All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Familial Exudative Vitreoretinopathy (FEVR) is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
ICD9:
- 362.12 Exudative retinopathy
ICD10:
- H35.02 Exudative retinopathy:
- H35.021 – right eye
- H35.022 – left eye
- H35.023 – bilateral
- H35.029 – unspecified eye
Disease
Familial Exudative Vitreoretinopathy (FEVR) defines a group of inherited retinal diseases characterized by abnormal retinal angiogenesis leading to incomplete vascularization of the peripheral retina with subsequent retinal ischemia. As seen in retinopathy of prematurity (ROP), it is believed the avascular retina in FEVR may lead to hypoxia and stimulation of neovascular growth into the vitreous leading to later vitreoretinal traction, subretinal exudation and hemorrhages, retinal folds, tractional retinal detachment, and foveal displacement. FEVR is also associated with increased permeability of vessels and can present with exudative retinal detachment.
Familial exudative vitreoretinopathy is a genetically heterogenous disorder that exhibits variable penetrance across patients. The clinical appearance of FEVR tends to be asymmetric and vary significantly even among affected members of the same family. Patients with mild disease may be asymptomatic while those with severe disease can present with severe vision loss. The disease can be inherited in an autosomal dominant, autosomal recessive, or X-linked recessive fashion. Thus far, mutations in about 11 genes have been associated with FEVR. However, all known pathogenic variants of these genes accounted for about 50% of all FEVR cases worldwide.
History
Familial exudative vitreoretinopathy was first reported by Criswick and Schepens in 1969.[1] They described a retinal vascular disease in 6 patients from two families that appeared similar to ROP; however, this disease occurred in patients without prematurity or use of oxygen and continued to progress several years after birth. Some of the principal features seen were organized vitreous membranes, traction on the retina, heterotopia of the macula with temporal traction, subretinal and intraretinal exudates in the periphery (generally in the temporal retina), and peripheral neovascularization.
In 1971, Gow and Oliver described FEVR in a large family exhibiting an autosomal dominant inheritance pattern.[2] They described three stages of the disease. Stage 1 featured areas of white with pressure and white without pressure associated with vitreous traction. Stage 1 had no evidence of subretinal exudation or abnormal retinal vessels. Stage 2 had dilated tortuous retinal vessels in the periphery, exudation, local retinal detachments in the temporal periphery and a dragged disc. Stage 3 had more extensive retinal detachments, massive subretinal exudates, and other end-stage disease findings.
In 1976, Canny and Oliver described the fluorescein angiography (FA) of patients with FEVR and its similarities to retinopathy of prematurity. The FA findings confirmed the presence of capillary nonperfusion in the periphery with associated neovascularization.[3] It was not until 1992 when Li discovered that the autosomal dominant form of FEVR mapped to chromosome 11q.[4] This was later discovered to harbor the FZD4 and LRP5 genes.[5][6][7] As of the most recent update of this article, at least 10 genes have been implicated in association with FEVR including FZD4,[6][7] NDP,[8][9] LRP5, [7][10][11] TSPAN12,[12][13][14] ZN408, [15] KIF11,[16][17] [18][19] CTNNB1,[20][21] JAG1,[22] RCBTB1,[23] ATOH7,[24] DOCK6,[25] ARHGAP31,[25] and SNX31.[26]
Epidemiology
FEVR is described as a rare inherited disorder. The prevalence is not known.
Molecular genetics of FEVR have been reported across different racial and ethnic groups including Caucasian (e.g. Dutch,[13][27] Canadian,[6] Italian[28]), Japanese,[29][30] [31] Chinese,[32][33][34] Korean,[35] Indian,[36][37] and Mexican. [38] Approximately 35% to 50% of patients diagnosed with FEVR are found to have a corroborating genetic mutation. The contribution of any given gene to the disease differs among study populations. [39] FEVR caused by NDP, FZD4, LRP5, and TSPAN12 mutations has reported from15 countries including USA, UK, China, Spain, India, Australia, Mexico, Japan, Netherlands, Italy, Canada, Korea, Sweden, Pakistan, and Israel. [40]
Stages
In 1998, Pendergast and Trese proposed a 5-stage clinical classification of FEVR based on ophthalmoscopic findings at the time of presentation.[41] [42] See Table 1. However, patients with FEVR may not progress through these stages in a stepwise fashion.
Table 1. Proposed FEVR Clinical Staging System 1998 | |
|---|---|
| Stage | Clinical Features |
| 1 | Avascular retinal periphery without extraretinal vascularization |
| 2 | Avascular retinal periphery with extraretinal vascularization |
| 2A | Without exudate |
| 2B | With exudate |
| 3 | Extramacular retinal detachment |
| 3A | Primarily exudate |
| 3B | Primary tractional |
| 4 | Macula-involving retinal detachment, subtotal |
| 4A | Primarily exudative |
| 4B | Primary tractional |
| 5 | Total retinal detachment |
| 5A | Open funnel |
| 5B | Closed funnel |
In 2014, using wide-field angiography, Kashani et al proposed a revised clinical staging system of FEVR that includes fluorescein angiography.[39] See Table 2.
Table 2. Revised FEVR Clinical Staging System 2014 | |
|---|---|
| Stage | Clinical Features |
| 1 | Avascular periphery or anomalous intraretinal vascularization |
| 1A | Without exudate or leakage |
| 1B | With exudate or leakage |
| 2 | Avascular retinal periphery with extraretinal vascularization |
| 2A | Without exudate or leakage |
| 2B | With exudate or leakage |
| 3 | Extramacular retinal detachment |
| 3A | Without exudate or leakage |
| 4 | Macula-involving retinal detachment, subtotal |
| 4A | Without exudate or leakage |
| 4B | With exudate or leakage |
| 5 | Total retinal detachment |
| 5A | Open funnel |
| 5B | Closed funnel |
Pathophysiology
Criswick and Schepens initially postulated that FEVR was caused by the “proliferation of fetal-type retinal vessels, much like those seen in retrolental fibroplasia.” [1] Later, from studies using fluorescein angiography the pathogenesis was proposed to be related to incomplete peripheral vascularization,[2] and secondary neovascularization with subsequent vitreous hemorrhage and retinal detachment.[42] More recently, studies using genetically -modified animal models provided evidence that the underlying pathogenesis was dysfunctional retinal angiogenesis.[43][44][45] Many studies have suggested a component of atypical membrane formation in FEVR with pathologic vitreoretinal traction. Yonekawa et al performed a structural OCT study, which showed atypical epiretinal membranes originating from an abnormal vitreous.[46] At this time, the underlying cause of the formation of these membranes is not fully understood.
Genetics
Genes associated with FEVR have been reported including:
- Frizzled-4 (FZD4, 11q14.2) [6][7]
- Norrie disease pseudoglioma (NDP, Xp11.3) [8][9][11]
- Low-density lipoprotein receptor related protein-5 (LRP5, 11q13.2) [7][10]
- Tetraspanin-12 (TSPAN12, 7q31.31) [12][13][14]
- Zinc finger protein-408 (ZNF408, 11p11.2) [15]
- Kinesin family member 11 (KIF11, 10q23.33) [16][17][18][19][47]
- Cadherin-associated protein, beta (CTNNB1, 3p22.1)[20][21]
- Jagged 1 (JAG1, 20p12.2)[22]
- RCC1 and BTB domain containing protein 1 (RCBTB1, 13q14.2)[23]
- Atonal bHLH transcriptation factor 7 (ATOH7, 10q21.3)[24]
- Dedicator of Cytokinesis 6 (DOCK6, 19p13.2)[25]
- RHO GTPase-activating protein 31 (ARHGAP31, 3q13.32-q13.33) [25]
- Sorting nexin 31 (SNX31, 8q22.3)[26]
FEVR exhibits three different forms of inheritance: autosomal dominant, autosomal recessive, and x-linked recessive.[48] Among these, autosomal dominant is the most common form.[49] Most of the known identified genes have been linked to certain forms of inheritance:
- Autosomal dominant: FZD4, LRP5, TSPAN12, ZNF408, KIF11, CTNNB1, JAG1, ARHGAP31, SNX31
- Autosomal recessive: FZD4, LRP5, TSPAN12, RCBTB1, ATOH7, DOCK6
- X-linked recessive: NDP
.
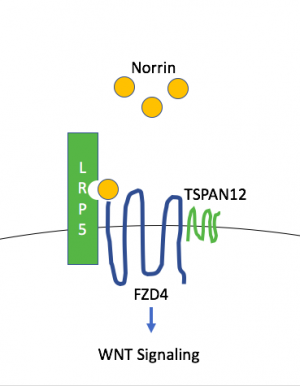
A biological pathway linked to FEVR is the WNT signaling pathway.[50] Norrin can be a ligand for WNT and trigger signaling through the same receptors. In the retina, the WNT signaling cascade regulates normal retinal vascular formation and involves activation of receptor FZD4 and LRP5 as well as TSPAN12.[51]
NDP, FZD4, LRP5, and TSPAN12 are part of the Norrin/β-catenin signaling pathway (also known as Norrin/Frizzled-4 pathway), which has been shown to be necessary for retinal angiogenesis. Variants in any of these genes has been associated with FEVR.[43][51] In retinal vascular development, Müller glia secrete Norrin (NDP gene), part of the TGF-beta superfamily that binds to Frizzled-4 (FZD4) on retinal endothelial cells and triggers WNT signaling. LRP5 is a WNT coreceptor, and TSPAN12 is believed to increase FZD4 multimerization, which enhances signaling. Mouse knockout models of the Norrin/Frizzled-4 pathway (Fzd4-/-, Ndp-/-, Lrp5-/-, and Tspan12-/-) show a stereotypic phenotypic pattern of retinal vascular defects including: absence of the intraretinal capillary plexi, artery and vein tortuosity, intraocular hemorrhage, and secondary delayed regression of the hyaloid vasculature. However, Lrp5-/- appear to have a less severe phenotype.[43][45][52]
Mutations in NDP are also seen in Norrie disease. Mutations in LRP5 are seen in Osteoporosis-pseudoglioma syndrome, which is characterized by the presence of low bone mass density. Patients with FEVR caused by LRP5 mutations should be evaluated for osteoporosis. [8][9][10]
Mutations in RCBTB1 are reported in association with Coats disease.[23] ATOH7 was identified as the gene associated with persistent fetal vasculature syndrome.[53][54] KIFF11 mutations, identified in approximately 5-8% of patients with FEVR, [19] are also associated with a rare autosomal dominant inherited syndrome called microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR). [16] Mutations of Adams-Oliver syndrome (AOS) genes: DOCK6 and ARHGAP31 were found in two patients presenting with FEVR and microcephaly.[25] Most recently, a mutation in SNX31, an intracellular scaffolding protein implicated in protein trafficking, was identified in a Chinese family without mutations in the other known FEVR genes.[26]
In a retrospective study of 89 patients with retinal folds associated with FEVR, it was observed that patients with mutations in NDP, TSPAN12, or KIF11 were more likely to have bilaterally symmetrical severe retinopathy in contrast to those with mutations in LRP5 and FZD4 who had milder but a broader phenotypic spectrum and greater asymmetry.[55] In a study involving a large cohort of 389 patients screened for genetic variants by a single clinic, patients with mutations in NDP and KIF11 had more severe disease than those with mutations in the other 4 genes evaluated (LRP5, FZD4, TSPAN12, ZNF408). [33] The same study also showed that variants in LRP5 and FZD4 exhibited more significant variation in phenotype than variants in TSPAN12 and NDP. However, the literature is not in agreement about genotype-phenotype relationships, and there is much variability in the phenotype of family members with the same mutation as well as in the same individual between eyes.
Diagnosis
History
The presence of bilateral avascular peripheral retina in the setting of full-term birth without supplemental oxygenation and a progressive clinical course distinguish FEVR from ROP. A positive family history provides additional evidence of the diagnosis. However, a negative family history is less useful, because a positive family history of FEVR can be present less than 10% of the time in some studies and up to 50% in others.[50] Furthermore, many family members with FEVR are asymptomatic and are not aware of the diagnosis.
In a retrospective chart review of 273 eyes in 145 patients, Ranchod et al described the clinical presentation of FEVR patients.[42] The mean age at presentation was 58.6 months. Compared to patients with ROP, the mean birth weight was 2798g and gestational age was 37.8 weeks. However, the range of birth weights was from 740-4763g and range of gestational ages was 25-42 weeks showing that there can be overlap with ROP. Twenty-six (18%) of patients with FEVR had a positive family history of FEVR. However, an additional 28 patients (19%) had a family history of ocular disease potentially representing undiagnosed FEVR. As FEVR can be highly asymmetric between eyes of the same patient, the study reported less than half (43%) of patients with the same clinical stage in both eyes, whereas 71% had bilateral disease within 1 stage of each other.
Physical Exam
Careful dilated fundus examination with wide angle fluorescein angiography is important in patients with FEVR for accurate clinical staging and management. An examination under anesthesia may be required for diagnosis, imaging acquisition, and treatment. Many of these patients will have clinical findings similar to retinopathy of prematurity, but full-term birth, normal birthweight, and no history of oxygen requirement should alert the physician to the possibility of FEVR.
Signs
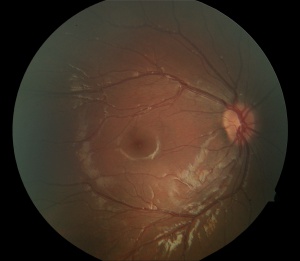
- Avascular peripheral retina - This can be appreciated on careful dilated fundus examination. However, in subtle cases or asymptomatic family members, wide angle fluorescein angiography is helpful in uncovering this finding. Classically, the temporal quadrant is most often involved with a v-shaped demarcation. However, the avascular zone can extend 360 degrees. The demarcation has been described as a "brush-border."
- Dragged retinal vessels and macula - Retinal arteries and veins can be dragged, usually temporally with apparent straightening of the vessels. In addition, the macula can be dragged temporally.
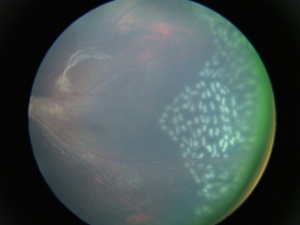
- Retinal (falciform) folds - Radial retinal folds were seen in 28% of eyes in one study. Retinal folds are most often seen in the temporal location, but they can be seen in any location.[42]
- Neovascularization -The avascular retina is believed to lead to retinal hypoxia, which can induce extraretinal neovascularization.
- Subretinal exudates - Variable amounts of subretinal exudation can be seen. Massive exudation can mimic Coats' disease.
- Retinal detachments - Tractional and/or rhegmatogenous retinal detachments can be seen in severe cases, occurring in 21–64% of affected individuals.[48] In the Ranchod et al series, of the 91 patients who presented with a retinal detachment in the worse eye, 62 (68%) presented with some degree of retinal detachment in the fellow eye as well.[42]
- Persistent fetal vasculature
- Epiretinal membranes
- Peripheral pigmentation particularly associated with peripheral avascular retina
Ancillary Tests
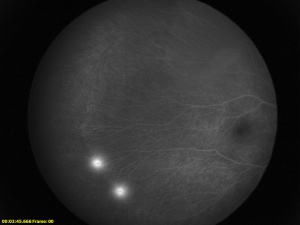
Fluorescein Angiography
Fluorescein angiography is helpful in the diagnosis and evaluation of FEVR especially in cases with only peripheral avascular retina. The avascular zone is classically described as a v-shaped pattern in the temporal periphery, typically larger than the rest of the periphery but can extend 360 degrees.[56] In a case series of 74 subjects from 17 families, Kashani et al described a variety of retinal vascular anomalies in FEVR using wide-field fluorescein angiography, including:[57]
- Telangiectasias in the macula or periphery
- Optic disc leakage
- Arterial tortuosity
- Peripheral capillary agenesis
- Anomalous vascularization or supernumerary vascular branching in areas of vascular-avascular junctions
- Aberrant circumferential vessels.
- Delayed AV transit
- Choroidal nonperfusion
- Venous-venous shunting
- Central macular edema
.
The majority of index patients (71%) had late-stage FEVR and poor visual acuity in the Kashani et al study. More than half of the asymptomatic relatives demonstrated clinical and angiographic findings consistent with early stages of the diseases and a third were found to have avascular retina with exudate or were at risk of developing exudate.
With the increasing use of wide-field fluorescein angiography, FEVR is increasingly recognized to have a broad range of appearances. This imaging modality is helpful for diagnosis and management of FEVR. Fluorescein leakage without clinical exudation raises concern for the exudative phase of the disease. Because exudation has been reported to complicate laser photocoagulation, detection of leakage on a fluorescein angiogram allows for timely treatment before exudation occurs.[57]
Optical Coherence Tomography
Optical coherence tomography (OCT) can show posterior segment microstructures present in FEVR including:[46]
- Posterior hyaloidal organization
- Vitreomacular traction
- Vitreopapillary traction
- Diminished foveal contour
- Persistence fetal foveal archiecture
- Cystoid macular edema
- Intraretinal exudates
- Distortion of the ellipsoid zone
OCT angiography (OCTA) has also been emerging as a noninvasive imaging modality to characterize FEVR. Chen et al analyzed OCTA images from 41 eyes of 41 FEVR patients and 37 eyes of 37 age-matched, control patients. Their findings demonstrated smaller foveal avascular zone, decreased vascular density in both the superficial and deep layers of parafoveal area, and a thicker fovea in eyes from FEVR patients compared to control patients.[58] Another more recent study of 46 eyes from 26 FEVR patients imaged by wide-field OCTA and fluorescein angiography found similar detection rates of peripheral retinal vascular abnormalities, peripheral retinal avascular zone, retinal neovascularization, macular ectopia, and temporal mid-peripheral vitreoretinal interface abnormalities.[59] In the same study, OCTA also identified vitreoretinal traction and small foveal avascular zone in 17 eyes (37%). Taken together, the data suggest that OCTA has the potential to detect structural and vascular changes associated with FEVR.
Genetic Testing
Genetic testing is offered for diagnostic confirmation and to screen family members if the genetic defect is known. In addition, it is helpful if the diagnosis is unclear such as in differentiating from other vitreoretinopathies with similarities to FEVR, such as Norrie disease, persistent fetal vasculature syndrome, incontinentia pigmenti, and retinopathy of prematurity. Results from genetic testing may help guide systemic management. For example, if a defect in the LRP5 gene is found, evaluation for osteoporosis should be performed.[10]
Mutations in genes implicated in FEVR thus far account for less than 50% of all FEVR cases. For example, a 2017 study of Chinese families found that mutations in six known genes at the time (LRP5, FZD4, TSPAN12, NDP, ZNF408, and KIF11) only accounted for 38.7% of patients with FEVR. [34] Thus, physicians and patients should be aware of the high likelihood of a negative genetic test suggesting there are other genes in the disease pathogenesis yet to be identified.
Differential Diagnosis
- Retinopathy of prematurity - In contrast to FEVR, there is a history of prematurity with possible use of oxygen. Family history is often absent. Subretinal or intraretinal exudation is less common.[60]The clinical course of ROP often is not progressive in the years after birth as is seen in FEVR.[42]
- Norrie disease - In contrast to FEVR, patients can have microphthalmia, corneal opacification, developmental delay and deafness. [51]
- Coats' disease - Usually unilateral and with a male predilection (80%). In contrast to FEVR, neovascularization and tractional membranes are not usually seen.[60]
- Incontinentia pigmenti - X-linked dominant and lethal in males. In contrast to FEVR, patients have pathognomonic skin findings at birth and can have neurologic issues including developmental delay, paralysis and seizures.
- Persistent fetal vasculature- Typically sporadic and unilateral. However, FEVR can manifest as persistent fetal vasculature, and genetic testing is often considered.
- Toxocara canis - Usually unilateral with associated uveitis.
Clinical Course
When it was first reported in 1969, FEVR was presented as a progressive disease.[1] In 1980, Ober et al presented a case series of 3 families affected by autosomal dominant FEVR and showed that the disease can be asymptomatic and nonprogressive.[61] It was observed that the disease was not more advanced in older compared to younger patients within families. In general, the disease tends to have a progressive course during childhood and adolescence and can become latent after 20 years of age. However, late progression with vision threatening complications, including vitreous hemorrhage and retinal detachment, can occur at any age after varying periods of quiescence.[62]
Benson evaluated the natural history of FEVR in 39 patients. It was found that patients who were diagnosed before the age of 3 had a more severe clinical course with very poor visual prognosis. The most rapid progression is generally seen in children and adolescents. If patients do not have progression before the age of 20, the disease can remain stable. In the study, 3 eyes of 4 patients who were asymptomatic to the age of 15 had a final visual acuity of counting fingers or worse. In addition, late deterioration can be seen with retinal detachments occurring 6 to 17 years after “apparent stabilization.”[60] A case series by Ranchod et al demonstrated a high propensity toward retinal detachment in the fellow eye in patients presenting with retinal detachment (62 out of 91, 68%). Pendergast and Trese showed that patients with Stage 1 disease did not progress to a more advanced stage with a mean follow up on 33 months. [41] Long-term follow up of these patients is important to detect reactivation.
Management
The management of FEVR depends on the clinical stage of the disease. Due to the vision threatening sequelae of neovascularization, an early diagnosis and treatment can be vision preserving. One study showed that 58% of asymptomatic family members had clinical and angiographic evidence of Stage 1 and 2 FEVR.[39] Therefore, screening of family members of patients with FEVR is recommended. Due to the association with osteoporosis-pseudoglioma syndrome caused by the LRP5 mutation, all FEVR patients who do have access to genetic testing should have DEXA scan to assess bone mineral density.[10] If positive for osteoporosis, then the patient should be referred to experts for consideration of bisphosphonate therapy.[63]
Patients with Stage 1 disease[41] can be observed and followed over time. For stage 2 disease, laser photocoagulation of the avascular zones is recommended to promote regression of neovascularization and resolution of exudation. Patients with more advanced disease with retinal detachments require surgical management. Scleral buckling and/or vitrectomy can be considered depending on the clinical scenario with laser photocoagulation of the peripheral avascular retina. Patients with Stage 3A or partial exudative retinal detachments have been reported to have favorable outcomes with scleral buckling alone.[41]
Few studies have evaluated the use of anti-VEGF in the treatment of FEVR. One case report of a patient with FEVR with neovascularization showed regression after one injection of bevacizumab with short term follow up.[64] In a larger study, intravitreal bevacizumab (IVB) was used as an adjunct therapy to either laser or surgical management. [65] Rapid progression of tractional retinal detachment occurred in 3 eyes of patients with stage 3B FEVR who were treated with IVB as an adjunct to surgery with scleral buckling and indirect diode laser (2 eyes) or vitrectomy with membrane peeling and endolaser (1 eye). In contrast, all 7 eyes with stage 2 or 3A FEVR in the same series achieved stabilization of disease with laser and IVB alone and did not develop progressive tractional retinal detachment. A case report of a patient with persistent subretinal exudation that was minimally responsive to intravitreal injections of anti-VEGF agents (aflibercept and ranibizumab) showed resolution in response to intravitreal injections of faricimab[66] . Therefore, further studies testing the efficacy of targeting VEGF and angiopoietin-2 receptors in the management of FEVR would need to be considered .
References
- ↑ 1.0 1.1 1.2 Criswick V, Schepens C. Familial Exudative Vitreoretinopathy. American Journal of Ophthalmology. 1969;68(4):578-594.
- ↑ 2.0 2.1 Gow J, Oliver GL. Familial exudative vitreoretinopathy. An expanded view. Arch Ophthalmol. 1971;86(2):150-5.
- ↑ Canny CLB, Oliver GL. Fluorescein Angiographic Findings in Familial Exudative Vitreoretinopathy. Archives of Ophthalmology. 1976;94(7):1114-1120.
- ↑ Li Y, Fuhrmann C, Schwinger E, Gal A, Laqua H. The gene for autosomal dominant familial exudative vitreoretinopathy (Criswick-Schepens) on the long arm of chromosome 11. Am J Ophthalmol. 1992;113(6):712-3.
- ↑ Li Y, Müller B, Fuhrmann C, et al. The autosomal dominant familial exudative vitreoretinopathy locus maps on 11q and is closely linked to D11S533. Am J Hum Genet. 1992;51(4):749–54
- ↑ 6.0 6.1 6.2 6.3 Robitaille J, MacDonald ML, Kaykas A, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32(2):326–30.
- ↑ 7.0 7.1 7.2 7.3 7.4 Toomes C, Bottomley HM, Jackson RM, et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74(4):721–30.
- ↑ 8.0 8.1 8.2 Plager DA, Orgel IK, Ellis FD, Hartzer M, Trese MT, Shastry BS. X-linked recessive familial exudative vitreoretinopathy. Am J Ophthalmol. 1992;114(2):145–8.
- ↑ 9.0 9.1 9.2 Chen ZY, Battinelli EM, Fielder A, et al. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet. 1993;5(2):180–3.
- ↑ 10.0 10.1 10.2 10.3 10.4 Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–23.
- ↑ 11.0 11.1 Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF. Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am J Hum Genet. 2004;75(5):878-84.
- ↑ 12.0 12.1 Poulter JA, Ali M, Gilmour DF, et al. Mutations in TSPAN12 cause autosomal-dominant familial exudative vitreoretinopathy. Am J Hum Genet. 2010;86(2):248–53.
- ↑ 13.0 13.1 13.2 Nikopoulos K, Gilissen C, Hoischen A, et al. Next-generation sequencing of a 40 Mb linkage interval reveals TSPAN12 mutations in patients with familial exudative vitreoretinopathy. Am J Hum Genet. 2010;86(2):240–7.
- ↑ 14.0 14.1 Poulter JA, Davidson AE, Ali M, et al. Recessive mutations in TSPAN12 cause retinal dysplasia and severe familial exudative vitreoretinopathy (FEVR). Invest Ophthalmol Vis Sci. 2012;53(6):2873–9.
- ↑ 15.0 15.1 Collin RW, Nikopoulos K, Dona M, et al. ZNF408 is mutated in familial exudative vitreoretinopathy and is crucial for the development of zebrafish retinal vasculature. Proc Natl Acad Sci USA. 2013;110(24):9856–61.
- ↑ 16.0 16.1 16.2 Robitaille JM, Gillett RM, LeBlanc MA, et al. Phenotypic overlap between familial exudative vitreoretinopathy and microcephaly, lymphedema, and chorioretinal dysplasia caused by KIF11 mutations. JAMA Ophthalmol. 2014;132(12):1393–9.
- ↑ 17.0 17.1 Hu H, Xiao X, Li S, Jia X, Guo X, Zhang Q. KIF11 mutations are a common cause of autosomal dominant familial exudative vitreoretinopathy. Br J Ophthalmol. 2016;100(2):278–83.
- ↑ 18.0 18.1 Chen C, Sun L, Li S, Huang L, Zhang T, Wang Z, et al. Novel variants in familial exudative vitreoretinopathy patients with KIF11 mutations and the Genotype-Phenotype correlation. Exp Eye Res. 2020;199:108165.
- ↑ 19.0 19.1 19.2 Li JK, Fei P, Li Y, Huang QJ, Zhang Q, Zhang X, et al. Identification of novel KIF11 mutations in patients with familial exudative vitreoretinopathy and a phenotypic analysis. Sci Rep. 2016;6:26564.
- ↑ 20.0 20.1 Sun W, Xiao X, Li S, Jia X, Wang P, Zhang Q. Germline mutations in CTNNB1 associated with syndromic FEVR or Norrie disease. Invest Ophthalmol Vis Sci. 2019;60:93-97.
- ↑ 21.0 21.1 Dixon MW, Stem MS, Schuette JL, Keegan CE, Besirli CG. CTNNB1 mutation associated with familial exudative vitreoretinopathy (FEVR) phenotype. Ophthalmic Genet. 2016;37(4):468-70.
- ↑ 22.0 22.1 Zhang L, Zhang X, Xu H, Huang L, Zhang S, Liu W, et al. Exome sequencing revealed Notch ligand JAG1 as a novel candidate gene for familial exudative vitreoretinopathy. Genet Med. 2020;22(1):77-84.
- ↑ 23.0 23.1 23.2 Wu JH, Liu JH, Ko YC, et al. Haploinsufficiency of RCBTB1 is associated with Coats disease and familial exudative vitreoretinopathy. Hum Mol Genet. 2016;25:1637-1647.
- ↑ 24.0 24.1 Kondo H, Matsushita I, Tahira T, Uchio E, Kusaka S. Mutations in ATOH7 gene in patients with nonsyndromic congenital retinal nonattachment and familial exudative vitreoretinopathy. Ophthalmic Genet. 2016;37:462-464.
- ↑ 25.0 25.1 25.2 25.3 25.4 Tao Z, Bu S, Lu F. Two AOS genes attributed to familial exudative vitreoretinopathy with microcephaly: Two case reports. Medicine (Baltimore). 2021 Mar 5;100(9):e24633. doi: 10.1097/MD.0000000000024633. PMID: 33655927; PMCID: PMC7939203.
- ↑ 26.0 26.1 26.2 Xu N, Cai Y, Li J, Tao T, Liu C, Shen Y, Li X, Zhang L, Zhao M, Shi X, Li J, Huang L. An SNX31 variant underlies dominant familial exudative vitreoretinopathy-like pathogenesis. JCI Insight. 2023 May 22;8(10):e167032. doi: 10.1172/jci.insight.167032. PMID: 37053012; PMCID: PMC10322688.
- ↑ Boonstra FN, van Nouhuys CE, Schuil J, de Wijs IJ, van der Donk KP, Nikopoulos K, et al. Clinical and molecular evaluation of probands and family members with familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2009;50(9):4379-85.
- ↑ Iarossi G, Bertelli M, Maltese PE, Gusson E, Marchini G, Bruson A, et al. Genotype-Phenotype Characterization of Novel Variants in Six Italian Patients with Familial Exudative Vitreoretinopathy. J Ophthalmol. 2017;2017:3080245.
- ↑ Omoto S, Hayashi T, Kitahara K, Takeuchi T, Ueoka Y. Autosomal dominant familial exudative vitreoretinopathy in two Japanese families with FZD4 mutations (H69Y and C181R). Ophthalmic Genet. 2004;25(2):81-90.
- ↑ Kondo D, Noguchi A, Takahashi I, Kubota H, Yano T, Sato Y, et al. A novel ZC4H2 gene mutation, K209N, in Japanese siblings with arthrogryposis multiplex congenita and intellectual disability: characterization of the K209N mutation and clinical findings. Brain Dev. 2018;40(9):760-7.
- ↑ Milhem RM, Ben-Salem S, Al-Gazali L, Ali BR. Identification of the cellular mechanisms that modulate trafficking of frizzled family receptor 4 (FZD4) missense mutants associated with familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2014;55(6):3423-31.
- ↑ Xu H, Zhang S, Huang L, Zhao P, Zhang X, Yang Z, et al. Identification of novel variants in the FZD4 gene associated with familial exudative vitreoretinopathy in Chinese families. Clin Exp Ophthalmol. 2020;48(3):356-6.
- ↑ 33.0 33.1 Li JK, Li Y, Zhang X, Chen CL, Rao YQ, Fei P, et al. Spectrum of Variants in 389 Chinese Probands With Familial Exudative Vitreoretinopathy. Invest Ophthalmol Vis Sci. 2018;59(13):5368-81.
- ↑ 34.0 34.1 Rao F-QQ, Cai X-BB, Cheng F-FF, et al. Mutations in LRP5,FZD4, TSPAN12, NDP, ZNF408, or KIF11 Genes Account for 38.7% of Chinese Patients With Familial Exudative Vitreoretinopathy. Invest Ophthalmol Vis Sci. 2017;58(5):2623–2629.
- ↑ Seo SH, Yu YS, Park SW, et al. Molecular characterization of FZD4, LRP5, and TSPAN12 in familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2015;56:5143‒5151.
- ↑ Musada GR, Jalali S, Hussain A, Chururu AR, Gaddam PR, Chakrabarti S, et al. Mutation spectrum of the Norrie disease pseudoglioma (NDP) gene in Indian patients with FEVR. Mol Vis. 2016;22:491-502.
- ↑ Shukla D, Singh J, Sudheer G, et al. Familial exudative vitreoretinopathy(FEVR). Clinical profile and management. Indian J Ophthalmol. 2003;51:323‒328.
- ↑ Toomes C, Downey LM, Bottomley HM, Mintz-Hittner HA, Inglehearn CF. Further evidence of genetic heterogeneity in familial exudative vitreoretinopathy; exclusion of EVR1, EVR3, and EVR4 in a large autosomal dominant pedigree. Br J Ophthalmol. 2005;89:194-197.
- ↑ 39.0 39.1 39.2 Kashani AH, Learned D, Nudleman E, Drenser KA, Capone A, Trese MT. High prevalence of peripheral retinal vascular anomalies in family members of patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(1):262–8.
- ↑ Xiao H, Tong Y, Zhu Y, Peng M. Familial Exudative Vitreoretinopathy-Related Disease-Causing Genes and Norrin/beta-Catenin Signal Pathway: Structure, Function, and Mutation Spectrums. J Ophthalmol. 2019;2019:5782536.
- ↑ 41.0 41.1 41.2 41.3 Pendergast SD, Trese MT. Familial exudative vitreoretinopathy. Results of surgical management.Ophthalmology. 1998;105(6):1015–23.
- ↑ 42.0 42.1 42.2 42.3 42.4 42.5 Ranchod TM, Ho LY, Drenser KA, Capone A, Trese MT. Clinical presentation of familial exudative vitreoretinopathy. Ophthalmology. 2011;118(10):2070–5.
- ↑ 43.0 43.1 43.2 Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883–95.
- ↑ Xia CH, Liu H, Cheung D, et al. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum Mol Genet. 2008;17:1605‒1612
- ↑ 45.0 45.1 Ye X, Wang Y, Cahill H, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285–98.
- ↑ 46.0 46.1 Yonekawa Y, Thomas BJ, Drenser KA, Trese MT, Capone A. Familial Exudative Vitreoretinopathy: Spectral-Domain Optical Coherence Tomography of the Vitreoretinal Interface, Retina, and Choroid.Ophthalmology. 2015;122(11):2270–7.
- ↑ Retinal Features of Family Members With Familial Exudative Vitreoretinopathy Caused By Mutations in KIF11 Gene. Kondo H, Matsushita I, Nagata T, Fujihara E, Hosono K, Uchio E, Hotta Y, Kusaka S.Transl Vis Sci Technol. 2021 Jun 1;10(7):18. doi: 10.1167/tvst.10.7.18.PMID: 34128965
- ↑ 48.0 48.1 Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies.Eye (Lond). 2015;29:1‒14.
- ↑ Sizmaz S, Yonekawa Y, Trese TM. Familial exudative vitreoretinopathy. Turk J Ophthalmol. 2015;45:164-168.
- ↑ 50.0 50.1 Tauqeer Z, Yonekawa Y. Familial Exudative Vitreoretinopathy: Pathophysiology, Diagnosis, and Management. Asia Pac J Ophthalmol (Phila). 2018 May-Jun;7(3):176-182
- ↑ 51.0 51.1 51.2 Nikopoulos K, Venselaar H, Collin RWJ, et al. Overview of the mutation spectrum in familial exudative vitreoretinopathy and Norrie disease with identification of 21 novel variants in FZD4, LRP5, and NDP. Hum Mutat. 2010;31:656‒666.
- ↑ Junge HJ, Yang S, Burton JB, et al. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139(2):299–311. doi:10.1016/j.cell.2009.07.048.
- ↑ Prasov L, Masud T, Khaliq S, Mehdi SQ, Abid A, Oliver ER, et al. ATOH7 mutations cause autosomal recessive persistent hyperplasia of the primary vitreous. Hum Mol Genet. 2012;21(16):3681-94.
- ↑ Kondo H. Complex genetics of familial exudative vitreoretinopathy and related pediatric retinal detachments. Taiwan J Ophthalmol. 2015;5(2):56-62.
- ↑ Wang Z, Chen C, Sun L, Zhang A, Liu C, Huang L, Ding X. Symmetry of folds in FEVR: A genotype-phenotype correlation study. Exp Eye Res. 2019 Sep;186:107720.
- ↑ Miyakubo H, Hashimoto K, Miyakubo S. Retinal vascular pattern in familial exudative vitreoretinopathy.Ophthalmology. 1984;91(12):1524–30.
- ↑ 57.0 57.1 Kashani AH, Brown KT, Chang E, Drenser KA, Capone A, Trese MT. Diversity of retinal vascular anomalies in patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(11):2220–7.
- ↑ Chen C, Liu C, Wang Z, Sun L, Zhao X, Li S, Luo X, Zhang A, Chong V, Lu L, Ding X. Optical Coherence Tomography Angiography in Familial Exudative Vitreoretinopathy: Clinical Features and Phenotype-Genotype Correlation. Invest Ophthalmol Vis Sci. 2018 Dec 3;59(15):5726-5734. doi: 10.1167/iovs.18-25377. PMID: 30513533.
- ↑ Wang Y, Lai Y, Zhou X, Zhang T, Sun L, Zhang Z, Huang L, Li S, Ding X. Ultra-wide-field optical coherence tomography angiography in mild familial exudative vitreoretinopathy. Retina. 2023 Jan 31. doi: 10.1097/IAE.0000000000003754. Epub ahead of print. PMID: 36809312.
- ↑ 60.0 60.1 60.2 Benson WE. Familial exudative vitreoretinopathy. Trans Am Ophthalmol Soc. 1995;93:473–521.
- ↑ Ober R, Bird A, Hamilton A, et al. Autosomal dominant exudativevitreoretinopathy. Br J Ophthalmol. 1980;64:112‒120.
- ↑ Tagami M, Kusuhara S, Honda S, et al. Rapid regression of retinal hemorrhage and neovascularization in a case of familial exudative vitreoretinopathy treated with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008;246:1787‒1789.
- ↑ Streeten EA, McBride D, Puffenberger E, Hoffman ME, Pollin TI, Donnelly P, et al. Osteoporosis-pseudoglioma syndrome: description of 9 new cases and beneficial response to bisphosphonates. Bone. 2008;43(3):584-90.
- ↑ Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Rapid regression of retinal hemorrhage and neovascularization in a case of familial exudative vitreoretinopathy treated with intravitreal bevacizumab.Graefes Arch Clin Exp Ophthalmol. 2008;246(12):1787–9.
- ↑ Henry CR, Sisk RA, Tzu JH, et al. Long-term follow-up of intravitreal bevacizumab for the treatment of pediatric retinal and choroidal diseases. J AAPOS. 2015;19(6):541–8.
- ↑ Ghoraba HH, DeBoer C, Moshfeghi DM. Rapid Improvement in Lipid Maculopathy Following Faricimab Therapy in Recalcitrant Familial Exudative Vitreoretinopathy. Ophthalmic Surg Lasers Imaging Retina. 2023 Jul;54(7):426-428. doi: 10.3928/23258160-20230609-01. Epub 2023 Jun 1. PMID: 37418669.