Geographic Atrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Geographic atrophy is a chronic progressive degeneration of the macula and can be seen as part of late-stage age-related macular degeneration (AMD). The condition leads to central scotomas and permanent loss of visual acuity.
Disease Entity
- ICD-9-CM: 362.51 Nonexudative senile macular degeneration
- ICD-10-CM: H35.31 Nonexudative age-related macular degeneration
Disease
Geographic atrophy (GA) is a chronic progressive degeneration of the macula as part of late-stage age-related macular degeneration (AMD). The disease is characterized by localized sharply demarcated atrophy of outer retinal tissue, retinal pigment epithelium (RPE), and choriocapillaris.[1] It typically starts in the perifoveal region and expands to involve the fovea with time, leading to central scotomas and permanent loss of visual acuity. It is bilateral in most cases. Over 8 million people are affected worldwide with GA, approximately 20% of all individuals with AMD. The incidence of GA is expected to rise as the age burden of developed countries increases.
Risk Factors
Several risk factors have been noted by several studies.
- The most pronounced risk factor is increasing age and family history of AMD. [2]
- Smoking history increases the risk of GA significantly. Both active smokers and former smokers are at greater risk of developing geographic atrophy. [3]
- One retrospective study through the Asian Eye Epidemiology Consortium found that GA is relatively uncommon in Asian populations when compared with those of European Ancestry. [4] This study also showed that the ratio of GA to neovascular AMD, another complication of advanced AMD, in the Asian population is lower (1:3) compared to that of the European population (1:1).
- Studies focusing on the United States have not found any gender difference in the prevalence of geographic atrophy, but the Asian Eye Epidemiology Consortium found a higher prevalence of GA in men. [2] [5][5]
- The Age-Related Eye Disease Study also found an increased risk of GA in users of thyroid hormones or antacids.
- People with higher education were at a lower risk of GA.
- Other studies have pointed out an increase in the risk of GA in patients with coronary heart disease as well as in patients with lens opacities or previous cataract surgery. [6]
- The Comparison of Age-Related Macular Degeneration Treatments Trial (CATT) found that older age, hypercholesterolemia, worse visual acuity, larger choroidal neovascularization (CNV) area, retinal angiomatous proliferation (RAP) lesion, GA in the fellow eye, and intraretinal fluid were associated with a higher risk of incident GA. They also found that thicker subretinal tissue complex and the presence of subretinal fluid were associated with slower development of GA. [7] One of the strongest risk factors was poor visual acuity at baseline, specifically visual acuity at or worse than 20/200 had almost a 3-fold risk of developing GA than those with a baseline of 20/25-20/40.
General Pathology
The pathogenesis of GA remains unclear. The natural course of AMD begins with early stages that are characterized by the presence of drusen, which are yellow deposits between the retinal pigment epithelium and Bruch’s membrane. Pigment displacement is also seen. [8] Late stages of AMD are either characterized by choroidal neovascularization or GA. GA is recognized as a sharply defined area in the posterior pole, with atrophy of the retinal pigment epithelium, the overlying photoreceptors, and the choriocapillaris. The defect in structures allows the observer to see the larger underlying choroidal vessels.[9] Reticular pseudodrusen are associated with the development of GA. [10]
The progression rate of GA varies but is relatively slow and progresses over the years. As the atrophic area expands, visual function decreases. [11] Clinically, the exudative and non-exudative AMD are very different, but these late stages of AMD are not mutually exclusive. Individuals with GA are at a high risk of developing choroidal neovascularizations, and patients with exudative AMD are at an increased risk of developing atrophic areas.
The cause of GA is not fully known, though it has been studied extensively. Genetic and environmental factors seem to contribute substantially. Complement factor H variant Y402H and ARMS2 have been associated with an increased risk of GA development. [12] [13] Drusen are shown to contain multiple complement components, [14][15] indicating that localized inflammation mediated by the complement system is an important element in AMD. This has been suggested to be a systemic immune dysfunction with retinal manifestation.[16] Oxidative stress and low-grade inflammation seem to play a role in AMD. [17] [18][19] In donor eyes with GA, choroidal T-lymphocytes and macrophages are noted to produce proinflammatory cytokines.[20] Furthermore, it has been found that mononuclear phagocytes are seen in abundance in the subretinal space in eyes with GA.[21] Their possible role in photoreceptor rescue or degeneration is unknown. Another recent study found that choroidal thickness may have a role in the pathogenesis of the unilateral form of GA, whereas Müller cells and their supported photoreceptors may play a role in bilateral GA. [22] New research is indicating that direct RNA toxicity, specifically Alu RNA mediated inflammation, might play a significant role in the pathogenesis of GA.[23]
Diagnosis
The diagnosis of geographic atrophy is clinical and can be made by ophthalmoscopy.
History
The typical patient with geographic atrophy is above 60 years of age, with gradually progressing loss of visual function.
Physical Examination
Ophthalmoscopy with visualization of the fundus, enables the trained ophthalmologist to observe drusen, as well as the atrophic area. In some cases, the atrophic area is unifocal, but in many cases, it shows as a multifocal disease within the macula area.
Signs
Geographic atrophy is one of the two forms of late stage AMD. The first sign is drusen, which can vary in size and number according to stage of disease. Small atrophic lesions begin to appear early on in the extrafoveal area that slowly expand into the fovea as the disease progresses.
Symptoms
In cases of GA, the fovea can be sparred for a long time, so that the measured visual acuity can remain nearly normal, but the contrast sensitivity as well as the ability to read suffers. The patient experiences relatively rapid function loss, when the fovea is involved due to loss of visual acuity.
Clinical Diagnosis
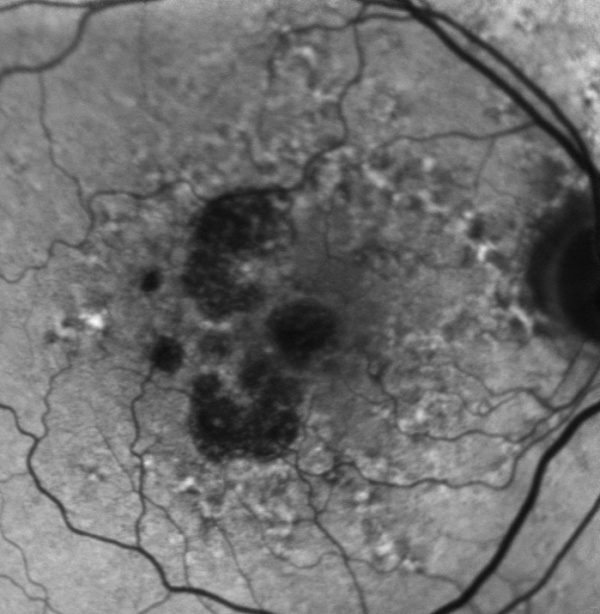
The diagnosis of geographic atrophy is clinical, and is made on ophthalmoscopy or on fundus photo. The ophthalmologist will see a macula decorated with drusen and a sharply demarcated area in the macular region with atrophic retina, lacking pigmentation and visible underlying choroidal vessels.
Diagnostic procedures
The clinical examination is key in diagnosing geographic atrophy, but other imaging techniques can be useful, especially in monitoring disease.
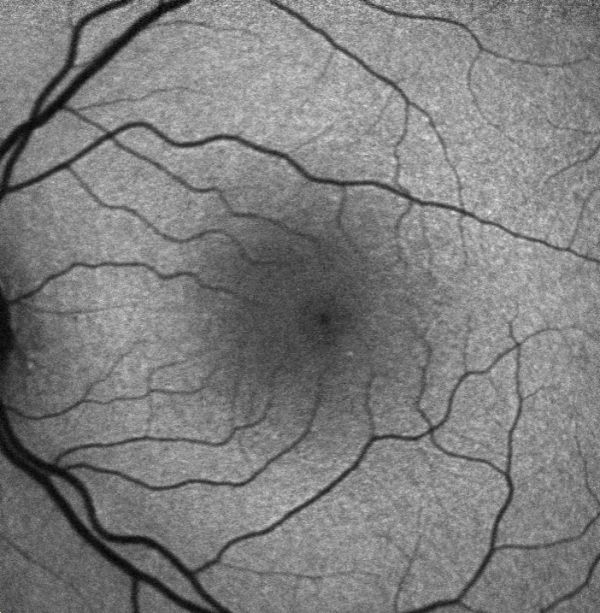
Fundus autofluorescence (FAF) is currently the standard imaging technology to visualize the retinal pigment epithelium (RPE) in geographic atrophy. Vital RPE contains intracellular lipofuscin. When exposed to light at a specific wavelength, lipofuscin absorbs this and emits light in another wavelength, from where the fluorescence signal originates. In the fovea, the signal diminishes physiologically due to absorption by the macular pigment. If atrophy of RPE occurs, this causes a distinct dark area due to the absence of lipofuscin-containing cells and, therefore, lack of autofluorescent signal. These sharp contrasts between completely dark and light grey have made it possible to introduce a semiautomated segmentation algorithm to detect and quantify the size of the atrophic area. Previously the preferred method was to outline the borders manually. Besides helping quantify the atrophic lesion, fundus autofluorescence also provides important information on the expected progression rate through the amount of hyperautofluorescence noted in the junctional zone of the lesion. Hyperautofluorescence is an accumulation of lipofuscin in the RPE cells and is thought to be due to suffering/dying cells. The amount of hyperautofluorescence correlates well with the rate of GA progression.[24][25]
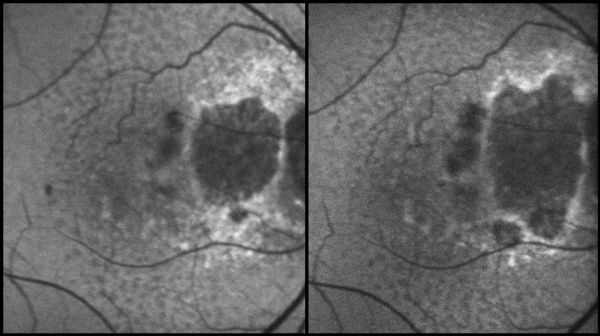
Optical coherence tomography (OCT) also provides important information. The atrophy of the retinal layers can be clearly seen with this non-invasive imaging technique; it has been demonstrated that some morphologic changes, such as a split between RPE and Bruch’s membrane in the junctional zone, may be an indication of fast progression. Patients suffering from GA are at a higher risk of developing choroidal neovascularizations, which can cause an even faster loss of visual function. Therefore, OCT can help in early recognition of intraretinal fluid, which is important in order to initiate treatment early. The ‘Classification of Atrophy Meetings (CAM)’ program provided a consensus definition of atrophy related to AMD.[26] The variants of macular atrophy according to this classification include complete RPE and outer retinal atrophy (cRORA), incomplete RPE and outer retinal atrophy (iRORA), complete outer retinal atrophy (cORA), and incomplete outer retinal atrophy (iORA).[26] The cRORA is defined on OCT by an area of hyper transmission of at least 250 microns diameter, RPE attenuation or disruption of minimum 250 microns diameter, evidence of photoreceptor degeneration, and absence of signs of RPE tear including the scrolled edge of RPE.[26]
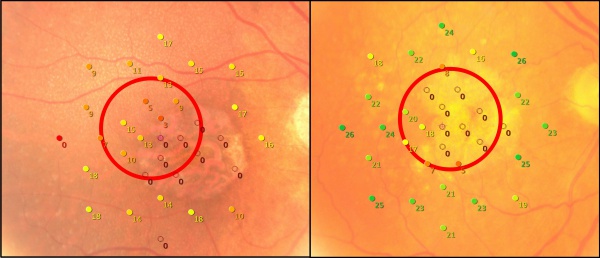
Measurement of visual acuity with a reading chart often provides poor information on the actual function of the retina due to foveal sparing and parafoveal scotomas. A better tool to evaluate the visual function is Microperimetry, a technique that stimulates the macula over 20 degrees in various spots with varying intensity of light, and the result depends on the patient’s ability to report recognition of the stimuli. In this way, low-luminance visual acuity and contrast sensitivity can also be measured. Studies using microperimetry have shown that the sensitivity of non-atrophic retina is decreased,[27] and that this loss correlates with the progression of GA over time.[28]
In Multifocal electroretinography, light stimuli is presented in patterns across the retina, and photoreceptor signaling is detected by an electrode. By varying the light stimuli, the retina is mapped with information on functionality and sensitivity.
Reading ability, or reading speed, can be quantified by the number of correctly read words in a limited amount of time. Radner and MNREAD reading charts are validated in several languages. Patients with GA and BCVA≥20/50 have shown to be significantly slower at reading compared to patients with intermediate AMD.[29]
Laboratory test
Geographic atrophy is a clinical diagnosis, and so far, there are no laboratory tests as part of the diagnostic or monitoring of disease.
Differential diagnosis
Atrophy of the retina due to other causes are in the differential of geographic atrophy, such as:
- atrophy secondary to anti-VEGF treatment,
- atrophy secondary to pattern dystrophy, maternally inherited diabetes and deafness (MIDD),[30] central areolar choroidal dystrophy, Stargardt disease, Best macular dystrophy,
- after macular surgeries,
- chronic central serous chorioretinopathy,
- Congenital infections including Zika virus, and
- Basal laminar arisen with pseudovitelliform detachment.
Management
General treatment
At this time, there is no treatment available, medical or surgical, that can halt or reverse the progression of geographic atrophy except pegcetacoplan injection. Visual rehabilitation is often necessary, even in cases of tolerable visual acuity, as contrast sensitivity and reading ability can suffer in cases of foveal sparing and parafoveal scotoma.[31][32] Monitoring of intra or subretinal fluid is important in early diagnosis of choroidal neovascularization, as this group of patients are at higher risk. The AREDS study showed that patients with visual loss due to AMD were at high risk of developing neovascular AMD and that AREDS2 vitamin supplements decrease the odds by 38% of developing neovascular AMD.[33] The study did not show any beneficial effects on slowing down the progression of geographic atrophy.
Several therapeutic agents for GA are in phase II and III clinical trials, targeting oxidative stress or inflammation or complement pathways to reduce the rate of GA progression.
The FDA, USA approved SYFOVRE™ (pegcetacoplan injection) on 17 February 2023 as the first approved therapy to slow the progression of GA secondary to AMD. Phase 3 OAKS and DERBY studies showed that monthly or every other month intravitreal injection of pegcetacoplan reduced the rate of growth of GA lesions in 2 years compared to sham.[34][35] It acts against complement factor 3 and had been previously approved for paroxysmal nocturnal hemoglobinuria (subcutaneous administration) by the FDA. The recommended dose is 15 mg in 0.1 ml injected intravitreally every 25 to 60 days. Like other intravitreal injections, there are risks of sub-conjunctival hemorrhage, endophthalmitis, retinal detachment, increased intraocular pressure, and vitreous hemorrhage. Other risks include an increased rate of conversion to wet AMD and intraocular inflammation. The phase 2 FILLY trial also showed a reduction in the growth of the area of GA.[36] The European Medicines Agency (EMA) 'confirmed its June 2024 negative opinion on the marketing authorization application of intravitreal pegcetacoplan for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration' on 20 September 2024.
The FDA, USA approved Izervay™ (avacincaptad pegol intravitreal solution) to reduce the progression of GA secondary to AMD on 4 August 2023. It is a complement C5 inhibitor. It is given intravitreally (2 mg) every month for up to 1 year.
Previously, other clinical trials found that lampalizumab, a selective complement factor D inhibitor, and tandospirone, a 5-HT1A agonist, were ineffective at reducing enlargement of lesions from GA secondary to AMD.[37][38]
Orally administered alpha-lipoic acid, an antioxidant, was evaluated in a phase 2 clinical trial but did not have any beneficial effects on GA lesion size nor best-corrected visual acuity. [39]
However, intravitreal brimonidine was shown to reduce GA lesion area growth within 3 months in a Phase 2 clinical trial.[40]
Ongoing treatment trials include neuroprotective agents (Ciliary neurotrophic factor, Tandospirone, tetracycline derivatives) and visual cycle inhibitors ( Fenretinide, Emixustat) as well as stem cell research; a few may hold future promise in halting the progression of geographic atrophy. [41]
Notable trials on GA include:[35]
- CHROMA-SPECTRI- intravitreal lampalizumab (anti-complement factor D),
- GATE- topical ocular tandospirone (neuroprotection, 5-HT1A agonist),
- GATHER - intravitreal Avacincaptad pegol (anti-complement factor 5),
- SAGA- oral ALK-001 (slows vitamin A dimerization),
- SEATTLE- oral emixustat hydrochloride (modulates visual cycle, anti-RPE65), and
- TOGA- oral doxycycline (anti-inflammatory).
Prognosis
The prognosis is poor and the disease is chronic and progressive. Progression of GA is associated with extensive decrease in visual acuity, a study has shown that 31% of patients with GA lose at least three lines of vision in 2 years,[42] and the growth rate median is 2.1 mm2/year, but with variation up to 10.2mm2/year.[43] The rate of progression differs between patients, but a sign of fast progression is high amounts of hyperautofluorescence shown on fundus autofluorescence and a decreased retinal function regarding contrast sensitivity or reading ability.
Additional Resources
- American Academy of Ophthalmology: http://www.aao.org
- http://Clinicaltrials.gov
References
- ↑ Fleckenstein, M., et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 125, 369-390 (2018).
- ↑ 2.0 2.1 Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224-32.
- ↑ 1. Chakravarthy U, Augood C, Bentham GC, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazolli L, Topouzis F, Vingerling JR, Vioque J, Young IS, Fletcher AE. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology 2007 jun;114(6):1157-63.
- ↑ Rim, T.H., et al. Prevalence and pattern of geographic atrophy in Asia: the Asian Eye Epidemiology Consortium. Ophthalmology 127, 1371-1381 (2020).
- ↑ 5.0 5.1 Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001;108:697-704.
- ↑ Fraser-Bell S, Donofrio J, Wu J, Klein R, Azen SP, Varma R et al. Sociodemographic factors and age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. American Journal of Ophthalmology 2005;139:30-8.
- ↑ Grunwald, J.E., et al. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology 124, 97-104 (2017).
- ↑ Young RW. Pathophysiology of age-related macular degeneration. Surv. Ophthalmol. 1987; 31:291-306.
- ↑ Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, et al. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Cis Sci 2011;52:1-6.
- ↑ Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7362-7369.
- ↑ Holz FG, Pauleikhoff D, Klein R, Bird AC: Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol 2004;137:504-510.
- ↑ Sepp T, et al. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci 2006;47:536-540.
- ↑ Cameron DJ, et al. HTRA1 variant confers similar risks to geographic atrophy and neovascular age-related macular degeneration. Cell Cycle 2007;6:1122-1125.
- ↑ Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 2001;20:705-732.
- ↑ Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 2010;29:95-112.
- ↑ Scholl HPN, Issa PC, Walier M, et al. Systemic complement activation in age-related macular degeneration.PLoS ONE 2008;3:e2593.
- ↑ Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000;45:115-134.
- ↑ Xu H, Chen M, Forrester JV: Para-inflammation in the aging retina. Prog Retin Eye Res 2009;28:348-368.
- ↑ Buschini E, Piras A, Nuzzi R, Vercelli A. Age-related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol 2011;95:14-25.
- ↑ Camelo S. Association of Choroidal Interleukin-17-producing T Lymphocytes and Macrophages with Geographic Atrophy. Ophthalmologica 2016;236:53-58.
- ↑ Sennlaub F, Auvynet C, Calippe B, Lavalette S, Poupel L, Hu SJ, et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in CX3CR1 deficient mice. EMBO Mol Med 2013;5:1775-1793.
- ↑ Pilotto, E., et al. Müller cells and choriocapillaris in the pathogenesis of geographic atrophy secondary to age-related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology 257, 1159-1167 (2019).
- ↑ Mao, X. & Liu, Q. An emerging role of Alu RNA in geographic atrophy pathogenesis: the implication for novel therapeutic strategies. Discovery medicine 22, 337-349 (2016).
- ↑ Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 2007;143:463-472.
- ↑ Bearelly S, Khanifar AA, Lederer DE, et al. Use of fundus autofluorescence images to predict geographic atrophy progression. Retina 2011;31:81-86
- ↑ 26.0 26.1 26.2 Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3 [published correction appears in Ophthalmology. 2019 Jan;126(1):177]. Ophthalmology. 2018;125(4):537-548. doi:10.1016/j.ophtha.2017.09.028
- ↑ Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci 2000;41:267-273.
- ↑ Meleth AD, Mettu P, Agron E, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci 2011;52:1119-1126.
- ↑ Sunnes JS, Rubin GS, Applegate CA et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology 1997;104:1677-1691.
- ↑ Tripathy K, Sarma B, Mazumdar S. Outer retinal tubulation and inner retinal pseudocysts in a patient with maternally inherited diabetes and deafness evaluated with optical coherence tomography angiogram. Indian J Ophthalmol. 2020;68(1):250-253. doi:10.4103/ijo.IJO_577_19
- ↑ Schmitz-Valckenberg S, Fleckenstein M, Helb HM, et al. Invivo imaging of foveal sparring in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci 2009;50:3915-3921.
- ↑ Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999;106:1768-1779.
- ↑ The Age-Related Eye Disease Study Research Group: A randomized, placebo-controlled clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol 2001;119(10):1439-1452
- ↑ Roger Goldberg, Jeffrey S Heier, Charles Clifton Wykoff, Giovanni Staurenghi, Rishi P Singh, Nathan Steinle, David S Boyer, Jordi Mones, Frank G Holz, Caleb Bliss, Federico Grossi, Ravi Metlapally, Ramiro Ribeiro; Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies.. Invest. Ophthalmol. Vis. Sci.2022;63(7):1500.
- ↑ 35.0 35.1 Biarnés M, Garrell-Salat X, Gómez-Benlloch A, et al. Methodological Appraisal of Phase 3 Clinical Trials in Geographic Atrophy. Biomedicines. 2023;11(6):1548. Published 2023 May 26. doi:10.3390/biomedicines11061548
- ↑ Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology. 2020;127(2):186-195. doi:10.1016/j.ophtha.2019.07.011
- ↑ Holz, F.G., et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA ophthalmology 136, 666-677 (2018).
- ↑ Jaffe, G.J., et al. Randomized trial to evaluate tandospirone in geographic atrophy secondary to age-related macular degeneration: the GATE study. American journal of ophthalmology 160, 1226-1234 (2015).
- ↑ Kim, B.J., et al. Orally administered alpha lipoic acid as a treatment for geographic atrophy: A randomized clinical trial. Ophthalmology Retina 4, 889-898 (2020).
- ↑ Kuppermann, B.D., et al. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (brimo dds) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina 41, 144-155 (2021).
- ↑ Kandaswamy R, Wickremasinghe S, Guymer R. New Treatment Modalities for Geographic Atrophy. Asia Pac J Ophthalmol 2017;6(6):508-513.
- ↑ Sunnes et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999; 106:1768-1779.
- ↑ Sunnes JS, Margalit E, Srikumaran D, Applegate CA, Tian Y, Perry D, Hawkins B, Bressler NM. The Long-term Natural History of Geographic Atrophy from Age-Related Macular Degeneration : Enlargement of Atrophy and Implications for Interventional Clinical Trials. Ophthalmology 2007;114(2):271-277.