Polypoidal Choroidal Vasculopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Polypoidal choroidal vasculopathy (PCV) is a disease of the choroidal vasculature. It is present in both men and woman of many ethnicities, characterized by serosanguineous detachments of the pigmented epithelium and exudative changes that can commonly lead to subretinal fibrosis. Evidence supports that symptomatic patients with PCV can have complete regression without severe vision loss with photodynamic therapy (PDT) and anti-VEGF treatment.
Disease Entity
Disease
The disease process known now as polypoidal choroidal vasculopathy (PCV) was described by Dr. L Yannuzzi during a presentation of cases at the American Academy of Ophthalmology in 1982[1]. He described patients with subretinal, vascular lesions associated with serous and hemorrhagic detachments of the retinal pigment epithelium (RPE)[1][2]. The name reflects the appearance of a network of branching choroidal vessels with terminal, polyp-like aneurysmal dilations.
Prevalence and Incidence:
PCV is described as a disease present in middle-aged black women[1][3], but it has been established that it can occur in both sexes and is most commonly diagnosed in patients between the ages of 50 and 65 years. In many Asian countries researchers have estimated the prevalence of PCV among their population; in Japan higher prevalence rates are present of approximately 23% to 54% in patients with presumed age-related macular degeneration (AMD)[3][4][5][6][7][8][9][10]. Prevalence rates of between 4% and 9.8% have been reported in Caucasian patients with presumed AMD[5].
Different clinical features in different populations:
The Japanese patients with IPCV are more commonly male, have macular involvement and unilateral disease as compared to white patients (more females, commonly bilateral, and less macular and more peripapillary involvement). see https://www.aao.org/topic-detail/polypoidal-choroidal-vasculopathy-pcv--asia-pacifi
Etiology
The etiology is not clearly understood. It has been proposed that there is a choroidal vasculature propensity for dilation and aneurysmal formation[5][11].
Pathophysiology
The pathogenesis of PCV is not clearly understood. Gross specimens are described in the literature for their histopathologic findings; Uyama et al described large vascular channels in their early pathological state, also observed by other researchers[2].
Primary Prevention
Unfortunately, there is no mechanism for prevention at this time. With more research in genetic markers there may be a time when risk of developing PCV can be better estimated and treatment can be tailored to individual patient needs.
Diagnosis
History
The best technique currently available from differentiating polypoidal choroid vasculopathy from other types of neovascularization is indocyanine green angiography (ICGA)[12]. The ICG molecule is excited by the absorption of infrared light in the range from 790 to 805 nm. The intravascular retention of the ICG molecule allows better resolution of the choroidal vasculature. Fluorescein angiography (FA) is not as useful because it lacks the same resolution of the choroidal vasculature as ICGA. However, it is able to show large polypoidal changes.
Physical Examination
Thorough history and dilated fundus exam are the first steps in diagnosis. On fundus examinations, one can see orange-reddish bulb-like lesions budding from choroid into the subretinal space. These lesions can be associated with recurrent and significant hemorrhagic and exudative detachments of retina and retinal pigment epithelium (RPE). Hard exudates are also common. On occasions, patients may present with break-through vitreous hemorrhage.
Signs/Symptoms
Common visual complaints can include blurred vision, central or paracentral scotoma, and dim vision in the affected eye.
Clinical Diagnosis
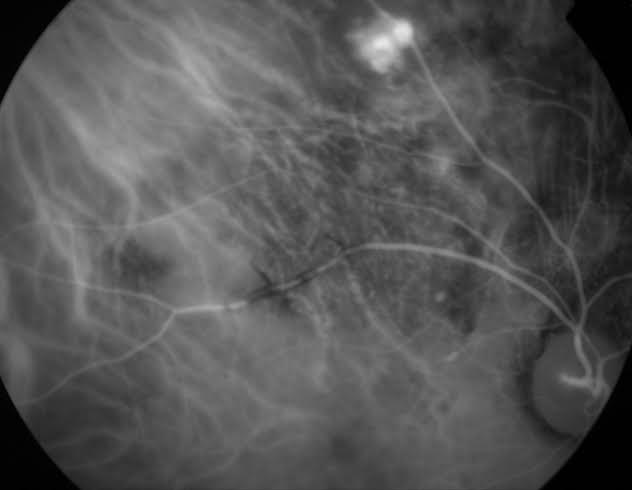
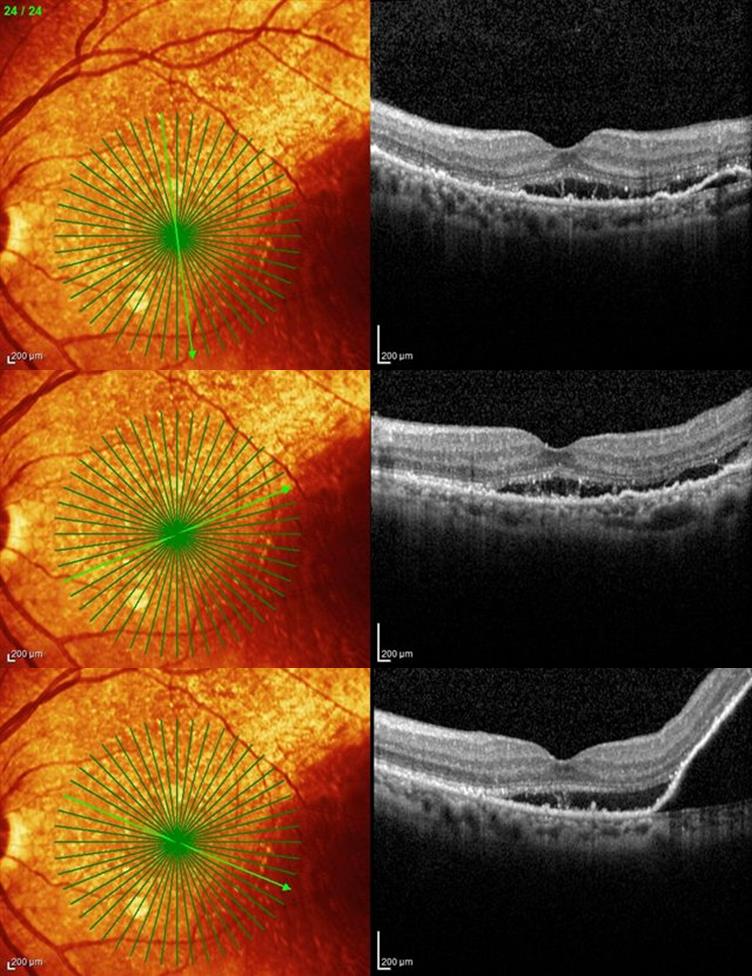
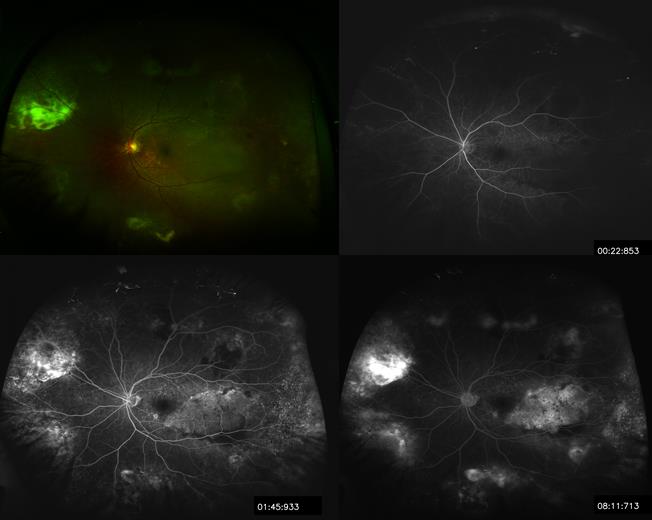
The constellation of clinical appearance, ICGA, FA in certain cases, along with high resolution optical coherence tomography OCT assist in making the diagnosis of this clinical entity (Figure 1-3).
Figure 1. ICGA of polyps in the choroidal vasculature. Image courtesy of Dr. Gelareh Abedi.
Figure 2. Spectral domain OCT® of the left macula of a patient with suspected PCV.
Figure 3. Wide field Optos® of left eye with fluorescein angiography at 22sec, 1:45min, and 8:11min demonstrating multiple areas of hemorrhages and serous retinal detachments.
Diagnostic procedures
Fluorescein angiography (FA)
PCV lesions on FA resemble occult CNVM lesions and when submacular, they can be mistaken for AMD. A peripheral notch at the margin of a serous pigment epithelial detachment may give a clue to the location of the polyp. RPE atrophy is manifested as window defects. Subretinal or sub-retinal pigment epithelial hemorrhage gives block fluorescence. FA also helps to rule out other differentials including CNV, microaneurysms and retinal angiomatous proliferans.
Indocyanine green angiography (ICGA)
ICGA with its ability to highlight choroidal vasculature is the standard for diagnosis of polypoidal lesions. On ICGA, the branching network of PCV is easily seen. The polyps present as focal hyperfluorescent spots. In later stages, reversal pattern of dye is seen with the center of the lesion becoming hypofluorescent and surrounding becoming hyperfluorescent. Finally, in the very last stage, there is “wash-out” of the lesion that is seen in non-leaking PCV lesions[13].
The EVEREST trial diagnosed PCV based on the following criteria:[14]
Early subretinal ICGA hyperfluorescence (appearing within the first 5 min of ICG dye injection) and at least one of the following diagnostic criteria:
- Nodular (elevated) appearance of the polyp on stereoscopic viewing
- Hypofluorescent halo around the nodule
- Abnormal vascular channel(s) supplying the polyps (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4413842/figure/BJOPHTHALMOL2014305674F1/)
- Pulsatile filling of polyps
- Orange subretinal nodules corresponding to the hyperfluorescent area on ICGA
- Massive submacular haemorrhage (>4 disc areas).
Based on ICGA features IPCV has been divided into 2 types:[15][16]
- Type 1 (polypoidal CNV): polyp/s with well defined branching vascular network (BVN, both feeder and draining vessels)
- Type 2 (typical PCV): polyp with absent BVN (neither feeder not draining vessels)
The abnormal vascular channels seen in IPCV are of 2 types (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4413842/figure/BJOPHTHALMOL2014305674F2/).[14] "A typical BVN originates from a feeder vessel and spreads outwards, with polyps typically located at the periphery. A second, less common type was interconnecting channels, which consisted of a network of crisscrossing choroidal vessels which supply the associated polyps. The dye has no specific point of origin or direction of flow, unlike a BVN."[14] The abnormal vascular channels are best seen using dynamic ICGA within 30 seconds and after 1 min it is difficult to identify these vessels as normal choroidal vessels also fill up. Dynamic ICGA is crucial to document the pulsatile filling of popyps. Stereo ICGA pair images at 1 min or 3 min is adequate to diagnose, locate and measure the greatest linear dimension (to guide photodynamic therapy) of the polyp and abnormal vascular channels.[14] Dilation of large choroidal vessel and choroidal vascular hypermeability may also be noted in ICGA.
Central Serous Chorioretinopathy and PCV share a common pachychoroid spectrum and clinical differentiation between the 2 may be difficult in clinical setting, though various factors including age, ICGA and OCT features guide the clinician for optimal management.
Optical coherence tomography (OCT)
OCT is not only helpful in identification of subretinal or sub-RPE fluid, it can also delineate polypoidal lesions. These lesions resemble dome-like elevations of RPE with moderate internal reflectivity. In most cases, there is also a highly reflective line just below these lesions consistent with location of vascular branching network[17]. The dual reflective layers are also called “double-layer sign,” and are seen in 59% of eyes with PCV[18]. In summary the OCT B scan features include
- Subretinal fluid
- Typically intraretinal cystoid spaces are seen less commonly- which may explain surprisingly good visual acuity in some cases[4]
- Pigment epithlial detachment (PED) may have fluid or hemorrhage beneath it
- Notched PED- a high PED connected with PED/s of lower height on the sides- wavy border of PEDs- corrugated or bumpy appearance[19] (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4159396/figure/f1-opth-8-1689/)- M shaped or QRS complex shaped PEDs (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4159396/figure/f2-opth-8-1689/)
- The polyp is seen as a round or oval structure connected to the outer surface of PED (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4159396/figure/f3-opth-8-1689/)
- The double layer sign is seen at the margin of PED elevation. It may be caused by either separation of RPE from the Bruch's membrane or separation of Bruch's membrane from the Choroid.[16] (https://www.karger.com/Article/Pdf/355488)
A recent article from the Asia-Pacific Ocular Imaging Society PCV Workgroup noted that 'For the diagnosis of PCV, the combination of 3 OCT-based major criteria (sub-retinal pigment epithelium [RPE] ring-like lesion, en face OCT complex RPE elevation, and sharp-peaked PED) achieved an area under the receiver operating characteristic curve of 0.90. Validation of this new scheme in a separate subset 80 eyes achieved an accuracy of 82%.'[20]
The OCT C scan features include
- Hematocrit sign[4] - round or oval polyp with blood corpuscles sedimented inferiorly giving rise to a horizontal level within the polyp between corpuscles and serous component of blood
- Bola sign[4]- multiple polyps connect to each other via abnormal choroidal vascular channels- prominently visible in 3D reconstruction using spectral domain OCT[21]
Laboratory test
No specific hematologic or aqueous biomarker testing exists for PCV. Park et al proposed a significant relationship between plasma MDA level and ARMS2 variants in patients with PCV and neovascular AMD in Korean patients[22].
Differential diagnosis
The differential includes other entities that can cause subretinal neovascularization. History and clinical presentation would differentiate PCV from the following limited list of disease processes including: age-related macular degeneration (type 1 or type 2), central serous chorioretinopathy, pathological myopia with neovascularization, and choroidal tumors or metastases.
Management
Medical therapy
Several studies have evaluated treatment for PCV. Treatment includes observation, photodynamic therapy, intravitreal injection of anti-VEGF therapy, or combination therapy. The EVEREST trial is a multi-center, double-masked trial compared three treatment regimens: verteporfin photodynamic therapy (PDT) plus the anti-VEGF agent ranibizumab (Lucentis), ranibizumab monotherapy, and PDT monotherapy. The patient population was sixty-one Asian patients with symptomatic PCV. The primary end point was complete polyp regression as assessed by ICG. PDT in combination with ranibizumab and PDT monotherapy showed a significantly higher proportion of patients with complete polyp regression at month 6 than the ranibizumab monotherapy group[23].
Laser therapy to the polyp and BVN occupying a small area away from fovea may be considered.
LAPTOP study[24] from Japan showed that ranibizumab resulted in visual gain from baseline at 24 months, the PDT group did not gain vision.
PLANET study[25]- at 12 months aflibercept monotherapy was non-inferior to aflibercept+PDT in visual outcome.
EPIC study[26]- at 6months aflibercept monotherapy stabilized vision and resulted in resolution of hemorrhagic and exudative complications regression of polyps in around 70% patients.
Medical follow up
Regardless of choice of treatment, patients should be followed on regular intervals to detect and prevent subretinal/subRPE fluid and hemorrhage.
Surgery
There is no current surgical management for PCV. If surgical management is needed, it would be tailored to complications and sequelae of PCV such as break-through vitreous hemorrhage.
Complications
The natural course of PCV depends on the size and extent of serosanguineous detachments of the pigmented epithelium and exudative changes, commonly leading to subretinal fibrosis. In a study by Uyama et al, high risk eyes were those with cluster of polypoidal lesions whereas eyes with solitary lesions had favorable prognosis[27]. Polypoidal lesions are usually located at the margin of the PED. Following the spontaneous resolution of the acute serosanguineous complications, there may be signs of subretinal fibrosis, pigment epithelial hyperplasia, and atrophic degeneration[18],[23],[28].
Prognosis
Depending on the extent of area involved prognosis is generally good. Even in patients with longer chronicity of disease, studies have shown halting of visual decline. Evidence supports that symptomatic patients with PCV can have complete regression without severe vision loss with PDT and anti-VEGF treatment[23].
Acknowledgements
- Dr. Gelareh Abedi
- Dr. C Armitage Harper III, Third Coast Retina Conference 2013
- Andres Sanchez, Texas Diabetes Institute Photographer for the UTHSCSA Department of Ophthalmology.
References
- ↑ 1.0 1.1 1.2 Yannuzzi LA. Idiopathic Polypoidal Choroidal Vasculopathy. Presented at: Macula Society Meeting, February 5, 1982, Miami, FL, USA.
- ↑ 2.0 2.1 Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990; 10:1–8.
- ↑ 3.0 3.1 Stern RM, Zakov ZN, Zegarra H, Gutman FA. Multiple recurrent serosanguineous retinal pigment epithelial detachments in black women. Am J Ophthalmol 1985; 100:560–569.
- ↑ 4.0 4.1 4.2 4.3 Imanmura Y et al. Polypoidal Choroidal Vasculopathy: A Review. Surv Ophthalmol 2010; 55:501–515.
- ↑ 5.0 5.1 5.2 Maruko I, Iida T, Saito M, et al. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol 2007; 144:15–22.
- ↑ Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997; 115:478–485.
- ↑ Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004; 49:25–37.
- ↑ Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 2008; 49:4729–4737.
- ↑ Liu Y, Wen F, Huang S, et al. Subtype lesions of neovascular age-related macular degeneration in Chinese patients. Graefes Arch Clin Exp Ophthalmol 2007; 245:1441–1445.
- ↑ Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003; 121:1392–1396.
- ↑ Byeon SH, Lee SC, Oh HS, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol 2008; 52:57–62.
- ↑ Spaide RF, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995; 15:100–110.
- ↑ Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49(1):25-37.
- ↑ 14.0 14.1 14.2 14.3 Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH; EVEREST Study Group. EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015 May;99(5):624-8. doi: 10.1136/bjophthalmol-2014-305674. Epub 2015 Mar 10. PubMed PMID: 25758601; PubMed Central PMCID: PMC4413842.
- ↑ Kawamura A, Yuzawa M, Mori R, Haruyama M, Tanaka K. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013 Sep;91(6):e474-81. doi: 10.1111/aos.12110. Epub 2013 Jul 15. PubMed PMID:23848133.
- ↑ 16.0 16.1 Honda S, Matsumiya W, Negi A. Polypoidal choroidal vasculopathy: clinical features and genetic predisposition. Ophthalmologica. 2014;231(2):59-74. doi: 10.1159/000355488. Epub 2013 Nov 21. Review. PubMed PMID: 24280967.
- ↑ Kamaeda T, Tsujikawa A, Otani A, et al. Polypoidal choroidal vasculopathy examined with en face optical coherence tomography. Clin Experiment Ophthalmol. 2007; 35(7):596-601.
- ↑ 18.0 18.1 Sato T, Kishi S, Watanabe G, et al. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007 27; 589-594.
- ↑ Alshahrani ST, Al Shamsi HN, Kahtani ES, Ghazi NG. Spectral-domain optical coherence tomography findings in polypoidal choroidal vasculopathy suggest a type 1 neovascular growth pattern. Clinical Ophthalmology (Auckland, NZ). 2014;8:1689-1695. doi:10.2147/OPTH.S68471.
- ↑ Cheung CMG, Lai TYY, Teo K, Ruamviboonsuk P, Chen SJ, Kim JE, Gomi F, Koh AH, Kokame G, Jordan-Yu JM, Corvi F, Invernizzi A, Ogura Y, Tan C, Mitchell P, Gupta V, Chhablani J, Chakravarthy U, Sadda SR, Wong TY, Staurenghi G, Lee WK. Polypoidal Choroidal Vasculopathy: Consensus Nomenclature and Non-Indocyanine Green Angiograph Diagnostic Criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup. Ophthalmology. 2021 Mar;128(3):443-452. doi: 10.1016/j.ophtha.2020.08.006. Epub 2020 Aug 11. PMID: 32795496.
- ↑ Abe S, Yamamoto T, Haneda S, Saito K, Miura H, Kirii E, Yamashita H. Three-Dimensional Features of Polypoidal Choroidal Vasculopathy Observed by Spectral-Domain OCT. Ophthalmic Surg Lasers Imaging. 2010 Mar 9:1-6. doi:0.3928/15428877-20100215-76. [Epub ahead of print] PubMed PMID: 20337297.
- ↑ Park DH, Shin JP, Kim IT. Association of plasma malondialdehyde with ARMS2 genetic variants and phenotypes in polypoidal choroidal vasculopathy and age-related macular degeneration. Retina 2013; 0:1–10.
- ↑ 23.0 23.1 23.2 Koh et al. EVEREST STUDY: Efficacy and Safety of Verteporfin Photodynamic Therapy in combination with Ranibizumab or Alone Versus Ranibizumab Monotherapy in Patients with Symptomatic Macular Polypoidal Choroidal Vasculopathy. Retina 2012; 32:1453-1464.
- ↑ Oishi A, Miyamoto N, Mandai M, Honda S, Matsuoka T, Oh H, Kita M, Nagai T, Bessho N, Uenishi M, Kurimoto Y, Negi A. LAPTOP study: a 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology. 2014 May;121(5):1151-2. doi: 10.1016/j.ophtha.2013.12.037. Epub 2014 Jan 29. PubMed PMID: 24484991
- ↑ Won Ki Lee, Yuichiro Ogura, Tomohiro Iida, Shih-Jen Chen, Tien Yin Wong, Paul Mitchell, Tatsuro Ishibashi, eric zhang, Sergio Leal; Efficacy and Safety of Intravitreal Aflibercept in Polypoidal Choroidal Vasculopathy: 12-Month Results of the PLANET Study. Invest. Ophthalmol. Vis. Sci. 2017;58(8):1199.
- ↑ Kokame GT, Lai JC, Wee R, Yanagihara R, Shantha JG, Ayabe J, Hirai K. Prospective clinical trial of Intravitreal aflibercept treatment for Polypoidal choroidal vasculopathy with hemorrhage or exudation (EPIC study): 6 month results. BMC Ophthalmol. 2016 Jul 27;16:127. doi: 10.1186/s12886-016-0305-2. PubMed PMID: 27465105; PubMed Central PMCID: PMC4964097.
- ↑ Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: Natural history. Am J Ophthalmol. 2002; 133(5):639-48.
- ↑ Lim TH, Laude A, Tan CS. Polypoidal choroidal vasculopathy: An Angiographic discussion. Eye (Lond) 2010; 24:483–490.