Rhabdomyosarcoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Rhabdomyosarcoma (RMS) is a rare childhood cancer with an estimated 250-350 new cases per year. The head and neck region and in particular, the orbit, represent a major anatomic site for RMS. Orbital RMS is the most common primary orbital malignancy in children with approximately 35 new cases per year.[2] Orbital rhabdomyosarcoma is a malignant neoplasm often seen initially by ophthalmologists, in which prompt diagnosis can save the life of the affected individual.
Primary Orbital RMS is mainly a disease of young children, with 90% occurring before the age of 16 years old with a mean age of onset of 5-7 years old.[3] While presentation within this age group is the most common, case reports have documented newborns and elderly patients with orbital RMS. There is a slight male to female predilection with a male:female ratio of 5:3.[2] Most authors agree that there is no racial predisposition for this malignancy.
Rhabdomyosarcomas are composed of cells with histologic features of striated muscle in various stages of embryogenesis that can occur in several sites in the body, including the ocular region. Most ocular rhabdomyosarcomas arise in the soft tissues of the orbit but they can rarely occur in other ocular adnexal structures and even within the eye. Further, RMS can gain access to the orbit secondarily by direct extension from the paranasal sinuses or nasopharynx. Very rarely, RMS can metastasize to the orbit from distant sites. Overwhelming however, the majority of ophthalmic RMS arises from the orbit. In a retrospective review by Shields et al, the primary site of ophthalmic RMS was the orbit in 76%, eyelid in 3%, conjunctiva in 12%, and the uveal tract in 9%.[4]
Prevalence
Rhabdomyosarcoma is the most common childhood soft tissue sarcoma, accounting for approximately 5% of all childhood cancers. It is the most common primary malignancy of the orbit in children. Approximately 250 new cases of rhabdomyosarcoma are diagnosed each year in the United States, of which approximately 10% occur primarily in the orbit.
Demographics
The average age of presentation for orbital rhabdomosarcomas is 7 to 8 years of age. While 70% occur in the first decade, it has been reported from birth to the seventh decade. In the orbit, some suggest a male predominance (1.3-1.6 to 1) and others indicate an equal sex distribution. There appears to be no difference based on race.
General Pathology
Microscopically, the four major histopathologic types of rhabdomyosarcoma are embryonal, alveolar, pleomorphic, and botryoid. The majority of orbital rhabdomyosarcomas are of the embryonal type, while the alveolar type is substantially less common. Embryonal types occur in childhood (mean age of presentation is 8 years), while alveolar types occur in adolescence and the rare pleomorphic type typically occurs in older individuals.
Etiology
Rhabdomyosarcomas develop in the orbital soft tissues, rather than in the extraocular muscles, which supports the theory that the tissue of origin is pluripotential or undifferentiated mesenchyme cells. The embryonal type corresponds to developing muscle at the 7 to 10 week fetal stage, and the alveolar type to the hollow tube stage. The pleomorphic type usually presents in older adults and is believed to occur de novo as a consequence of dedifferentiation of developed adult muscle.
Risk Factors
Most cases arise spontaneously, but the disease has been associated with familiar syndromes (Neurofibromatosis and Li-Fraumeni syndrome), the p53 tumor suppressor gene, and congenital malformations (Beckwith-Wiedemann syndrome). Occurrences as a second tumor with hereditary retinoblastoma have also been cited.
Anatomic locations
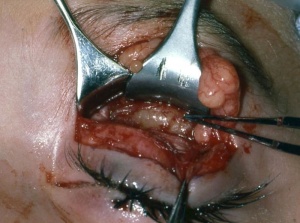
The orbit is the primary site for 10% of rhabdomyosarcomas. Other primary sites include genitourinary (22%), extremities (18%), parameningeal (16%), and other head and neck locations (10%), such as the scalp, face, buccal mucosa, oropharynx, larynx, and neck. Rhabdomyosarcomas can also invade the orbit by direct extension from the nasopharynx or the paranasal sinuses. Less commonly, ocular rhabdomyosarcomas can present in the conjunctiva, eyelid, or in the anterior uveal tract. Presentations in the eyelid and conjunctiva are rarely confined to these areas, but rather an extension from the anterior orbit (Fig. 1).
In a series of 33 cases of ocular rhabdomyosarcoma reported by Shields, there were 4 that initially presented in the forniceal conjunctiva, but upon further investigation all had contiguous anterior orbital involvement. In contrast there are reports of rhabdomyosarcomas that are only present in the iris or ciliary body, as a much slower growing mass than typical rhabdomyosarcomas that can become loosely cohesive and diffusely seed the anterior chamber, leading to secondary glaucoma. While rare, there is a case report of a known primary extraocular rhabdomyosarcoma that metastasized to the orbit.
Diagnosis
Signs and Symptoms
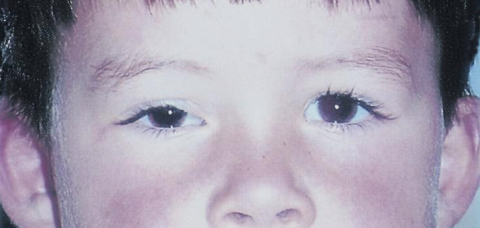
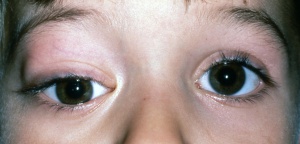
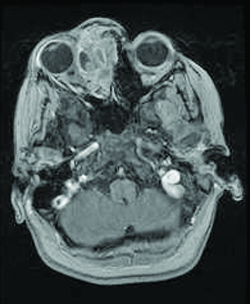
Patients generally present with proptosis (80-100%), globe displacement (80%), blepharoptosis (30-50%), conjunctival and eyelid swelling (60%), palpable mass (25%), ptosis (25%) and pain (10%) (Shields). Typically, orbital rhabdomyosarcomas present with rapidly developing unilateral exophthalmos over the course of weeks, however some cases may have a slower onset, with chronic eyelid and conjunctival edema being present before proptosis and globe displacement. Two thirds are superior or superonasal (Fig. 2), causing downward and outward displacement of the globe. Roughly ten percent arise from the paranasal sinuses (Fig. 3), nasal cavity, pterygopalatine fossa, and parapharyngeal space and secondarily invade the orbit, thus presenting with features of sinusitis, nasal obstruction, or epistaxis. Pain and decreased vision are minimal and are symptoms of more advanced disease. Epiphora is uncommon although nasolacrimal duct obstruction has been rarely reported.
While usually diagnosed due to the symptoms listed above, if allowed to progress, it can invade orbital bone and extend intracranially, producing neoplastic meningitis. Metastatic disease is rare, but it most often spreads hematogenously to bone and lung. The orbit contains few lymphatics, however anterior tumors in the conjunctiva and eyelid can metastasize to regional lymph nodes.
Clinical Exam
Obtaining a thorough history for the above symptoms, and performing a careful ocular examination in the office may assist in the diagnosis. Examination findings correlate with the size and location of the orbital tumor. The most common location for orbital RMS is the superior or superonasal orbit and therefore the most important examination findings are proptosis and inferior or inferotemporal displacement of the globe.[4] Superior tumors are also commonly associated with blepharoptosis. Anteriorly located tumors are commonly associated with edema and erythema of the eyelids as well as conjunctival congestion and chemosis. Further, these are typically associated with a palpable subcutaneous mass. Tumors located anterior and nasal have been shown to be associated with nasolacrimal duct obstruction. Posteriorly located tumors are more commonly associated with choroidal folds, retinal detachment, retinal vessel tortuosity, and optic disc edema with optic neuropathy. Larger tumors are more likely to cause extraocular motility restriction, severe proptosis, and optic nerve compression. As discussed previously, conjunctival RMS usually appears as a fleshy pink grapelike mass in the conjunctival fornix. Uveal RMS most commonly appears as a single fleshy iris mass that may be associated with seeding of the anterior chamber with secondary glaucoma.
Differential Diagnosis
The clinical differential diagnosis includes progressive rapidly developing masses and inflammatory conditions of childhood, such as neuroblastoma, chloroma, lymphangioma, infantile hemangioma, cellulitis, and nonspecific inflammatory diseases. The histopathologic differential diagnosis of childhood round cell tumors include neuroblastoma, neuroepithelioma, Ewing’s sarcoma, angiosarcoma, synovial sarcoma, malignant melanoma, granulocytic sarcoma, rhabdoid tumor, alveolar soft part sarcoma, and malignant lymphoma. Some inflammatory conditions include nodular fasciitis and destructive inflammations affecting muscle.
Imaging
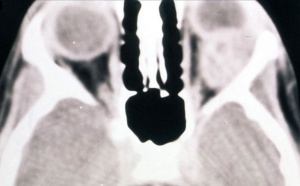
On CT scan (Fig. 3 & 4), the tumors appear as homogenous, well-circumscribed, round to ovoid masses, which are isodense to muscle. They are usually within the orbital soft tissues and do not appear to arise from the extraocular muscles. Earlier tumors do not invade bone, however larger tumors are less well defined and may erode bone and extend into the nasopharynx or sinuses. Areas of focal hemorrhage or necrosis may appear heterogeneous on CT scan. Rhabdomyosarcomas demonstrate moderate to marked contrast enhancement.
On MR T1-weighted imaging, the tumor appears hypointense compared with orbital fat, but isointense with respect to extraocular muscles. On T2-weighted imaging, the lesions are hyperintense to orbital fat and extraocular muscles. Areas of chronic hemorrhage may show focal areas of increased signal on T1- and T2-weighted images. They typically demonstrate moderate contrast enhancement with gadolinium injection, particularly with fat suppression.
Biopsy
Biopsy is necessary to establish a diagnosis. Fine needle aspiration biopsy does not provide sufficient tissue. Based on the findings of clinical examination and imaging studies, the clinician must decide whether to perform an excisional or incisional biopsy. Excisional biopsy is appropriate if surgical removal can be achieved without significant damage to vital structures, such as the optic nerve and extraocular muscles. If the suspected rhabdomyosarcoma is large and located in the posterior orbit, making a complete excision difficult without damage to vital orbital structures, an incisional biopsy is appropriate. In both cases, after histopathologic confirmation, radiation and chemotherapy are administered.
Pathology
Central to the diagnosis is the demonstration of rhabdomyoblasts via light microscopy, immunohistochemistry and/or electron microscopy.
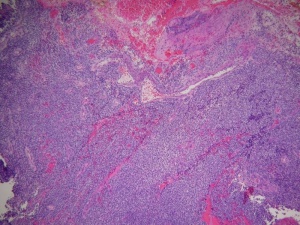
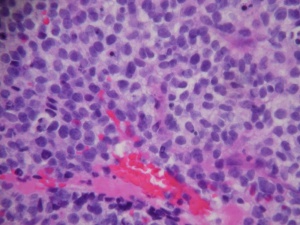
The histopathologic classification favored by the Intergroup Rhabdomyosarcoma Study divides these tumors into embryonal, alveolar, undifferentiated, and anaplastic types. By light microscopy, the embryonal subtype, which makes up 80% of orbital rhabdomyosarcomas and presents most commonly in young children (mean age 7-8), is characterized by spindle-shaped cells in various stages of differentiation (Fig. 5 and 6) with highly eosinophilic cytoplasm (Fig. 6). The alveolar subtype exhibits small, round, densely appearing cells that are loosely arranged, with septae that are similar to the alveoli of the lung. The botryoid variant of embryonal rhabdomyosarcoma, seen most commonly in infants, is defined by the presence of subepithelial aggregates of tumor cells and is named due to the common “grape-like” appearance of these cells. Immunohistochemisty is useful in identifying skeletal muscle proteins, such as alpha-actin, myosin, desmin and myoglobin. Electron microscopy can favor the diagnosis by demonstrating actin-myosin bundles or the A, I or Z band proteins, specific to muscle.
Further, cytogenetics is important in the diagnosis of RMS and differentiation between alveolar and embryonal variants. Alveolar RMS is distinguished from Embryonal RMS by the presence of one or two recurrent chromosomal translocations including t(2;13)(q35;q14) and t(1;13)(p36;q14). Embryonal RMS does not have recurrent structural chromosomal rearrangements but rather has frequent chromosomal gains and losses. In particular, there is a higher frequency of loss of one or two alleles often at chromosome 11 loci particularly in the 11p15.5 region.[5][2]
Treatment
Surgery
Orbital exenteration was considered the treatment of choice up until the 1960s; however, the mortality rate was roughly 70%. Today, the goal of surgery is to obtain tissue either through an excisional or incisional biopsy (Fig. 1) as described above, to provide pathological confirmation of the diagnosis and histopathologic typing. Frozen sections intraoperatively are difficult to differentiate rhabdomyosarcoma from several other neoplasms and inflammatory processes. If the diagnosis is confirmed, the child should be referred to pediatric oncologists for appropriate management with chemotherapy and possible radiation.
The current staging system of the Intergroup Rhabdomyosarcoma Study (IRMS) is listed below. Staging is done by review of imaging, clinical examination, and the amount of tumor remaining after initial surgery. In a study of 30 patients with Orbital Rhabdomyosarcoma by Shields et al, 2 (7%) had Group I, 11 (37%) had Group II, 16 (53%) had Group III and 1 (3%) had Group IV.
| Staging of Rhabdomyosarcoma by the Intergroup Rhabdomyosarcoma Study | |
|---|---|
| Group | Description |
| I | Completely resected localized disease implying both gross impression resection and microscopic confirmation of complete resection and absence of regional lymph node involvement |
| Ia | Confirmed to muscle or organ of origin |
| Ib | Contiguous involvement outside the muscle or organ of origin |
| II | Residual disease and/or regional lymph node involvement |
| IIa | Grossly resected localized tumor with microscopic residual disease and no evidence of gross residual tumor or regional lymph node involvement |
| IIb | Completely resected regional disease with no microscopic residual tumor |
| IIc | Grossly resected regional disease with microscopic residual tumor |
| III | Incomplete resection with biopsy or gross residual disease |
| IV | Distant metastatic disease present at onset |
In addition, the patient should be worked up for metastases with chest X-ray, full blood count, renal and liver function test, bone marrow aspiration for cytology, and bone scan. The cerebral spinal fluid should be cytologically examined if there is any suggestion of meningeal spread. Metastases from orbital sites are to lung and bone with very rare lymphatic extension.
Radiation
The poor prognosis for patients with orbital rhabdomyosarcoma following orbital exenteration (reported to be 70% mortality) prompted the use of orbital irradiation, later combined with chemotherapy for selected patients. Patients in Groups II, III, and IV usually receive radiation, generally in the range of 4000 to 5000 cGy over 4 to 5 weeks. When there is evidence of intracranial spread, whole cranial irradiation and intrathecal chemotherapy may be indicated.
Chemotherapy
Patients in whom a localized tumor has been completely resected (group I) are usually treated with chemotherapy alone. Groups II, III and IV receive combination chemotherapy and radiation therapy. The staging determines the length and number of chemotherapeutic agents used for treatment. Vincristine and actinomycin are the traditional agents that have shown to have very favorable outcomes, however newer agents, such as ifosfamide and etoposide are beneficial as well. Encouraged by results with newer methods of chemotherapy, some investigators have suggested that radiation be reserved for patients who do not achieve a complete response to chemotherapy, given the adverse side effects of radiation. It is important that the patient’s treatment regimen is managed by an experienced oncologist.
Follow up
In addition to close follow-up by the pediatric oncologist, the child should have a comprehensive ocular examination after completion of treatment every 3–4 months initially. Assessment should include:
- Best corrected visual acuity examination
- External ocular examination for dry eye, proptosis, masses, and motility disturbance
- Slit-lamp biomicroscopy to detect cataract
- Ophthalmoscopy to rule out radiation retinopathy
- Orbital CT or MRI to evaluate for any residual or recurrent tumor
After the first year the child should be examined every 4–6 months for several years and then yearly with periodic orbital CT or MRI, depending on the clinical findings.
Shields states that that radiologists and pediatric oncologists often express concern about residual tumor and they recommend an additional orbital biopsy. They believe a biopsy should be withheld until there is evidence of tumor regrowth as determined by clinical findings and serial CT or MRI. Additional management should consider that the biopsy of a small, residual orbital tumor in a child is often surgically difficult with a higher complication risk, and that the viability of residual tumor can be difficult to interpret by a pathologist.
Complications
The wide utilization of the IRMS treatment guidelines for rhabdomyosarcoma has led to a significant increase in the number of patients surviving and leading longer tumor-free lives. Secondarily, there is an increasing need for health care providers, in particular the ophthalmologist caring for these patients, to have a thorough understanding of the ophthalmic comorbidities associated with these tumors and specifically their treatment. The chemotherapeutic agents used for the treatment of RMS are known to have ophthalmic complications. For example, cyclophosphamide is most commonly associated with keratoconjunctivitis sicca and blepharoconjunctivitis as well as, albeit less frequently, lacrimal duct stenosis and cataract. Similarly, ifosfamide is associated with conjunctivitis and blurred vision. Etoposide has been associated with central retinal artery occlusion. Lastly, doxorubicin has been associated with acute reversible maculopathy.[6]
Radiation employed for orbital rhabdomyosarcoma (4,000 to 5,000 cGy) can be associated with ocular complications. Most notable are radiation cataract (55%), dry eye (36%), orbital hypoplasia (24%), blepharoptosis (9%), and radiation retinopathy (90%). There is a case report of an orbital malignant melanoma, observed 45 years after irradiation for orbital rhabdomyosarcoma. Long-term visual outcome in the Shields study, was 20/20 to 20/40 (39%), 20/50-20/100 (18%), and 20/200 to no light perception in (43%). Other secondary malignancies resulting from chemotherapy are rare, but include lymphoblastic leukemia and osteogenic sarcoma.
Prognosis
The introduction of the combination of surgery, adjuvant chemotherapy, and radiotherapy in the late 1960s improved the overall prognosis from survival of roughly 30% to over 90%. Factors that determine prognosis include: anatomic location, stage of the disease at diagnosis, tumor morphology, and patient age. Tumors in the orbit produce early ocular signs and symptoms compared to tumors in more occult locations, permitting an earlier diagnosis. Additionally, there are fewer lymphatics in the orbital area, therefore disease is usually discovered before distant metastasis. Tumor morphology is an important prognostic indicator. Patients with alveolar cell type show a 74% 5-year survival rate, whereas those with the more common embryonal cell type (80%) demonstrate a 94% 5-year survival rate. Even though age at diagnosis has not proven to be a predictor of tumor-related death, infants under 1 year of age with orbital rhabdomyosarcoma tend to have a worse prognosis with death in 46% of patients.
References
- ↑ American Academy of Ophthalmology. Rhabdomyosarcoma in a 4-year-old boy. https://www.aao.org/image/rhabdomyosarcoma-in-4yearold-boy-2 Accessed July 17, 2019.
- ↑ Jump up to: 2.0 2.1 2.2 Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol.2003;48:39–57.
- ↑ Wharam M, Beltangady M, Hays D, et al. Localized orbital rhabdomyosarcoma. An interim report of the Intergroup Rhabdomyosarcoma Study Committee. Ophthalmology 1987;94:251-4.
- ↑ Jump up to: 4.0 4.1 Shields C, Shields J, Honavar S, et al. Clinical Spectrum of Primary Ophthalmic Rhabdomyosarcoma. Ophthalmology 2001;108:2284-2292.
- ↑ Gandhi P, Fleming J, Haik B,Wilson M. Ophthalmic complications following treatment of paranasal sinus rhabdomyosarcoma in comparison to orbital disease. Ophthal Plast Reconstr Surg 2011;0: 1-6.
- ↑ Schmid K, Kornek G, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol 2006;51:19-40.
- Abramson DH, Ellsworth RM, Tretter P, et al: The treament of orbital rhabdomyosarcoma with irradiation and chemotherapy. Ophthalmology 86:1330-5, 1979
- Burkat CN, Lucarelli MJ: Rhabdomyosarcoma masquerading as acute dacryocystitis. Ophthal Plast Reconstr Surg. 2005 Nov; 21(6):456-8.
- Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4:34–44.
- Diller L, Sexsmith E, Gottlieb A et al. Germline mutations are frequently detected in young children with rhabdomyosarcoma. J Clin Invest 1995;95: 1606-1611
- Elsas FJ, Mrokzek EC, Kelly DR, Specht CS. Primary rhabdomyosarcoma of the iris. Arch Ophthalmol 1991;109:982–4
- Fekrat S, Miller NR, Loury MC: Alveolar rhabdomyosarcoma that metastasized to the orbit. Arch Ophthalmol 111:1662-4, 1993
- Heyn R, Ragab A, Raney RB Jr, et al: Late effects of therapy in orbital rhabdomyosarcoma in children. A report from the Intergroup Rhabdomyosarcoma Study. Cancer 57:1738-43, 1986
- Leff SR, Henkind P: Rhabdomyosarcoma and late malignant melanoma of the orbit. Ophthalmology 90:1258-60, 1983
- Rootman, Jack. Diseases of the orbit: a multidisciplinary approach. Lippincott Williams & Wilkins, Philadelphia, 2nd ed, 2003.
- Rousseau P, Flamant F, Quintana E, et al: Primary chemotherapy in rhabdomyosarcoma and other malignant meschymal tumors of the orbit: results of the International Society of Pediatric Oncology MMT 84 Study. J Clin Oncol 12:516-21, 1994
- Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol.2003;48:39–57
- Shields CL, Shields JA, Honavar SG, Demirci H: Clinical spectrum of primary ophthalmic rhabdomyosarcoma. Ophthalmology 108:2284-92, 2001