All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Surgical Procedure
Exploration, Excision, Decompression on the Orbit of the Ocular Adnexa CPT 67400-67450
Background
Orbital decompression is a surgical procedure that is used to treat exophthalmos (also known as proptosis), a condition in which the eye protrudes anteriorly out of the orbits.[1] Orbital decompression involves careful removal or thinning of the orbital walls (and orbital fat) to expand the space in the orbit, allowing for accommodation of abnormally enlarged muscles and tissue that exceed the native volume of the orbit. Decompression can be customized according to the severity of proptosis, along with considerations for functional and cosmetic requirements. In some cases, the increase in orbital contents places pressure on the optic nerve and surrounding vasculature, leading to exposure keratopathy, optic neuropathy, and other visual disturbances. The most common indication for orbital decompression is thyroid eye disease (TED), which affects 25 to 50% of patients with Graves’ disease.[2] Outcomes are dependent on the knowledge of underlying pathogenesis and choice of surgical techniques.
History
The first description of orbital decompression was reported in 1911 by Dollinger et al,[3] in which a lateral wall technique achieved minimal decompression into the temporal fossa. Since then, a variety of techniques have surfaced, each removing one or more orbital walls and even orbital fat. The method by which access is gained into the orbit also varies. Kennedy et al[4] recently introduced an endoscopic transnasal approach, which has increasingly replaced other techniques. However, as with the other techniques, there are limitations. Despite studies that attempt to prove superiority of one approach over another, customization on an individual basis is preferred, with a focus on specific anatomical and clinical findings to select the appropriate plan.
Anatomy
Structure and Function
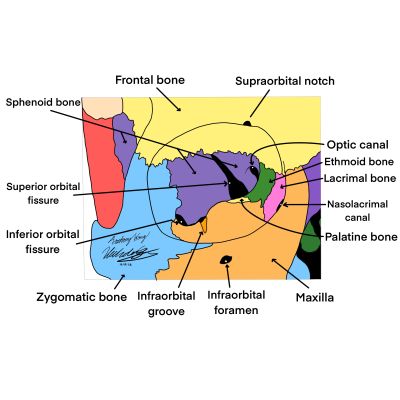
The orbits are bony structures of the skull that allow for the protection, three dimensional movement, and neural communication of the eye. The orbit as a whole houses the globe, fat, muscles, vessels, and nerves. The lacrimal, ethmoid, zygomatic, palatine, frontal, sphenoid, and maxillary bones form the four walls of each orbit[5]. The medial wall is composed of the lacrimal, ethmoid, maxillary, and lesser wing of sphenoid bones. The lateral wall is made of the zygomatic and greater wing of sphenoid bones. The roof is derived from the frontal and lesser wing of the sphenoid bones. Finally, the floor comprises the palatine, maxillary, and zygomatic bones. Several foramen and canals perforate the structure, transmitting vital nerves and blood vessels. Each wall also anchors muscles and ligaments that facilitate movement of the eye.
All of the orbital walls have a curvilinear shape that serves as a cushion against blunt forces. Connective tissue and orbital fat that surround the extraocular muscles provide additional protection to the orbital contents. The orbit is surrounded by the maxillary sinus inferiorly, ethmoid sinus medially, brain superiorly, and temporalis muscle temporally. The main lacrimal gland is present near the superior lateral portion of the orbital roof and the accessory lacrimal gland is found within the lamina propria of the conjunctiva. Their secretory products include tears, which lubricate the eye and maintain the proper microenvironment for ocular function.
Blood Supply and Lymphatics
The major artery within the orbit is the ophthalmic artery, which branches from the internal carotid artery just as it exits the cavernous sinus. After entering the orbit via the optic canal, it courses medially and over the optic nerve, continuing along the medial wall. Its many branches include the central retinal artery (retina), lacrimal artery (eyelids, lacrimal gland, superior cheek, conjunctiva), and superior and inferior muscular arteries (superior rectus, superior oblique, inferior rectus, medial rectus).
The inferior orbital fissure transmits the infraorbital artery, a branch of the maxillary artery, and the infraorbital vein, which drains into the pterygoid plexus. Both accompany the infraorbital nerve within the orbit before exiting through the infraorbital foramen.
The superior ophthalmic veins run with the ophthalmic artery and course through the superior orbital fissure, joining the inferior ophthalmic vein before draining into the cavernous sinus.
Lymphatic drainage of the orbital and periorbital regions have differing descriptions within the literature. For many years, the orbit was believed to have sparse drainage; however, new findings support greater lymphatics within the area, particularly in the lacrimal gland.[6]
Nerves
Cutaneous innervation to the maxillary region of the face is provided by the infraorbital nerve, a branch of the maxillary division of the trigeminal nerve. The infraorbital nerve originates from the inferior orbital fissure, where it courses along the orbital floor before entering the infraorbital canal and emerging from the infraorbital foramen.
The superior orbital fissure serves as a channel for cranial nerves (CN) III (oculomotor), IV (trochlear), V1 (ophthalmic branch of trigeminal), and VI (abducens). CN III provides innervation to the superior rectus, medial rectus, inferior rectus, and inferior oblique muscles. CN IV innervates the superior oblique muscle and CN VI innervates the lateral rectus muscle. Sensation to the upper face, mucous membranes, and scalp is supplied by the ophthalmic branch of the trigeminal nerve.
Slightly medial to the superior orbital fissure lies the optic canal, through which the optic nerve (CN II) transmits visual input from the retina to the central nervous system.
Muscles and Attachments
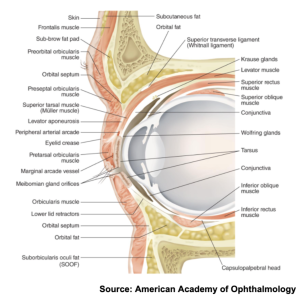
The orbicularis oculi muscle is a broad, flat muscle that encircles the orbit and serves to close the eyelids and compress the lacrimal sac. The muscle may be divided into the orbital, palpebral, and lacrimal part. Just beneath the orbicularis oculi is a membranous sheet extending from the periosteum called the septum orbitale. This layer attaches to the rim of the orbit and plays a role in reducing the spread of infection.[6] Past the septum orbitale lie muscles attaching to the tarsus, which are thick pads of dense connective tissue within the lid margins. The aponeurosis of the levator muscle in the upper lid attaches to the tarsus near the lid margin. Deep to this aponeurosis lies the inferior tarsal muscle (Mueller’s muscle) that attaches to the capsulopalpebral fascia and inserts into the tarsus. In the lower lid, the inferior rectus fascia connects to the orbicularis oculi muscle, inferior tarsus, and subcutaneous tissues.
The superior rectus, inferior rectus, lateral rectus, medial rectus, superior oblique, inferior oblique, and levator palpebrae superioris constitute the seven extraocular muscles of the orbit. Except for the inferior oblique, all other extraocular muscles originate at the annulus of Zinn. The four rectus muscles attach anteriorly to the globe at their respective positions. Meanwhile, the superior oblique travels in the superomedial corner of the orbit, where it becomes tendinous and passes through the trochlea and curves posterolaterally to attach to the sclera. Similarly, the inferior oblique ascends posterolaterally from its origin and inserts onto the sclera between the inferior and lateral recti muscles.
Differential Diagnosis
Exophthalmos is defined as a 2mm or greater difference in protrusion between eyes. Depending on the laterality and age of the patient, several different clinical entities exist on the differential diagnosis.
Although not exhaustive, the differential diagnosis for proptosis without specific age related considerations should include the following:[7]
- Orbital cellulitis
- Developmental anomalies
- Primary or metastatic neoplasm
- Metabolic disease
- Thyroid eye disease
- Cavernous sinus thrombosis
- Orbital myositis
- Metastatic neuroblastoma
- Obesity-related increase in orbital fat
- Cushing’s syndrome
- Idiopathic orbital inflammation (Orbital Pseudotumor)
- Vascular structural abnormalities (such as aneurysm, fistula (Carotid Cavernous Fistula), thrombosis)
- Infiltrative/Autoimmune disorders (such as IgG4 Disease)
- Physiologic Asymmetry of the orbits
- Asymmetric axial orbital length
- Contralateral enophthalmos (due to blow out fractures, scirrhous tumors)
Note: Relevant anatomy should be considered when creating the differential diagnosis as eyelid retraction and globe malposition can mistakenly give the illusion of proptotic eyes.
As a guideline the differential diagnoses by age may consider the following: [8]
- Ages 1-5: Dermoid, Epithelial cyst, Hemangioma, Sphenoid wing meningioma, Metastatic neuroblastoma, Rhabdomyosarcoma, and Glioma of the optic nerve
- Ages 5-10: Retinoblastoma, Malignant lymphoma or leukemia, Dermoid, Hemangioma, and Pseudotumor
- Ages 10-30: Mucocele, Meningioma, Pseudotumor, Lacrimal gland tumor, Malignant lymphoma or leukemia, Dermoid, Hemangioma, and TED
- Ages 30-50: Mucocele, Malignant lymphoma or leukemia, Hemangioma, Lacrimal gland tumor, Pseudotumor, and TED
- Over age 70: Pseudotumor, Melanoma, Lymphoma, and Mucocele
Thyroid Eye Disease
Epidemiology
Thyroid eye disease, also known as Grave’s orbitopathy, Graves Ophthalmopathy, and thyroid associated orbitopathy, is the leading cause of proptosis that requires orbital decompression. Despite an annual incidence of 16 in 100,000 in women and 3 in 100,000 in men, only about 25% of individuals present with overt signs of thyroid eye disease.[9] Most patients with thyroid eye disease have underlying thyroid dysfunction with excessive circulating levels of antithyroid antibodies like TSH-Receptor-Thyrotropin receptor antibodies (TRAb) and Anti-Thyroperoxidase (TPO) Antibodies. Although TRAb titers are more specific and used for diagnosis, Anti-TPO Antibodies are the most prevalent and are present in 80-90% of patients with Hashimoto’s Thyroiditis, and 65-75% of Grave’s Disease. They are also present in 10-20% of patients with nodular goiters, but even 10-15% of individuals without pathology can present with elevated antibody levels. Only about 10% of patients have either euthyroid antibody levels or autoimmune hypothyroidism develops.
Thyroid disease can manifest in the eye bilaterally and symmetrically, or more rarely it may present unilaterally or sequentially. The sequential progression from one eye to another may occur over years. Eye manifestations usually appear concurrently with thyrotoxicosis about 40% of the time (20% preceding thyrotoxicosis, and 20% occurring after treatment with radioactive iodine).[9] Not limited to Grave’s disease, thyroid eye disease can also occur with Hashimoto’s autoimmune thyroiditis.
Risk Factors
Although age is a typical risk factor in many diseases, thyroid eye disease is not beholden to individuals of any specific age group. However, it typically presents in the fifth to seventh decades of life. Despite the propensity to affect women more frequently, men typically present with more severe manifestations of the disease.[9] Smoking is a widely recognized and well-established risk factor with a direct correlation to severity of disease. It is thought that smoking produces an immunomodulatory effect and increases the volume of the connective tissues within the orbit by upregulating adipogenesis and stimulating hyaluronic acid production by orbital fibroblasts.[9]
There is a noted association between thyroid eye disease severity and progression with increased titers of anti thyroid stimulating hormone receptor antibodies (Anti-TSH). Extreme physical or psychological stress, noted by Perros et al., is also noted to be a risk factor for thyroid eye disease.[10] As mentioned previously, treatment with radioactive iodine can also precipitate ocular manifestations of disease in patients with thyrotoxicosis.
Disease Course, Pathogenesis, and Manifestations
Thyroid ophthalmopathy follows a generalized pattern first described in the literature in 1945 by Rundle and Wilson.[11] The pathogenesis of thyroid eye disease is made of humoral and cellular components. It is thought that antibodies have a direct effect on the orbital tissues, causing infiltration of lymphocytes, upregulation of fibroblast activity, and deposition of glycosaminoglycans within the structures of the orbit.[12]
The disease manifests first with an active phase that lasts typically from 6 to 24 months (extending up to 3 years). During this time, there is progressive inflammation, congestion, and noticeable worsening of symptoms. Overt disease presents as proptosis, eyelid swelling, diplopia, dry eye, and ocular discomfort.
The second phase is a plateau phase, and manifestations of the disease begin to stabilize.
Finally, the third phase is known as an inactive or partial remission stage. Although this is the general pattern, each individual’s natural course may vary. Perros et al., found that with respect to ocular symptoms, 15% of patients progress, 20% remain unchanged, and 75% regress spontaneously.[10]
Clinically significant disease presents in 50% of patients, and can become sight-threatening via compressive optic neuropathy or corneal surface disease in around 3-5 % of patients. The common extra-thyroid ocular physical exam findings are as follows:[13]
- Proptosis
- Periorbital edema
- Chemosis
- Ocular pain
- Eyelid lag
- Eyelid retraction
- Scleral injection
- Increased lacrimation
- Diplopia
- Pain with extraocular movements
- Dysfunctional extraocular movements
- Exposure keratitis
- Optic neuropathy
The prevalence of the physical exam findings were found in a particular study to be 92% with eyelid retraction, 62% with exophthalmos, 43% with extraocular muscle dysfunction, 30% with ocular pain, 23% with increased lacrimation, and 6% with optic neuropathy.[14]
It is important to note that without treatment, 30% of patients with compressive optic neuropathy will suffer permanent vision loss.[12]
Symptoms
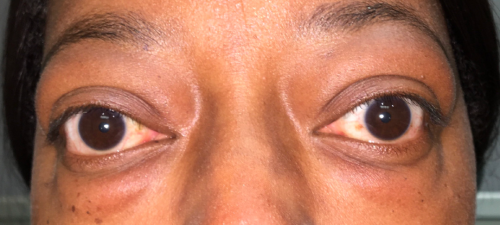
The degree of proptosis varies from subtle to very severe, with symptoms ranging from none to various symptoms. Proptosis can be subtle without any symptoms. Oftentimes the patient will notice some "bulging" of their eyes in photos or as noted by friends or family members. Patient may also note tearing which is worse with proptosis due to exposure of the cornea and increased surface area of the eye, resulting in increase evaporation of the tear film. Some patients with thyroid eye disease note pain around the eye which is thought to occur from pressure on the sensory nerves around the orbit. More severe symptoms of proptosis may include the inability to close the eyes (lagophthalmos), diplopia, and decreased vision.
Physical examination
Clinical examination of the eye, orbit, and surrounding structures is critical to determine the etiology of the proptosis, severity of the proptosis, and other contributing factors.
The Physical exam should include the following components:
- A Full eye exam which should include visual acuity, extraocular motility (inferior rectus most frequently affected first in thyroid eye disease), confrontational visual fields, a pupillary exam, intraocular pressure (often elevated), color vision assessment, an anterior segment exam, and a dilated fundus exam
- Note: anterior segment exam should pay particular attention to the cornea, conjunctiva, and caruncle
- An examination of the eyelid which includes assessment of eyelid position, assessment of eyelid function and presence of lid lag or lagophthalmos
- An examination of the orbit which includes examination of the bony structures of the orbital rim, presence of any masses, ability to retropulse the eye
- Symmetry and positioning of the eyes should be assessed, and abnormal positioning, such as hypoglobus, should be noted
- Quantitative measurements of proptosis should be taken using a Hertel Exophthalmometer
- The periorbital skin and tissues should also be examined and should note erythema, chemosis, caruncular injection or swelling, edema, masses, or any notable abnormalities
When compressive optic neuropathy occurs there may be visual field defects, color vision loss, an afferent pupillary defect, and vision loss. Examination of the cornea may reveal punctate epithelial erosions from dry eyes. If the erosions are concentrated in the inferior portion of the cornea it may be a sign that the patient is experiencing lagophthalmos, or inability to fully close the eyelids.
Clinical Activity Score (CAS)
In cases of Thyroid associated orbitopathy, the disease burden should be measured by a clinically validated scoring system first proposed by Mourits et al.[15] This system is centered around inflammatory physical exam findings and symptoms and is useful for distinguishing inflammatory from non-inflammatory thyroid eye disease. Its utility lies in the high predictive value for treatment response to immunosuppressants depending on the patient’s score.
Each positive response warrants 1
| Finding | Score |
| Spontaneous orbital pain | 1 |
| Gaze evoked orbital pain | 1 |
| Eyelid erythema | 1 |
| Eyelid swelling | 1 |
| Redness of the conjunctiva | 1 |
| Swelling of the conjunctiva (chemosis) | 1 |
| Swelling/inflammation of the caruncle | 1 |
Scores greater than or equal to 3 signify significant active inflammatory disease (and greater than 4 at follow up visits). Scores less than 3 signify non-inflammatory disease. Based on the European Groups on Graves’ Orbitopathy (EUGOGO), a score of greater than or equal to 3 has a positive predictive value of 65% and a negative predictive value of 56% response to radiotherapy. [16]
It is important to note, however, that even patients with a score of 1 or 2 showed a significant response to immunosuppressive therapy in the seminal study by Mourtis et al.,[15] Additionally, the cutoff of 3 did not prove appropriate for Asian populations.
Overall, the clinical activity score is a useful diagnostic tool, but should be used in concert with imaging studies to improve diagnostic precision. Characteristics found on imaging can also help distinguish between active and inactive inflammatory thyroid eye disease.
Physical Exam Findings between mild and severe ophthalmopathy
Used in conjunction with the clinical activity score and other diagnostic modalities, the following table is helpful for practitioners to distinguish between mild ophthalmopathy and severe ophthalmopathy given physical exam findings
From Bartalena et al., 2008[16]
| Finding | Mild | Severe |
| Eyelid retraction (mm) | <2 | >/= 2 |
| ≥2 Exophthalmos (mm) | <3 | >/=3 |
| Soft tissue involvement | mild | Moderate to severe |
| Diplopia | None or intermittent | Inconstant or constant |
| Corneal involvement | Absent or mild | moderate |
Diagnostic tests/procedures
Laboratory Workup
Recognizing the many causes of exophthalmos is crucial for proper diagnosis and appropriate designation for possible orbital decompression. Basic laboratory workup should include a complete blood count (CBC), renal function test, and C-reactive protein, as they may add value depending on the etiology of the proptosis. Ordering a thyroid panel, including T3, T4, TSH, Thyroid-stimulating immunoglobulins (TSI), and anti-thyroid antibodies (Anti-thyroid peroxidase (TPOAb), anti-thyroglobulin (TgAb), TSH receptor antibodies (TrAb)) may also be very useful for diagnosis. If infection is suspected, blood cultures and appropriate wound or nasopharyngeal swabs are indicated.
Imaging
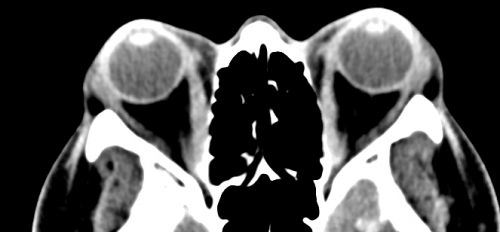
Computed tomography (CT) scans and magnetic resonance imaging (MRI) serve as the main diagnostic tools to further characterize the proptosis. Features visualized on imaging that pertain to the location and specificity of swelling, as in the case of orbital pseudotumor vs thyroid eye disease, will help to diagnose and treat the underlying cause of the disease. However, there will remain cases that, due to clinical similarities, will necessitate tissue biopsies to confirm a diagnosis.[1][8]
Furthermore, there is utility in orbital and head imaging using CT and/or MRI when planning for a customized orbital decompression because it allows for visualization of the position and thickness of various orbital bones and surrounding structures. Imaging also adds practical value if a surgeon decides to utilize stereotactic guidance during surgery. This preoperative imaging allows for reconstruction of a three dimensional image of anatomy that corresponds to an external guidance system. This guidance system can be used to compare the 3D model against the real surgical field to assist with real-time spatial awareness of structures that may not be visually apparent during surgery. Vascular lesions may warrant arteriography and venography.
Indications for Orbital Decompression
The primary indication for orbital decompression is related to potential damage to the eye or optic nerve due to proptosis or compressive optic neuropathy. As aforementioned, thyroid eye disease is the most common indication for orbital decompression surgery. Congenitally shallow orbits, relative maxillary hypoplasia, orbital tumors, and trauma resulting in orbital hemorrhage have been documented as indications for orbital decompression. However, it is rarely performed for the latter two etiologies.[17]
Surgery is typically performed when patients fail to respond to noninvasive therapy and prompt orbital decompression should be performed when there are severe surgical indications such as optic neuropathy, dramatic ocular surface disease, and subluxation of the globe.[12] Non-urgent orbital decompression indications are diplopia, orbital pain, proptosis, and ocular hypertension as a mechanical result of proptosis. It is important to note that non-urgent orbital decompression should not be performed within the active phase of thyroid eye disease. Furthermore, strabismus surgery to correct for diplopia or misalignment should occur after orbital decompression has been completed.
Although the historical context for orbital decompression has been for functional (medical) reasons, interest in cosmetic decompression has emerged. Because of the improvements in technique and subsequent decreases in postoperative complications, there has been a rise in popularity in patients undergoing surgery for cosmetic purposes.
Consideration of Alternatives
When urgent surgical indications are not present, medical and therapeutic alternatives should be considered prior to orbital decompression surgery.
For mild symptoms related to exposure keratopathy, first treatment attempts may be made with lubrication (preservative free artificial tears, gel tears, warm compresses). Smoking cessation should always be counseled for the patient. For diplopia, a manifest refraction and addition of fresnel prisms or ocular occlusion may provide relief. Other supportive therapies for proptosis include taping of the eyelids and botulinum toxin injections. Sunglasses may help with photosensitivity, and sleeping with a raised head may also help to reduce orbital congestion.[18]
There is evidence in the literature that suggests selenium supplementation (100mcg twice daily for 6 months) may have a beneficial effect on the disease course of thyroid eye disease and the quality of life of the patient. It may prove to have even greater effects when the patient is selenium deficient. However, evidence around the long term results of selenium supplementation is ill-defined. Further studies are required to fully understand the benefit of selenium, especially as it is associated with a greater risk of developing type 2 diabetes mellitus.[19]
If disease burden is great enough to warrant medical therapy, the first line treatment involves high dose pulses of methylprednisolone.[20] Although both IV (500mg/week for 6 weeks, followed by 250mg/week for 6 weeks) and oral (up to 100mg daily) preparations of corticosteroids may be used, the intravenous route is more efficacious and better tolerated.[21]
Radiation therapy has also proven to be effective for mild-moderate thyroid eye disease and is particularly useful for improving limited extraocular motility. The dose is 2Gy per day for a total of 20 Gy. If tumors are the underlying cause of proptosis, radiation is particularly useful. Combination therapy with steroids and radiation is synergistic and provides more therapeutic benefit than each treatment alone.
Tepezza (teprotumumab-trbw) is a human monoclonal antibody against the insulin-like growth factor type I receptor (IGF-IR) that is FDA approved medication for the treatment of adults with thyroid eye disease. When compared to placebo, multiple studies highlight its effectiveness in reducing the activity and severity of thyroid eye disease related symptoms including proptosis and scoring on the clinical activity scale.[22] Adverse reactions include muscle spasm, nausea, alopecia (hair loss), diarrhea, fatigue, hyperglycemia (high blood sugar), hearing loss, dry skin, dysgeusia (altered sense of taste) and headache. Contraindications include pregnancy; pregnancy should be prevented during treatment and for 6 months following the last dose of Tepezza.[23]
Preoperative Evaluation
The preoperative evaluation prior to orbital decompression is crucial, as it allows surgeons to create individualized plans and utilize knowledge of different surgical approaches for optimal results.
The following is an stepwise evaluation suggested by the author that may aid surgeons in preoperative planning for each unique case (Some steps adapted from Cheng et al.[24])
Comprehensive Ocular Examination
The preoperative evaluation should include a thorough ocular examination. Examination will include various functionality tests such as pupillary response, visual acuity (VA), refraction, color vision, biomicroscopy, intraocular pressure, fundoscopy, and visual field analysis.[25]
Periorbital Examination
Measurements of upper and lower lid retraction, ocular motility, lagophthalmos, and corneal/scleral exposure are of particular importance during the periorbital exam. During this step, reviewing and taking photographs of the patient before disease onset and at the current presentation are helpful in determining to what extent the proptosis should be reduced. It is also advisable to obtain photographic documentation throughout the progression of the disease course.
Imaging
In conjunction with functional ocular testing and a complete clinical exam, imaging such as MRI and CT scans should be employed to give a visual representation of the existing anatomical abnormalities and potential routes for surgical planning. MRI and CT imagery should include the extraocular muscles, orbital fat compartment, orbit, paranasal sinuses, and optic nerve appearance.[25] The functional ocular exams congruent with imaging will aid the surgeon in disclosing the severity of compression within the ocular orbit.
Measurement of Proptosis
Quantitative measurement of proptosis should be measured via Hertel exophthalmometry.
Orbital decompression can be performed by removing any combination of the lateral, inferior, or medial walls of the orbit, as well as fat. The number of walls that undergo operation typically has an effect on the overall amount of proptosis reduction. Based on the severity of proptosis, the surgeon may consider the following general resultant reductions in proptosis with respect to approach:[26][27]
- Medial wall decompression has shown to reduce proptosis by 2.5-3.1mm with endoscopy and 1-4mm without endoscopy
- Lateral wall decompression has reported a 2.7-4.8mm reduction of proptosis
- Balanced decompression of both medial and lateral walls achieves a greater decompression of 4-5.5mm
- When medial wall and orbital floor decompression are combined, proptosis reduces by 4-6mm.
- The greatest range of decompression can be achieved through a three-wall decompression of the lateral wall, medial wall, and floor, in which proptosis has been reported to be reduced by 4.5-7.5mm.
- Resection of orbital fat has been correlated with retrobulbar volume change and proptosis reduction of 4.1 ± 0.9mm.
Presence of preoperative diplopia
As the most common postoperative complication of orbital decompression, diplopia should be discussed with patients when weighing the benefits of surgery. Additionally, primary gaze diplopia may develop in patients with preoperative diplopia.
Cases of medial wall, combined medial wall and floor, and balanced medial and lateral wall decompressions exhibit similar new-onset diplopia rates of 14-19%, 10-35%, and 10-20%, respectively. The three-wall decompression mentioned earlier demonstrated the highest rate of newly acquired diplopia at 57%, owing to the increased change in orbital anatomy associated with multiple wall decompression. At the other end, lateral wall and orbital fat decompression show low ranges of postoperative diplopia of 0-6% and 3.3%, respectively.
Decompression technique selection
After the previous steps are complete, the surgeon may then compile the information to devise an informed surgical plan.
- Preoperative diplopia and mild proptosis (Hertel < 22mm)
Orbital fat decompression is indicated due to low rates of newly acquired diplopia. Though effective, it is not appropriate for larger cases of proptosis.
- Preoperative diplopia and moderate/severe proptosis (Hertel ≥ 22mm)
For patients with preexisting diplopia but more severe proptosis, a combined medial wall and orbital floor decompression is preferred, as it leads to greater proptosis reduction without increasing the risk for diplopia exacerbation.
- No preoperative diplopia and mild/moderate proptosis (Hertel < 24mm)
If a patient does not present with diplopia and requires a milder correction of proptosis, orbital fat decompression is appropriate to avoid postoperative diplopia.
- No preoperative diplopia and severe proptosis (Hertel < 26mm)
Increased severity of proptosis typically requires the removal of more orbital walls. However, three-wall decompression is usually reserved for very severe cases, so a balanced medial and lateral wall decompression is recommended based on decreased complication rates.
- No preoperative diplopia and very severe proptosis (Hertel ≥ 26mm)
Although three-wall decompression presents with the highest rate of complications, the method is indicated for very severe proptosis.
Further Considerations/ Risk Management
The lateral approach, if eyelid crease incisions are utilized, results in aesthetically preferential outcomes and is best used when pathology is lateral to optic nerve.[28] Consequently, there is risk with lateral incisions in damaging facial nerves.[29]
When pathology is located medially, Endonasal Endoscopic Approach (EEA) is preferred due to improved access to the desired field. Additionally, a posterior medial decompression is particularly effective for resolution of compressive optic neuropathies.[12] A transcaruncular approach to the medial wall is also more effective at proptosis reduction than optic nerve decompression. Anatomical considerations with the medial approach are the proximity to the medial rectus muscle, which may worsen diplopia, as well as the proximity to the ethmoid air cells. Prior to surgery, each patient should be notified of the potential need for strabismus surgery after decompression.
It is to be noted that both lateral and medial surgical techniques have similar outcomes in the reduction of exophthalmos[28]
A systematic review by Leong et al compared rates of different approaches. Although each case will warrant a different approach for its own reason, the rates of complications can be considered before adopting the approach. It was found that for an inferomedial wall decompression, rates of complications were as follows:[30]
- Transantral - 15.6%
- Transnasal - 5.2%
- Transconjunctival - 4.2%
- Transcaruncular - 5.8%
- Transcutaneous - 12.8%
It is important to note that coronal approaches also showed greater incidence of complications, and are thus rarely pursued today.
Surgical Technique
Orbital decompression is performed under general anesthesia. Orbital decompression is performed for either functional or cosmetic reasons. More orbital decompression (greater amount of bony and fat removal or debulking) is usually necessary for functional (medically necessary) orbital decompression and usually less is needed for cosmetic orbital decompression. The surgical principle is the same in both, where various amounts of orbital fat and orbital bone is removed. The best and safest first orbital wall to remove (or thin out) is the lateral orbital wall, followed by the medial wall, and last the orbital floor. More reduction with added risk is taken as more walls are decompressed. Incisions are hidden in the lateral upper eyelid crease (for lateral orbital decompression), caruncle or transcaruncular (for medial wall decompression) and lower eyelid conjunctiva (for orbital floor decompression).
Orbital Fat Decompression
First described in the early 1990s by Olivari, fat decompression has become an adjunctive step in orbital decompression surgeries.[27] Removing fat theoretically gives orbital structures space to prolapse anteriorly and relieve pressure posteriorly. Fat can be removed transcutaneously, transconjunctivally, and from the intraconal or extraconal space.
Transconjunctival approach (Adapted from Braun et al., and Ediriwickrema et al.,)[31][12]
- General anesthesia is induced. Preoperative IV antibiotics and corticosteroids are given.
- Inject 1% lidocaine with 1:100,000 epinephrine at the incision site
- Create a transconjunctival or transcutaneous incision based on preference and anatomical position of the globe to the eyelid
- Superior fat - Superior lid blepharoplasty incision
- Lower fat - inferior lid blepharoplasty Incision 3 mm below the tarsus
- Dissect deep to the Orbicularis oculi and orbital septum and visualize the fat pads
- Remove orbital fat with scissors or cautery while ensuring protection of the globe (malleable retractors may be employed)
- When removing inferior fat, take care to avoid inferior oblique injury
- Achieve hemostasis
- If superior fat removal is required, create an upper eyelid crease incision and perform fat removal in a similar manner (superior fat removal may reduce proptosis by another 0.5mm)
- Note: When removing superior fat, take care to avoid injuring the lacrimal artery, lacrimal nerve, supratrochlear nerve, and ethmoidal arteries
- With absorbable sutures, close the conjunctiva or skin depending on the initial approach
Lateral Wall Decompression
The first lateral wall approach that accessed the orbit was performed in 1889 by the Swiss surgeon Kronlein.[32] It first involved a large incision that ran from the lateral canthus from the hairline. The lateral approach to the orbit has since evolved, undergoing iterations to reduce invasiveness and scarring. Coronal flaps, incisions into the eyebrow and canthal-splitting incisions have all been used. Now, the lateral eyelid crease is commonly employed as it provides notable access to the lateral orbit and the scar is cosmetically favorable.
Lateral Canthotomy Approach (Adapted from Braun et al., and Ediriwickrema et al., )[12][31]
- General anesthesia is induced. Preoperative IV antibiotics and corticosteroids are given.
- Inject 1% lidocaine with 1:100,000 epinephrine at the incision site
- Create a lateral canthotomy incision and a small upper eyelid crease incision
- Dissect deep to expose the lateral orbital rim
- Expose the lateral orbital rim periosteum with monopolar cautery and a Freer elevator
- To prevent excessive blood loss, cauterize the vasculature of the zygomaticotemporal and zygomaticofacial neurovascular bundles
- Create space within the orbit laterally by using a surgical drill (75,000 RPM)/rongeurs to sculpt along the internal orbital bone
- Note: The heat generated during drilling can create a secondarily beneficial cauterizing effect to the vascular structures during the surgery. Careful irrigation can be used to taper the heat and prevent unwanted thermal spread to delicate structures
- Remove bone superior to the lacrimal fossa, lateral to the infraorbital canal, and laterally until the temporalis muscle is visualized
- In the posterior aspect, the trigonal bone of the greater wing of the sphenoid can be resected.
- Note: There is some debate in the literature as to the risk versus benefit of this step. Some authors argue the reduction in proptosis from removal of the inner table of the greater wing of the sphenoid is minor compared to the risk of dural penetration.[31]
- If this step is pursued, extra care must be taken during this portion as there is exposure of the dura as well as the potential for blood loss during this step. The marrow space underlying the bone is particularly vascular
- Superior and inferior incisions to the lateral rectus muscle may be made to create volume within the orbit
- The inferolateral fat pocket can also be removed at this stage
- If more reduction in proptosis is needed, floor decompression may occur with cantholysis of the lateral canthal tendon and an inferior transconjunctival incision to make a swinging lower eyelid flap
- Close the periosteum with 4-0 Vicryl suture and reattach the lateral canthal tendon with absorbable suture
Removal of the lateral orbital rim is typically reserved only for severe cases of proptosis. If necessary, osteotomies can be made at the frontozygomatic suture and above the zygomatic arch.
Medial Wall Decompression
The medial wall approach to orbital decompression has historically been via a Lynch incision or a coronal incision. The Lynch incision occurs between the nasal bridge and the medial canthus. The coronal approach involves creating a flap and manipulation of many structures (supraorbital nerve, trochlea, lacrimal sac). As orbital decompression techniques evolved, these approaches lost favor to techniques with less complications and that were less invasive, but equally efficacious.
Transcaruncular Approach (Adapted from Braun et al., and Ediriwickrema et al.,)[12]
- General anesthesia is induced. Preoperative IV antibiotics and corticosteroids are given.
- Inject 1% lidocaine with 1:100,000 epinephrine at the incision site
- Create a transcaruncular incision with Wescott scissors
- Dissect deep toward the posterior lacrimal crest and identify the medial wall and the periosteum
- Using cautery and a Freer elevator, open the periosteum
- Remove the appropriate amount of bone posterior to the posterior lacrimal crest with Kerrison rongeurs
- Allow the anterior section of the medial wall to remain intact
- Close the incision in the caruncle with 6-0 Plain gut suture
Orbital Floor Decompression
Orbital floor decompressions are rarely performed in isolation. They are usually accompanied by a medial wall decompression to provide for a combined inferomedial evacuation.
Transconjunctival Approach (Adapted from Braun et al., and Ediriwickrema et al.,)[12]
- General anesthesia is induced. Preoperative IV antibiotics and corticosteroids are given.
- Inject 1% lidocaine with 1:100,000 epinephrine at the incision site
- Create a transconjunctival incision from the caruncle to the lateral canthus (3-4mm below the tarsus along the palpebral conjunctiva)
- Reflect the conjunctiva superiorly and dissect deep toward the arcus marginalis that sits at the inferior orbital rim
- Take care to avoid injury to the infraorbital nerve
- Note: In some cases, detaching and later reattaching the inferior oblique muscle to gain greater access may be necessary
- With a Freer elevator and cautery, open the periosteum off the orbital floor to enter the posterior portion of the orbital floor
- While leaving the maxillary-ethmoid strut and anterior orbital floor intact, use Kerrison rongeurs to remove bone that lies medial to the infraorbital nerve
- The posterior inferior medial wall is reserved as the last wall to be removed for decompression due to increased risk of diplopia, hypoglobus, and sinusitis
- Leave patency in the maxillary sinus ostium to decrease the risk of maxillary sinusitis in the postoperative period
- Close the conjunctiva with absorbable sutures
Variation: A lateral canthotomy and cantholysis with an inferior fornix incision can allow for a swinging eyelid approach. This will permit favorable exposure in cases that require access to the inferior or lateral orbit
Endoscopic Orbital Decompression
Endoscopic decompression is a more recent approach that can be used to combine a medial with a lateral or inferior wall approach. In addition to the minimally invasive nature of the approach, it allows for excellent exposure to the medial wall to the apex, and can decompress the optic nerve if needed. This can also be combined with stereotactic guidance to facilitate surgery. Limitations involve potentially limited floor decompression lateral to the infraorbital nerve and need for endoscopic proficiency.
Surgical Technique Adapted from Braun et al.,[12]
- General anesthesia is induced. Preoperative IV antibiotics and corticosteroids are given.
- Topical oxymetazoline on cotton pledgets is applied to the nasal mucosa to aid with vasoconstriction. Inject lidocaine into the lateral nasal wall
- Assess the middle meatus. If there is insufficient space to accommodate the volume of the orbital contents, consider performing an uncinectomy
- If this approach is selected, complete a maxillary antrostomy and uncinectomy, which provides good exposure of the posterior maxillary wall and orbital floor
- Locate and take care to avoid the infraorbital nerve
- Perform a complete endoscopic sphenoethmoidectomy to expose the medial orbital wall from the sphenoid sinus, down to the crista ethmoidalis and superiorly to the skull base
- Use a periosteal elevator or spoon curette to open the medial orbital wall and/or inferior wall
- Use Blakesley forceps to remove bone fragments
- Fracture the medial orbital floor with a spoon curette, and remove the subsequent orbital floor medial to the infraorbital nerve
- Once exposure of the periorbita has been achieved by incision, the orbital structures may shift into the ethmoid and maxillary cavities
Postoperative regimen
Orbital decompression is typically completed on an outpatient basis, usually one eye at a time; however, both eyes can be done together. Some surgeons have listed intraorbital steroid injections at the end of the surgery as a method of combating inflammation and disease recurrence. The literature suggests that monthly retrobulbar or subconjunctival injections of 10-15 mg of methylprednisolone can further improve resolution of symptoms including eyelid retraction, residual exophthalmos, and strabismus. It is important, however, to remember the risk of intraocular pressure increase, vascular occlusion, scleral melt, and retrobulbar hemorrhage.[33]
After surgery, the patient should undergo a brief eye examination (visual acuity, pupils, intraocular pressure, motility). They should then be watched for several hours before being discharged. Per surgeon preference, a pressure patch may be placed over the surgical eye for removal at the postoperative day one visit. Pain can be managed with oral pain medication and the patient should be educated regarding expectations about pain and bruising for at least 2 weeks. A preventative medication course to reduce inflammation such as methylprednisolone can be given in a 3 day dose, along with oral antibiotics and antibiotic ointment.[34]
Due to postoperative orbital recovery time, the patient should limit physical activity, heavy lifting for 10 days and maneuvers that may increase ocular pressure. The patient should also be educated on sinus precautions, and should limit any activity (drinking from straws, blowing their nose) that may cause pressure fluctuations of the sinuses. The patient should watch for signs of CSF leak, persistent hemorrhage, and unrelenting orbital pain that may necessitate evacuation.
The follow up period should consist of a postoperative day one visit, one week visit, one month visit, and regularly spaced visits within the next 3-6 month period. During this time, ancillary testing may be performed to confirm ocular health. Photographic documentation and other objective data (Hertel measurements, repeat imaging) should be collected to compare preoperative against postoperative states.[34]
Possible Complications
The overall complication rate after orbital decompression is 9.3%.[30]
- Hemorrhage, infection, surgical failure
- These are common postoperative complications that can be divided into patient factors and procedural factors. Usually, they can be prevented through careful consideration of rules, guidelines, and proper technique.
- Periorbital ecchymosis
- Though rarely caused by surgical interventions, capillaries may be stretched during orbital procedures, leading to blue or purple discoloration of the eyelids.[35]
- Edema
- Postoperative orbital swelling is typically considered inflammatory in origin, but other diagnoses should be considered as failure to treat may lead to loss of vision or life.
- Vision loss
- With any invasive intervention into the orbit, there is a risk of vision loss. While loss of vision can be caused by a variety of factors, any changes in vision postoperatively should be promptly investigated. Some common causes are excessive traction on the globe and optic nerve, contusion of the optic nerve, infection, and hemorrhage.
- Sinusitis
- Orbital fat may herniate and block the maxillary sinus ostium. The frontal sinus is less commonly involved in postoperative sinusitis.[36]
- Cerebrospinal fluid leak
- Reports of CSF leakage are rare and may be complicated by mixture with blood or nasal secretion. Beta-2-transferrin presence is the gold standard in CSF leakage diagnosis. Leaks can be repaired by flaps or grafts in the defective area.
- Trigeminal nerve hypoesthesia
- Most, if not all patients report temporary injury to the peripheral branches of the trigeminal nerve, particularly the V1 and V2 divisions. For some, hypoesthesia remains a permanent complication.[37]
- Diplopia (up to 30% of cases), worsening of motility, strabismus, visual field defects
- The rates of diplopia and strabismus vary in the literature. Postoperative onset of diplopia ranges, but is typically reported in the range of 15%-25%
- Visual Field Defects
- Supraorbital anesthesia
- Fat decompression can has been shown to produce supraorbital anesthesia in 1.5-6%.[27] This complication can be reduced by minimizing dissection in the superomedial orbit or avoiding extraconal fat resection
- Temporal Wasting
- Coronal approaches may result in damage to musculature and result in temporal wasting
Outcome and Prognosis
Orbital decompression surgery is a viable treatment modality for some patients with proptosis. It has been shown to provide noticeable improvement in proptosis and successfully treats urgent indications for decompression. Due to the lack of consistent endpoints and reporting, lack of comparative trials, and large number of varying techniques, it is difficult to identify one superior approach. However, the aggregate consensus is a favorable outcome with a marked potential for reducing proptosis.
For urgent cases of compressive optic neuropathy, visual acuity is the main consideration and outcome. Visual acuity has been shown to improve in up to 82-88% of these patients postoperatively[20] For non-urgent cases of decompression, visual acuity improved in 44-55% of patients, remain stable in 27-36% of patients, and worsened in 18-20% of patients. [12]
Postoperative resolution of diplopia associated with proptosis ranged in some studies from moderate (20-30%) to as high as 90%.[27]
Reductions in Proptosis
The following Table lists the estimated range of proptosis reductions per approach:[26][27]
| Approach | Reduction in Proptosis | Other Outcomes |
| Medial Wall Decompression (with endoscopy) | 2.5-3.1 mm | |
| Medial Wall Decompression (without endoscopy) | 1-4 mm | |
| Other endoscopic approaches | 3.7- 4.7 mm | |
| Lateral Wall Decompression | 2.7 - 4.8 mm | Causes less strabismus |
| Medial and Lateral Wall (Balanced) | 4-5.5 mm | |
| Medial Wall and Orbital Floor (inferomedial) | 4-6 mm | |
| 3 walls (medial, lateral, floor) | 4.5-7.5 mm | |
| Orbital Fat | 4.1 mm ± 0.9mm , some reports up to 4.7mm | Reduction in IOP up to 3.4mm Hg |
The timing of the surgery and the use of steroids also plays a large role in the outcome of orbital decompression. One study compared the visual recovery in patients with IV steroids vs patients not receiving steroids after orbital decompression. The IV steroid group achieved greater visual recovery (56%) versus the group not receiving treatment (17%). However, there were increased reports of adverse outcomes, albeit transient, such as weight gain, hypertension, and elevated blood glucose levels. Overall the study concluded that the improvement in visual acuity trumped the drawback from the temporary side effects.
Ultimately, orbital decompression is a useful tool for reducing proptosis. The goal of orbital decompression should not lie in the desire to create the greatest volumetric expansion. Rather, it should be to create a customized and feasible surgical plan that appropriately achieves decompression with success and few complications. The aesthetic result should always be a consideration in urgent and non-urgent cases, and should influence the operative plan.
Additional resources
References
- ↑ Jump up to: 1.0 1.1 Butt S, Patel BC. Exophthalmos. [Updated 2022 Jun 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559323/
- ↑ Victores AJ, Takashima M. Thyroid Eye Disease: Optic Neuropathy and Orbital Decompression. Int Ophthalmol Clin. 2016 Winter;56(1):69-79. doi: 10.1097/IIO.0000000000000101. PMID: 26626933.
- ↑ Dollinger J.. Die Druckentlastung der Augenhöhle durch Entfernung der äuβeren Orbitalwand bei hochgradigem Exophtalmus (Morbus Basedowii) und konsekutiver Hornhauterkrankung. Dtsch Med Wochenschr. 1911; 37: 1888
- ↑ Kennedy DW, Goodstein ML, Miller NR, Zinreich SJ. Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg. 1990 Mar;116(3):275-82. doi: 10.1001/archotol.1990.01870030039006. PMID: 2306344.
- ↑ Luibil N, Lopez MJ, Patel BC. Anatomy, Head and Neck, Orbit. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539843/
- ↑ Jump up to: 6.0 6.1 Turvey TA, Golden BA. Orbital anatomy for the surgeon. Oral Maxillofac Surg Clin North Am. 2012 Nov;24(4):525-36. doi: 10.1016/j.coms.2012.08.003. PMID: 23107426; PMCID: PMC3566239.
- ↑ Karra E, Yousseif A, Rose GE, Baldeweg SE. Clinical assessment of patients with thyroid eye disease. Br J Hosp Med (Lond). 2016 Jan;77(1):C2-5. doi: 10.12968/hmed.2016.77.1.C2. PMID: 26903467.
- ↑ Jump up to: 8.0 8.1 Topilow NJ, Tran AQ, Koo EB, Alabiad CR. Etiologies of Proptosis: A review. Intern Med Rev (Wash D C). 2020 Mar;6(3):10.18103/imr.v6i3.852. doi: 10.18103/imr.v6i3.852. PMID: 32382689; PMCID: PMC7204542.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 Karra E, Yousseif A, Rose GE, Baldeweg SE. Clinical assessment of patients with thyroid eye disease. Br J Hosp Med (Lond). 2016 Jan;77(1):C2-5. doi: 10.12968/hmed.2016.77.1.C2. PMID: 26903467.
- ↑ Jump up to: 10.0 10.1 Perros P, Crombie AL, Kendall-Taylor P. Natural history of thyroid associated ophthalmopathy. Clin Endocrinol (Oxf). 1995 Jan;42(1):45-50. doi: 10.1111/j.1365-2265.1995.tb02597.x. PMID: 7889631.
- ↑ RUNDLE FF, WILSON CW. Development and course of exophthalmos and ophthalmoplegia in Graves' disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5(3-4):177-94. PMID: 21011937.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 Braun TL, Bhadkamkar MA, Jubbal KT, Weber AC, Marx DP. Orbital Decompression for Thyroid Eye Disease. Semin Plast Surg. 2017 Feb;31(1):40-45. doi: 10.1055/s-0037-1598192. PMID: 28255288; PMCID: PMC5330789.
- ↑ Smith TJ, Hegedüs L. Graves' Disease. N Engl J Med. 2016 Oct 20;375(16):1552-1565. doi: 10.1056/NEJMra1510030. PMID: 27797318.
- ↑ Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. Clinical features of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996 Mar;121(3):284-90. doi: 10.1016/s0002-9394(14)70276-4. PMID: 8597271.
- ↑ Jump up to: 15.0 15.1 Mourits MP, Prummel MF, Wiersinga WM, et al. Clinical activity score as a guide in the management of patients with Graves' Ophthalmopathy. Clin Endocrinol. 1997;47(1):9–14.
- ↑ Jump up to: 16.0 16.1 Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits MP, Perros P, Boboridis K, Boschi A, Currò N, Daumerie C, Kahaly GJ, Krassas G, Lane CM, Lazarus JH, Marinò M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid. 2008 Mar;18(3):333-46. doi: 10.1089/thy.2007.0315. PMID: 18341379.
- ↑ Wehrmann D, Antisdel JL. An update on endoscopic orbital decompression. Curr Opin Otolaryngol Head Neck Surg. 2017 Feb;25(1):73-78. doi: 10.1097/MOO.0000000000000326. PMID: 27846020.
- ↑ “Graves' Disease.” National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Department of Health and Human Services, https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease#radioiodine-therapy.
- ↑ Winther, K. H., Rayman, M. P., Bonnema, S. J., & Hegedüs Laszlo. (2020). Selenium in thyroid disorders — essential knowledge for clinicians. Nature Reviews.Endocrinology, 16(3), 165-176. doi:https://doi.org/10.1038/s41574-019-0311-6
- ↑ Jump up to: 20.0 20.1 Ismailova DS, Belovalova IM, Grusha YO, Sviridenko NY. Orbital decompression in the system of treatment for complicated thyroid eye disease: case report and literature review. Int Med Case Rep J. 2018 Oct 1;11:243-249. doi: 10.2147/IMCRJ.S164372. Erratum in: Int Med Case Rep J. 2019 Jan 30;12:21. PMID: 30319289; PMCID: PMC6171517.
- ↑ Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011 Feb;96(2):320-32. doi: 10.1210/jc.2010-1962. Epub 2011 Jan 14. PMID: 21239515.
- ↑ Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, Fleming JC, Fowler BT, Marcocci C, Marinò M, Antonelli A, Dailey R, Harris GJ, Eckstein A, Schiffman J, Tang R, Nelson C, Salvi M, Wester S, Sherman JW, Vescio T, Holt RJ, Smith TJ. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N Engl J Med. 2020 Jan 23;382(4):341-352. doi: 10.1056/NEJMoa1910434. PMID: 31971679.
- ↑ Commissioner, Office of the. “FDA Approves First Treatment for Thyroid Eye Disease.” U.S. Food and Drug Administration, FDA, https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-thyroid-eye-disease.
- ↑ Cheng AMS, Wei YH, Liao SL. Strategies in Surgical Decompression for Thyroid Eye Disease. Oxid Med Cell Longev. 2020 Sep 9;2020:3537675. doi: .1155/202010/3537675. PMID: 32963693; PMCID: PMC7501557.
- ↑ Jump up to: 25.0 25.1 Cubuk MO, Konuk O, Unal M. Orbital decompression surgery for the treatment of Graves' ophthalmopathy: comparison of different techniques and long-term results. Int J Ophthalmol. 2018 Aug 18;11(8):1363-1370. doi: 10.18240/ijo.2018.08.18. PMID: 30140642; PMCID: PMC6090113.
- ↑ Jump up to: 26.0 26.1 Liao SL, Huang SW. Correlation of retrobulbar volume change with resected orbital fat volume and proptosis reduction after fatty decompression for Graves ophthalmopathy. Am J Ophthalmol. 2011 Mar;151(3):465-9.e1. doi: 10.1016/j.ajo.2010.08.042. Epub 2011 Jan 12. PMID: 21232731.
- ↑ Jump up to: 27.0 27.1 27.2 27.3 27.4 Olivari N. Transpalpebral decompression of endocrine ophthalmopathy (Graves' disease) by removal of intraorbital fat: experience with 147 operations over 5 years. Plast Reconstr Surg. 1991 Apr;87(4):627-41; discussion 642-3. PMID: 2008461.
- ↑ Jump up to: 28.0 28.1 Baskaran Ketharanathan, Mikkel Schou Andersen, Christian Bonde Pedersen, Peter Darling, John Jakobsen, Laleh Dehghani Molander, Rikke Hedegaard Dahlrot, Nina Nguyen, Frantz Rom Poulsen, Bo Halle, Endonasal Endoscopic Approach for Minimally Invasive Orbital Decompression in Nonthyroid Proptosis—A Scoping Review, World Neurosurgery, Volume 162, 2022, Pages 85-90, ISSN 1878-8750, https://doi.org/10.1016/j.wneu.2022.03.075. (https://www.sciencedirect.com/science/article/pii/S1878875022003667)
- ↑ Baskaran Ketharanathan, Mikkel Schou Andersen, Christian Bonde Pedersen, Peter Darling, John Jakobsen, Laleh Dehghani Molander, Rikke Hedegaard Dahlrot, Nina Nguyen, Frantz Rom Poulsen, Bo Halle, Endonasal Endoscopic Approach for Minimally Invasive Orbital Decompression in Nonthyroid Proptosis—A Scoping Review, World Neurosurgery, Volume 162, 2022, Pages 85-90, ISSN 1878-8750, https://doi.org/10.1016/j.wneu.2022.03.075. (https://www.sciencedirect.com/science/article/pii/S1878875022003667)
- ↑ Jump up to: 30.0 30.1 Leong SC, Karkos PD, Macewen CJ, White PS. A systematic review of outcomes following surgical decompression for dysthyroid orbitopathy. Laryngoscope. 2009 Jun;119(6):1106-15. doi: 10.1002/lary.20213. PMID: 19358198.
- ↑ Jump up to: 31.0 31.1 31.2 Ediriwickrema LS, Korn BS, Kikkawa DO. Orbital Decompression for Thyroid-Related Orbitopathy During the Quiescent Phase. Ophthalmic Plast Reconstr Surg. 2018 Jul/Aug;34(4S Suppl 1):S90-S97. doi: 10.1097/IOP.0000000000001119. PMID: 29771754.
- ↑ Rootman DB. Orbital decompression for thyroid eye disease. Surv Ophthalmol. 2018 Jan-Feb;63(1):86-104. doi: 10.1016/j.survophthal.2017.03.007. Epub 2017 Mar 24. PMID: 28343872.
- ↑ Ediriwickrema LS, Korn BS, Kikkawa DO. Orbital Decompression for Thyroid-Related Orbitopathy During the Quiescent Phase. Ophthalmic Plast Reconstr Surg. 2018 Jul/Aug;34(4S Suppl 1):S90-S97. doi: 10.1097/IOP.0000000000001119. PMID: 29771754.
- ↑ Jump up to: 34.0 34.1 Zhang Yinghong, Zhou Jichao, Zhidi Zhang, Xu Chiyu, Zhou Haipeng, Ren Yanrong, Zhu Li, Wang Yi, Combined endonasal and orbital approach for annulus of Zinn area decompression in dysthyroid optic neuropathy, American Journal of Otolaryngology, 2022, 103692, ISSN 0196-0709, https://doi.org/10.1016/j.amjoto.2022.103692. (https://www.sciencedirect.com/science/article/pii/S0196070922003192)
- ↑ Nasiri J, Zamani F. Periorbital Ecchymosis (Raccoon Eye) and Orbital Hematoma following Endoscopic Retrograde Cholangiopancreatography. Case Rep Gastroenterol. 2017 Mar 17;11(1):134-141. doi: 10.1159/000456657. PMID: 28611566; PMCID: PMC5465703.
- ↑ Suresh R, Soparkar CN, Alford EL. Sinonasal complications associated with endoscopic orbital decompression. Laryngoscope Investig Otolaryngol. 2021 Jan 29;6(1):71-76. doi: 10.1002/lio2.531. PMID: 33614932; PMCID: PMC7883623.
- ↑ Sellari-Franceschini S, Dallan I, Bajraktari A, Fiacchini G, Nardi M, Rocchi R, Marcocci C, Marinò M, Casani AP. Surgical complications in orbital decompression for Graves' orbitopathy. Acta Otorhinolaryngol Ital. 2016 Aug;36(4):265-274. doi: 10.14639/0392-100X-1082. PMID: 27734978; PMCID: PMC5066461.