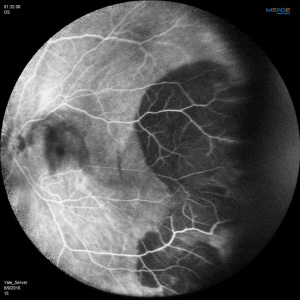
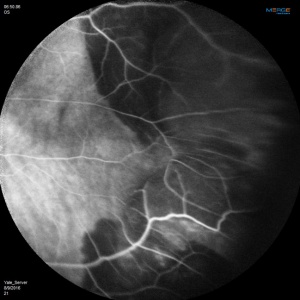
Angiographic studies should be performed when possible. Technical limitations may be incurred by media opacification from vitreous hemorrhage or lesions too far in the periphery. The use of Optos Ultra-Widefield imaging system or scanning laser ophthalmoscope (SLO) Heidelberg Retina Angiograph (HRA)[22] may circumvent the challenges of imaging lesions in the far periphery.
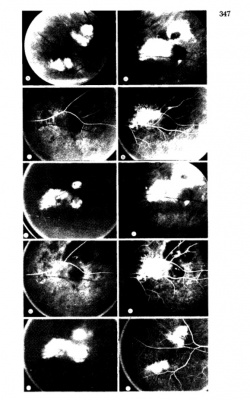
Fluorescein Angiographic Findings
Blockage of choroidal fluorescence related to hemorrhage (sub-retinal, sub-RPE) and window defect from peripheral atrophic RPE changes are the most common FA findings. Other findings include diffuse peripheral hyper- or hypofluorescence that correlate to RPE atrophy or hyperplasia. Choroidal neovascularization is seen on rare occasions (in 2 out of 173 eyes in the case series by Shields[5] and in series by Bec et al[23]), but even in eyes without choroidal neovascularization, ICG angiography may reveal abnormal choroidal neovascular network. Instances of abnormal retinal circulation--peripheral patches of nonperfusion, ischemic hemi-central vein occlusion, and delayed retinal filling--were seen in a few patients in Mantel's 2012 case series[13] but without clear significance or relevance.
ICG Angiographic Findings
In Mantel's 2009 series of 56 eyes with PEHCR[7], 20 eyes underwent ICG-A, and among them, 6 (30%) had pathologic choroidal vascular networks that "resembled those observed in polypoidal choroidal vasculopathy" (none of them had associated FA findings of neovascularization). Late hyperfluorescence was seen in 12 eyes, and in two eyes hypofluorescence resulting from hemorrhage-related blockage was seen. In Mantel's 2012 case series of 48 eyes in 40 patients[13], the proportion of eyes with pathologic choroidal vascular network was 50% (24 eyes, again none of the eyes had CNV seen on FA), albeit the ICG-A system in use was different in 2012 series compared to 2009 case series. Additionally, peripheral poly-like choroidal telangiectases were seen in nearly 70% of the eyes along with dilated choroidal veins.
Mantel claims that his angiographic findings objectively establish commonalities between PEHCR and PCV and stops short of asserting that PEHCR is a peripheral manifestation of or caused by PCV. Note that the neovascular origin of PEHCR is a hypothesis and not yet backed up by histologic evidence.
Differential diagnosis
The clinical picture of PEHCR has limited differential that includes retinal capillary hemangioma, vasoproliferative tumor, macroaneurysm, Coats-like reaction, familial exudative vitreoretinopathy, traumatic choroidal rupture, choroidal hemangioma, uveal lymphoma, large choroidal tuberculoma, choroidal metastatic tumor, and choroidal melanoma.[24]
 Retinal capillary hemangioma with prominent retinal feeder vessels[26] |
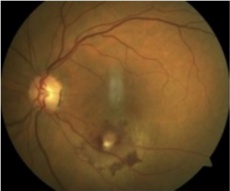 Retinal macroaneurysm[27] |
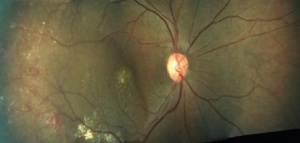 Coat's disease with telangiectasias in the temporal periphery with the presence of exudates and subretinal fluid[28] |
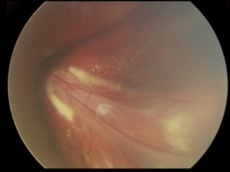 Familial exudative vitreoretinopathy with subtotal macular detachment (stage 4)[29] |
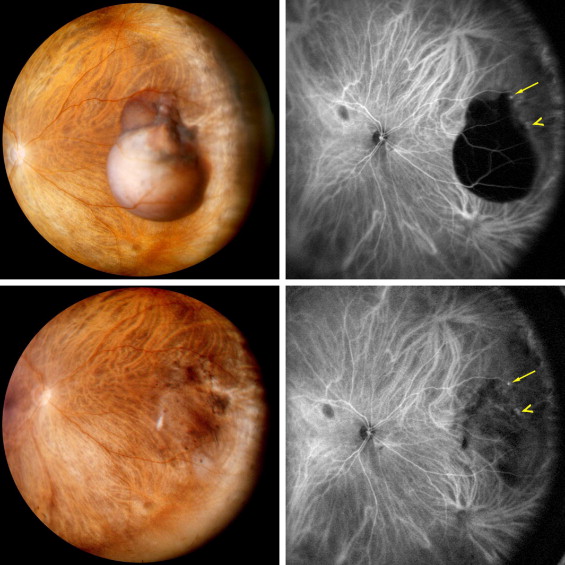
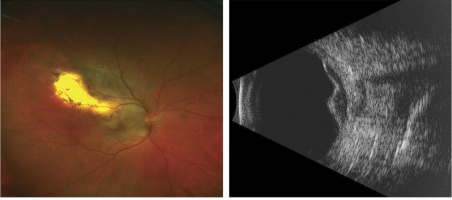 Choroidal melanoma fundus photo and B scan. This tumor was initially treated as choroidal hemangioma. [30] |
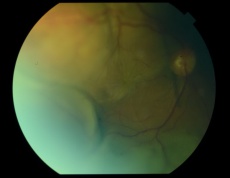
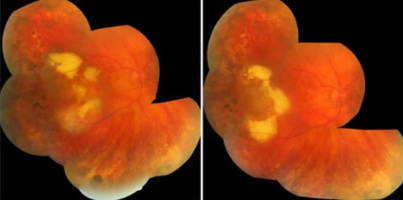 PEHCR in temporal quadrant of OD. Left: before treatment with anti-VEGF (left); Right: 4 months after anti-VEGF[31] |
While the aforementioned differential diagnoses are all important to consider, perhaps the most feared missed differential for PEHCR is choroidal melanoma. In fact, 100% of the cases in the largest case series to date by Shields et al were referred to the clinic either with the diagnosis of choroidal melanoma or request to rule out choroidal melanoma[5]. In light of this fact, a few important distinguishing features between PEHCR and choroidal melanoma are highlighted below:
| PEHCR | Choroidal Melanoma | |
|---|---|---|
| Age of occurrence | Greater than 65, average age between 77-83 | Wide range between birth to the 9th decade [32] but with average age of 59-62 in America[33][34] and 45-55 in East Asian population studies[35][36] |
| Sex | Female predominance | Male predominance especially when age-adjusted[37] |
| Association with systemic HTN | Yes | No |
| Familial occurrence | No | Yes, with BAP1 gene [38] |
| Location of appearance | Temporal between equator and the ora (89%) | At the macula or between the macula and the equator (83%) |
| Bilateral Occurrence | In one-third of cases | In about 0.2% of cases[39] |
| Dimensions | Median basal dimension of 10mm and a mean thickness of 3.0mm | Median basal dimension of 11 mm and a mean thickness of 4.5 mm |
| Findings on indirect ophthalmoscopy | Subretinal hemorrhage, sub-RPE hemorrhage or fluid, RPE detachment or RPE tear, subretinal fibrosis, lipid exudation. Usually apepars dark due to hemorrhage | Pigmented/darkappearing in about 85% of cases, the rest amelanotic[40]. Orange pigment at the level of retinal pigment epithelium, exudative retinal detachment (usually with melanomas greater than 4 mm in thickness), rarely hemorrhagic |
| OCT findings | Difficult to capture due to the peripheral location of these lesions. When PEHCR causes associated vision decrease, it may be due to subretinal fluid/hemorrhage visible on OCT. | Serous retinal detachments around and overlying the tumor, intra-retinal cystic spaces in the overlying retina, loss of normal retinal architecture overlying the tumor [41] |
| Ultrasound | (+)Retraction cleft
(-)Vascular pulsation (-)Choroidal excavation (-)Shadowing in the orbit (-)Classically associated internal reflectivity (nothing reflectivity is classic for PEHCR) |
(-)Retraction cleft
(+)Vascular pulsation (+)Choroidal excavation (+)Shadowing in the orbit, (+)low to medium internal reflectivity |
| Fluorescein angiography | Blockage of choroidal fluorescence related to hemorrhage (sub-retinal, sub-RPE) , window defect from peripheral atrophic RPE, diffuse peripheral hyper- or hypofluorescence that correlate to RPE atrophy or hyperplasia. Choroidal neovascularization possible. | Some patterns are characteristic but not pathognomonic. Patterns include intrinsic tumor circulation (double circulation), extensive leakage with progressive fluorescence, late staining of the lesion and multiple pinpoint leaks(hot spots) at the level of the retinal pigment epithelium. [42] |
| Prognosis | Often regresses spontaneously, but may lead to decreased vision from vitreous hemorrhage, foveal subretinal fluid/hemorrhage that may respond to treatments such as anti-VEGF, laser photocoagulation, or cryotherapy . No known systemic complications. | Often progresses without treatment. Associated with metastasis and possible death. Every millimeter increase in thickness leads to a 5% increased risk for metastasis.[43] |
Management
Asymptomatic patients should be observed because the majority of PEHCR lesions (89% of cases in a series by Shields[5]) stabilize or regress with time. Associated vitreous hemorrhage and visual impairment may call for vitrectomy. Sub-foveal extension with subretinal blood/fluid may also decrease vision, and the use of anti-vascular endothelial growth factor has been tried with variable success in restoring visual acuity[44][45][46]. The use of laser photocoagulation, in combination with anti-VEGF, has been advocated as the treatment of choice for choroidal neovascular membrane associated with subretinal fluid in the macular region[47], but Kim et al[8] have also commented that their experience with cryotherapy may have precipitated massive subretinal hemorrhage that disrupted the fovea and thus resulted in poor vision. In general, there is no standard of care for this uncommon disease entity, and methods of management other than observation is largely exploratory and anecdotal. Further studies are needed to show efficacy and safety of the aforementioned treatment modalities for PEHCR.
Prognosis
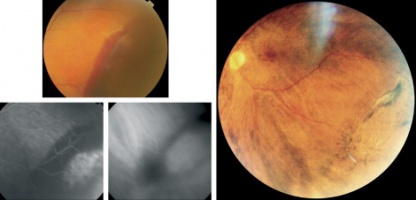
The vast majority of the lesions stabilize or regress spontaneously (see section on management). In the regressed state, the lesions most often exhibit subretinal fibrosis, RPE hyperplasia or atrophy, with variable visual outcomes.
For those lesions that present with or progress to recurrent hemorrhage or exudation with involvement of the fovea or vitreous and thus with decreased vision, treatment with modalities mentioned in management may restore vision. Surveillance for recurrence of lesion and/or progression of co-existing ARMD or PCV may be warranted to preserve vision[5].
Additional Resources
- Porter D, Vemulakonda GA. Blood Pressure. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/anatomy/blood-pressure-list. Accessed January 06, 2023.
References
- ↑ Jump up to: 1.0 1.1 Shields JA, Mashayekhi A, Ra S, Shields CL. Pseudomelanomas of the posterior uveal tract: the 2006 Taylor R. Smith Lecture. Retina. 2005 Sep;25(6):767-71
- ↑ Ghassemi F, Bazvand F, Hosseini SS. Pseudomelanoma at a referral center in iran. J Ophthalmic Vis Res. 2014 Jan;9(1):50-3.
- ↑ Reese AB, Jones IS. Hematomas under the retinal pigment epithelium. Trans Am Ophthalmol Soc, 59 (1961), pp. 43–69
- ↑ Jump up to: 4.0 4.1 4.2 Annesley WH. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc, 78 (1980), pp. 321–364
- ↑ Jump up to: 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 Shields CL, Salazar PF, Mashayekhi A, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy simulating choroidal melanoma in 173 eyes. Ophthalmology. 2009 Mar;116(3):529-35.
- ↑ Mirshahi A, Höhn F, Baatz H, Müller M, Hattenbach LO. Peripheral exudative haemorrhagic chorioretinopathy: clinical and angiographic findings. Klin Monbl Augenheilkd. 2009 Aug;226(8):659-63.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical, angiographic, and histologic study. Am J Ophthalmol. 2009 Dec;148(6):932-8.
- ↑ Jump up to: 8.0 8.1 8.2 Kim YT, Kang SW, Lee JH, Chung SE. Peripheral exudative hemorrhagic chorioretinopathy in Korean patients. Jpn J Ophthalmol. 2010 May;54(3):227-31.
- ↑ Jump up to: 9.0 9.1 Bloome MA, Ruiz RS. Massive spontaneous subretinal hemorrhage. Am J Ophthalmol, 86 (1978), pp. 630–637
- ↑ Bardenstein DS, Char DH, Irvine AR, Stone RD. Extramacular disciform lesions simulating uveal tumors. Ophthalmology, 99 (1992), pp. 944–951
- ↑ Seibel I, Hager A, Duncker T, Riechardt AI, Nürnberg D, Klein JP, Rehak M, Joussen AM. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016 Apr;254(4):653-9.
- ↑ Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the US: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief, No. 133. Hyattsville, MD: National Center for Health Statistics, Centers for Disease Control and Prevention, US Dept of Health and Human Services, 2013.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 Mantel I, Schalenbourg A, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: polypoidal choroidal vasculopathy and hemodynamic modifications. Am J Ophthalmol. 2012 May;153(5):910-922.e2.
- ↑ Jump up to: 14.0 14.1 14.2 Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: a report of 10 eyes. Retina. 2013 Jan;33(1):48-55.
- ↑ Tsui I, Jain A, Shah S, Schwartz SD, McCannel TA. Ultra widefield imaging of peripheral exudative hemorrhagic chorioretinopathy. Semin Ophthalmol. 2009 Jan-Feb;24(1):25-8.
- ↑ Mashayekhi A, Shields CL, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy: a variant of polypoidal choroidal vasculopathy? J Ophthalmic Vis Res. 2013 Jul;8(3):264-7.
- ↑ Mazal Z. Peripheral exudative hemorrhagic chorioretinopathy. Cesk Slov Oftalmol. 2019 Summer;75(2):80-84.
- ↑ Jump up to: 18.0 18.1 Alforja MS, Sabater N, Giralt J, Adán A, Pelegrín L, Casaroli-Marano R. Intravitreal bevacizumab injection for peripheral exudative hemorrhagic chorioretinopathy. Jpn J Ophthalmol. 2011 Jul;55(4):425-7.
- ↑ Nagori S, Furuta M, Shields CL. Posterior uveal melanoma thickness at diagnosis correlates with tumor location. American Academy of Ophthalmology, New Orleans, Louisiana (2007) November 12–13
- ↑ Shroff D, Sharma M, Chhablani J, Gupta P, Gupta C, Shroff C. PERIPHERAL EXUDATIVE HEMORRHAGIC CHORIORETINOPATHY-A NEW ADDITION TO THE SPECTRUM OF PACHYCHOROID DISEASE? Retina. 2021 Jul 1;41(7):1518-1525.
- ↑ New York Eye Cancer Center. Ultrasound Images. [https://eyecancer.com/eye-cancer/image-galleries/ultrasound-images/# Website
- ↑ Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol, 123 (2) (2005), pp. 244–252
- ↑ Bec P, Secheyron P, Arne JL, Aubry JP. La néovascularisation sous-rétinienne périphérique et ses conséquences pathologiques. J Fr Ophtalmol, 2 (1979), pp. 329–336.
- ↑ Elwood KF, Richards PJ, Schildroth KR, Mititelu M. Peripheral Exudative Hemorrhagic Chorioretinopathy (PEHCR): Diagnostic and Therapeutic Challenges. Medicina (Kaunas). 2023 Aug 22;59(9):1507.
- ↑ Shields CL, Kaliki S, Al-Dahmash S, Rojanaporn D, Shukla SY, Reilly B, Shields JA. Retinal vasoproliferative tumors: comparative clinical features of primary vs secondary tumors in 334 cases. JAMA Ophthalmol. 2013 Mar;131(3):328-34.
- ↑ Singh AD, Nouri M, Shields CL, Shields JA, Smith AF. Retinal capillary hemangioma: a comparison of sporadic cases and cases associated with von Hippel-Lindau disease. Ophthalmology. 2001 Oct;108(10):1907-11.
- ↑ Erol MK, Dogan B, Coban DT, Toslak D, Cengiz A, Ozel D. Intravitreal ranibizumab therapy for retinal arterial macroaneurysm. Int J Clin Exp Med. 2015 Jul 15;8(7):11572-8.
- ↑ Rishi E, Rishi P, Appukuttan B, Uparkar M, Sharma T, Gopal L. Coats' disease of adult-onset in 48 eyes. Indian J Ophthalmol. 2016 Jul;64(7):518-23.
- ↑ Ranchod TM, Ho LY, Drenser KA, Capone A Jr, Trese MT. Clinical presentation of familial exudative vitreoretinopathy. Ophthalmology. 2011 Oct;118(10):2070-5.
- ↑ Campagnoli TR, Medina CA, Singh AD. Choroidal melanoma initially treated as hemangioma: diagnostic and therapeutic considerations. Retin Cases Brief Rep. 2016 Spring;10(2):175-82.
- ↑ Seibel I, Hager A, Duncker T, Riechardt AI, Nürnberg D, Klein JP, Rehak M, Joussen AM. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016 Apr;254(4):653-9.
- ↑ Yousef YA, Alkilany M. Characterization, treatment, and outcome of uveal melanoma in the first two years of life. Hematol Oncol Stem Cell Ther. 2015 Mar;8(1):1-5.
- ↑ Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond). 2016 Dec 2.
- ↑ Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 2011; 118(9): 1881–1885.
- ↑ Park SJ, Oh CM, Kim BW, Woo SJ, Cho H, Park KH. Nationwide Incidence of Ocular Melanoma in South Korea by using the National Cancer Registry Database (1999–2011). Invest Ophthalmol Vis Sci 2015; 56(8): 4719–4724.
- ↑ Biswas J, Kabra S, Krishnakumar S, Shanmugam MP. Clinical and histopathological characteristics of uveal melanoma in Asian Indians. A study of 103 patients. Indian J Ophthalmol 2004; 52(1): 41–44.
- ↑ Nichols EE, Richmond A, Daniels AB. Disparities in Uveal Melanoma: Patient Characteristics. Semin Ophthalmol. 2016;31(4):296-303.
- ↑ Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330(6009):1410–3.
- ↑ Sturm V1, Richard G. The prevalence of bilateral malignant uveal melanoma. Klin Monbl Augenheilkd. 2007 Oct;224(10):770-4.
- ↑ Parul Singh and Abhishek Singh. Choroidal melanoma. Oman J Ophthalmol. 2012 Jan-Apr; 5(1): 3–9.
- ↑ Materin MA, Raducu M, Bianciotto C, Shields CL. Fundus Autofluorescence and Optical Coherence Tomography Findings in Choroidal Melanocytic Lesions. Middle East Afr J Ophthalmol. 2010 Jul-Sep; 17(3): 201–206.
- ↑ Gass JD. Problems in the differential diagnosis of chorodal nevi and malignant melanomas.The XXXIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1977;83:299–323.
- ↑ Shields CL1, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: clinical features, classification, and top 10 pseudomelanomas. Curr Opin Ophthalmol. 2014 May;25(3):177-85.
- ↑ Pinarci EY, Kilic I, Bayar SA, Sizmaz S, Akkoyun I, Yilmaz G. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013 Jan;27(1):111-2.
- ↑ Gonzales JA, Kapoor KG, Gibran SK. Peripheral exudative hemorrhagic chorioretinopathy: a clinical, angiographic, and histologic study, comment on. Am J Ophthalmol. 2010 Jun;149(6):1013-4;
- ↑ Barkmeier AJ, Kadikoy H, Holz ER, Carvounis PE. Regression of serous macular detachment due to peripheral exudative hemorrhagic chorioretinopathy following intravitreal bevacizumab. Eur J Ophthalmol. 2011 Jul-Aug;21(4):506-8.
- ↑ Takkar B, Roy S, Sodhi PK, Azad S, Bajwa GS.Peripheral choroidal neovascular membrane in a case of peripheral exudative hemorrhagic chorioretinopathy managed with combination therapy. Int Ophthalmol. 2016 Jun 16.