Sphenoid Wing Meningioma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Sphenoid wing meningiomas are slow growing tumors that originate from outer arachnoid meningeal epithelial cells. They are the most common tumor of the intracranial space to spread to the orbit.[1][2]
Disease Entity
Incidence
The annual incidence of symptomatic meningiomas is approximately 2 cases per 100,000 individuals. Meningiomas of the anterior skull base constitute 40% of all intracranial meningiomas, and sphenoid wing meningiomas constitute 11-20% of intracranial meningiomas.[3] Sphenoid wing meningiomas with secondary orbital extension are rare. [4][2]
Sphenoid wing meningiomas are classified as either globoid tumors with a nodular shape or an en plaque tumor which is flat and spreads along the entire sphenoid ridge.[5] The globoid tumors include 3 groups depending on their location: inner (medial), middle, and lateral (pterional).[5] Medial sphenoid wing meningiomas have a higher morbidity, mortality, and recurrence rate compared to other meningiomas due to their involvement with anterior visual pathways, anterior intracranial arteries, and the cavernous sinus.[5]
Etiology
Sphenoid wing meningiomas are slow growing tumors that originate from outer arachnoid meningeal epithelial cells.[1]
Currently no definite environmental risk factors exist. [4][6]
Genetically, the most well characterized and common alteration is the loss of the NF2 gene (NF2) on chromosome 22q. NF2 encodes a tumor suppressor known as merlin. Approximately 60% of sporadic meningiomas were found to have mutations in NF2. Meningiomas can also be associated with other genetic syndromes such as Gorlin and Rubinstein-Taybi syndromes, but the association is not as strong as with NF2.[4][6]
Systemic Associations
Meningiomas can be multiple, particularly when they associated with neurofibromatosis type 2 (NF2). [6]
General Pathology and Classification
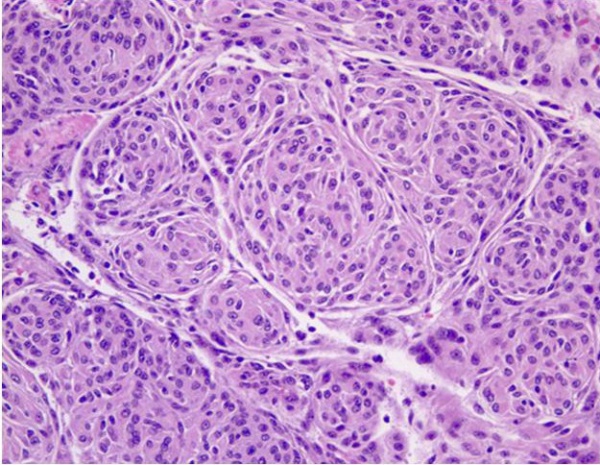
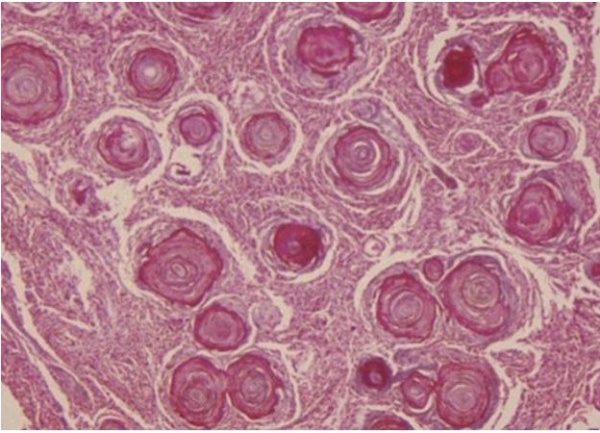
There is substantial pathologic variation in the appearance of sphenoid wing meningioma specimens. There are many variants encountered including secretory, microcystic, clear cell, lymphoplasmocyte rich, chordoid, atypical, malignant, papillary and anaplastic variants.[7][8] In general, the typical pathologic features that characterize most meningiomas are whorls of meningothelial cells composed of epithelioid type cells with eosinophilic cytoplasm and ovoid nuclei with or without vacuoles or pseudo-inclusions. Meningiomas may contain calcium deposition in the center of the whorls of cells called psammoma bodies. Figures 1 and 2 represent typical examples of histopathologic specimens of meningiomas.[7][8][2]
The pathological and clinical classification of meningiomas is based upon the WHO classification system for brain tumors.[7][8]
- WHO Grade I – Includes secretory, microcystic, clear cell, lymphoplasmocyte rich, and chordoid variants. Despite invasion of the adjacent bony structures, grade I meningiomas do not invade the brain parenchyma.
- WHO Grade II – Includes atypical variant. This type of meningioma shows frequent mitosis and an increased nuclear-cytoplasmic ratio.
- WHO Grade III/IV - Includes malignant, papillary and anaplastic variants. This type of meningioma shows even greater mitosis, necrosis, and invasion of brain parenchyma.
In a large retrospective study of 1663 patients who underwent surgery for meningioma, 90% were benign WHO grade I tumors while 10% were atypical or anaplastic variants, WHO grade II or III.[9]
Diagnosis
Clinical Features
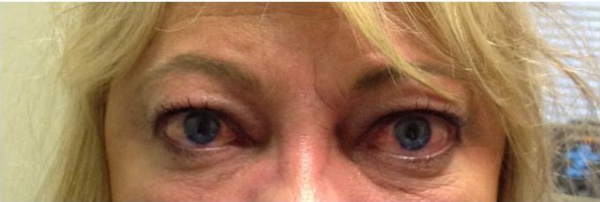
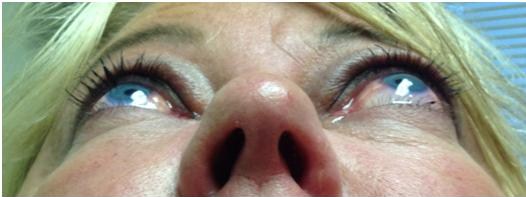
The ophthalmic manifestations of sphenoid wing meningiomas vary depending on the location of the primary tumor. The tumor can extend from the intracranial space into the orbit through bone, the superior orbital fissure or the cavernous sinus. They can present with progressive symptoms of an orbital or temporal fossa mass, including temporal fullness, proptosis, globe displacement, ptosis, and impaired extraocular motility. Tumors near the sella or optic nerve can cause visual field defects, optic disc edema or atrophy. Eyelid edema and chemosis are also commonly encountered.[1] Average age of onset is 50 years with a higher incidence in women and Caucasians.[4][6][1][2][10]
Diagnostic Imaging
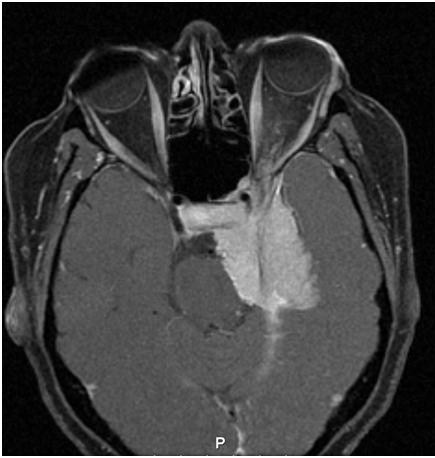
Imaging of sphenoid wing meningiomas demonstrates a thick homogenous tumor bed with relative sparing of anatomic structures. The tumors are slow growing and have a tendency to conform around structures and cause compression rather than tissue invasion. The average annual growth rate of meningiomas are 1–3 mm per year.[11] On CT, meningiomas are isoattenuating to slightly hyperattenuating and exhibit homogenous and intense enhancement after injection of iodinated contrast. On MRI imaging, T1- and T2-weighted sequences have variable signal intensity, but they enhance intensely and homogeneously after injection of gadolinium. They also tend to exhibit hyperostosis and calcifications which can be seen on either CT or MRI imaging. Additionally, the presence of a dural extension (also known as a dural tail) is helpful in distinguishing a meningioma from fibrous dysplasia.[4][6][1][2]
Management
The cornerstones of therapy for sphenoid wing meningioma involve observation, surgery, radiation therapy and chemotherapy. Early surgical intervention is often preferred for younger patients and healthy elderly patients, while observation is typically preferred for asymptomatic older patients with multiple medical problems.[12]
Surgery
Meningioma resection has long been considered the primary and definitive treatment for meningioma.8[13] The surgery for sphenoid wing meningiomas is difficult because of their complicated intracranial, intraosseous and intraorbital growth patterns that lead them in close proximity to major nerves and arteries.[14] Benefits to this approach include: Immediate removal of the lesion, rapid reduction of an intracranial mass effect, and the possibility to perform a precise pathological diagnosis. Surgery is typically performed using multi- stage procedures leveraging the benefits of different approaches (e.g. endoscopic endonasal surgery for anterior skull base lesions). This technique may minimize the morbidity associated with resection of the entire tumor in one operation.[13][15] [16] [17]Rates of gross total resection are approximately 50%. Surgical complications range from 1-18%.[13][15][16][17] The 5 year recurrence free survival for WHO Grade I meningiomas is 88%.[13][15][16][17] A study of 53 patients with microsurgically treated sphenoid wing meningiomas found that intentional incomplete resection of the tumor had a favorable impact on the postoperative quality of life, primarily due to avoiding neurological defects when the tumor involved neurovascular structures[14]. In these cases of incompletely resected tumors, postoperative radiotherapy or radiotherapy are considered beneficial. Other factors improving postoperative quality of life in patients with sphenoid wing meningiomas treated with microvascular surgery include poor blood supply and lack of tumor adhesion to adjacent structures.[14]
Radiation Therapy
Radiation is widely used in the management of sphenoid wing meningiomas. In the past, radiation therapy was reserved for malignant meninigomas or for recurrences.[17][18] Improvement in radiation therapy technology has altered this paradigm. Treatment decisions are individualized and multimodal using both surgery and radiation for patients with symptomatic tumors.[18] The various radiation therapy options for sphenoid wing meningiomas include: stereotactic radiosurgery, fractionated stereotactic radiotherapy (FSRT), intensity modulated radiotherapy (IMRT), and particle radiotherapy (also known as “Proton Beam”). In stereotactic radiosurgery, all of the radiation is pulsed at the tumor in one fraction while in FSRT and IMRT the dose is given in multiple fractions over time. The main difference between FSRT and IMRT is that IMRT can accommodate more complex shapes and larger tumors because it improves the ability of the radiation to conform to the tumor edge and minimize damage to important normal structures.[18] Clinically, radiosurgery is a safe alternative to skull-base meningioma resection in patients who are not good surgical candidates; however, limitations exist for tumors in close proximity to critical and radiosensitive structures. FSRT and IMRT are generally used when the tumor is around radiosensitive structures such as the optic nerve or chiasm.[17][18] For radiation therapy as a whole, local control rates of 92-100% have been reported. Rates of permanent neurological deficits range from 1.6% to 9.8%.[17][18] After radiation therapy, tumor volume decreases by 33% at 2 years and 36% at 3 years.[18]
Chemotherapy
Chemotherapy has also been used in the treatment of meningiomas that are progressive, recurrent or inoperable. Many agents have been tried including typical cytotoxic agents and mifepristone. In general, chemotherapy does not play a significant role in the management of meningioma as significant systemic toxicity is typically encountered with modest or no tumor regression seen.[11] Combination chemotherapy with hydroxyurea is currently being evaluated. Inhibiting angiogenesis has become one the key mechanisms to treating meningiomas since they are heavily vascular tumors[19]. In vitro studies using targeted molecular therapies against PDGF (Platelet derived growth factor), VEGF (vascular endothelial growth factor), EGF (epidermal growth factor), and MAPK (MAP Kinase) appear promising for recurrent meningioma.[11]
Treatment Summary
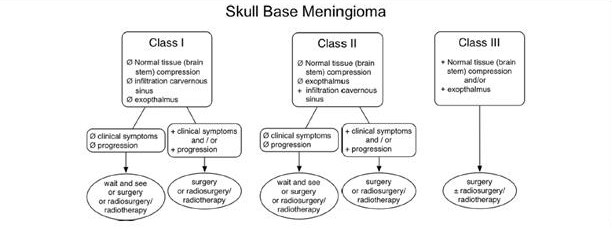
All of the treatment modalities can play a role in the management of sphenoid wing meningioma. In a review article published by Coombs, et al[18], they suggested a relative general standard for treating skull base meningiomas. Figure 6 is a suggested decision tree for the treatment of skull base meningiomas. The flow chart describes consideration of surgery and/or radiotherapy in the presence of symptoms, tissue compression, exophthalmos or cavernous sinus infiltration. In the absence of symptoms or signs, observation may be appropriate.[18]
Orbital Bony Reconstruction
There is currently no consensus regarding orbital bony reconstruction after tumor resection. [20] [21] Proponents for spheno-orbital reconstruction advocate for its structural and cosmetic benefits, claiming that it can prevent postoperative complications such as pulsating enophthalmos, meningocele formation, temporal muscle atrophy, diplopia from extraocular muscle fibrosis, orbital pain, and restrictive ptosis.[22][23][24][25][26][20] Reconstruction of dural defects is often performed to prevent CSF leaks and reduce the risk of wound infection.[20][21] In addition, detailed reconstruction of the soft tissue and bony landmarks may aid postoperative imaging and subsequent reoperation in the case of tumor recurrence.[23] Surgeons often opt for reconstruction in cases involving significant orbital bone removal, [22][23][26][27] but criteria for reconstruction vary.
Some reports suggest bony reconstruction is unnecessary in most cases, especially with tailored tumor resection and orbital decompression. [21][28][21][29][30][31] Watertight dural reconstruction has also been noted to be unnecessary unless entry into the sphenoid or ethmoid sinuses was required for tumor resection. [30][32] These studies argue that non-reconstruction of the orbit still results in acceptable functional and cosmetic outcomes and does not seem to correlate with higher prevalence of post-resection enophthalmos. [21][28][29][30] In a recent series of 33 patients with spheno-orbital meningioma over a 10-year period managed without orbital bony reconstruction, Kim et al. reported satisfactory cosmetic and visual outcomes and no cases of pulsatile enophthalmos, even among 20 patients who required entrance into their periorbita for reconstruction. [29]
Reconstructive Techniques
Surgeons may employ a variety of reconstructive techniques that utilize a variety of materials: split calvaria, rib grafts, iliac crest, titanium mesh, and other synthetic materials.[20] [24][25] A systematic review and meta-analysis of surgical management of spheno-orbital meningioma showed that the most commonly utilized materials for bony reconstruction include titanium mesh (37%), inner calvarial grafts (29%), and polymethylmethacrylate (26%).[20] The most used materials for dural defects were fascial grafts (18%) and pericranium (16%), although artificial dural reconstruction materials are becoming more common. [20] Abdominal fat grafts have been used to fill surgical dead space as a result of extensive tumor resection (24%).[20] In 11 patients with large volume tumor removal, Kim et al. reported that the use of an autologous fat graft without bony orbital reconstruction was sufficient to prevent postoperative pulsatile enophthalmos. [29]
Titanium mesh
Titanium mesh implants have many advantages as a reconstructive option. They are widely available due to their use for traumatic injury repairs and are generally well-tolerated by patients.[19] They also avoid the need to harvest graft tissue from a second site and are deformable to allow matching of orbital volume with the contralateral uninvolved orbit.[19][24] Pace et al. demonstrated safety and efficacy of deformable titanium mesh implants in orbital reconstruction, resulting in reduced proptosis, improved visual outcomes, minimized ocular motility disturbance, and few postoperative complications.[24] Using intraoperative image guidance technology, titanium mesh was secured to the orbital rim anteriorly, and a layer of Duraform or Gelfoam was placed between the titanium mesh and the orbital contents to avoid entrapment of the orbital tissues.[24]Some complications or drawbacks associated with titanium mesh include orbital adherence syndrome, signal interference with MRI, and recurrent meningioma growing through the implant, but none were seen in this study.[19][24]
Inner calvarial grafts
Autologous bone grafts have traditionally been the standard technique for craniofacial reconstruction due to their advantages of using primarily native material and avoidance of using more expensive implants. [19] However, they are associated with increased risk of donor site morbidity, increased operative time, possibility for a larger incision during tumor resection, and difficulty remodeling rigid graft material to the curved orbit. In addition, although a biologically inert native graft theoretically poses a lower risk of infection, this may not be accurate due to use of a titanium plating system to fixate the graft. Iliac crest autografts have fallen out of favor due to high rates of donor site morbidity. Calvarial autografts are the most common option and are produced by splitting the frontal or parietal bone and using the inner table to reconstruct the orbit. Honeybul et al. used inner table calvarial bone grafts positioned using intraoperative comparisons of the globe position with the contralateral eye for reconstruction of the lateral wall and orbital roof.[23] Results from this study showed 8 out of 11 patients with globe position accurate within 2 mm, 2 patients with 3 mm exophthalmos that was undetectable to the patients, and 1 patient with significant enophthalmos requiring additional surgical correction.
Polymethylmethacrylate
Polymethylmethacrylate implants are reported to be advantageous due to their non-resorbability, similar hardness to bone, compatibility with screw and drill fixation, and porosity which allows for blood product penetration and vascular ingrowth.[25] Pritz et al. used custom-made polymethylmethacrylate implants created using a CT-guided preoperative planning model to mark areas ipsilateral to the lesion and portions of the orbit, sphenoid, and zygoma contralateral to the tumor.[25] The polymethylmethacrylate implant was noted to restore the 3D structure of resected bone more accurately than bone grafts and resulted in decreased surgical time, good functional globe position and excellent cosmesis. However, these implants were more expensive than other options and required extensive planning of the bony resection prior to the procedure.[19]
Prognosis
Overall, malignant potential is low for skull base meningiomas (including sphenoid wing meningiomas). In a large retrospective study of 1663 patients who were operated on for meningioma, 90% were benign (WHO Grade I) tumors while only 10% were atypical or anaplastic variants (WHO Grade II or III). The major risk factors for higher WHO grade were non skull base location (OR 1.7), and age >= 65 years (OR 1.5). Male gender also conferred a 2x risk of higher WHO grade.[9] Recurrence free survival in WHO Grade I meningioma with surgery, radiotherapy or combined treatment is nearly 90%.[13][15][16][17][18] Morbidity varies after therapy with the incidence of permanent neurological deficits ranging from 1.6-9.8%.[17][18]Morbidity from the tumor and therapeutic interventions depends on the location of the tumor and its proximity to vital neurological and ocular structures
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Basic Clinical Science Series. Orbit, Eyelids, and Lacrimal System. American Academy of Ophthalmology. 2011-2012
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Fathi, et al. Meningioma... Curr Neurol Neurosci Rep. 2013 Apr;13(4):337. doi: 10.1007/s11910-013-0337-4.
- ↑ Güdük, M., Özduman, K. & Pamir, M. N. Sphenoid Wing Meningiomas: Surgical Outcomes in a Series of 141 Cases and Proposal of a Scoring System Predicting Extent of Resection. World Neurosurgery 125, e48–e59 (2019).
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 http://emedicine.medscape.com/article/1156552-overview#a0199 (Accessed 10/2013)
- ↑ Jump up to: 5.0 5.1 5.2 Nakamura, M., Roser, F., Jacobs, C., Vorkapic, P. & Samii, M. Medial Sphenoid Wing Meningiomas: Clinical Outcome and Recurrence Rate. Neurosurgery 58, 626–639 (2006).
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 http://emedicine.medscape.com/article/1156552-overview#a0199 (Accessed 10/2013)
- ↑ Jump up to: 7.0 7.1 7.2 Louis, DN, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007 Aug; 114(2):97-109. Epub 2007 Jul 6.
- ↑ Jump up to: 8.0 8.1 8.2 Wrobel, G, et al. Microarray-based gene expression profiling of benign, atypical and anaplastic meningiomas identifies novel genes associated with meningioma progression. International Journal of Cancer. Volume 114, Issue 2, pages 249–256, 20 March 2005
- ↑ Jump up to: 9.0 9.1 Cornelius, et al. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta Neurochir (Wien). 2013 Mar;155(3):407-13. doi: 10.1007/s00701-012-1611-y. Epub 2013 Jan 15
- ↑ Deshmukh S, Das D, Bhattacharjee H, Kuri GC, Magdalene D, Gupta K, Multani PK, Paulbuddhe V, Dhar S. Profile of brain tumors having ocular manifestations in a Tertiary Eye Care Institute: A retrospective study. TNOA J Ophthalmic Sci Res 2018;56:71-5.
- ↑ Jump up to: 11.0 11.1 11.2 Sherman, et al. Chemotherapy: what is its role in meningioma? Expert Rev. Neurother. 12(10), 1189–1196 (2012)
- ↑ Peele, K. A. et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology 103, 1761–1766; discussion 1766-1767 (1996).
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 Oostra, et al. Extended endoscopic endonasal skull base surgery: from the sella to the anterior and posterior cranial fossa. ANZ J Surg. 2012 Mar;82(3):122-30. doi: 10.1111/j.1445-2197.2011.05971.x. Epub 2012 Jan 17.
- ↑ Jump up to: 14.0 14.1 14.2 Ouyang, T., Zhang, N., Wang, L., Li, Z. & Chen, J. Sphenoid wing meningiomas: Surgical strategies and evaluation of prognostic factors influencing clinical outcomes. Clinical Neurology and Neurosurgery 134, 85–90 (2015).
- ↑ Jump up to: 15.0 15.1 15.2 15.3 Kotomotar, et al. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg. 2012 May-Jun; 77(5-6):713-24. Epub 2011 Nov 7.
- ↑ Jump up to: 16.0 16.1 16.2 16.3 Di Maio, et al. Evolution and future of skull base surgery: the paradigm of skull base meningiomas. World Neurosurg. 2012 Sep-Oct; 78(3-4):260-75. Epub 2011 Nov 7.
- ↑ Jump up to: 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 Pechivanis, et al. Evidence level in the treatment of meningioma with focus on the comparison between surgery versus radiotherapy. A review. J Neurosurg Sci. 2011 Dec; 55(4):319-28.
- ↑ Jump up to: 18.0 18.1 18.2 18.3 18.4 18.5 18.6 18.7 18.8 18.9 Combs, et al. State-of-the-art treatment alternatives for base of skull meningiomas: complementing and controversial indications for neurosurgery, stereotactic and robotic based radiosurgery or modern fractionated radiation techniques. Radiation Oncology 2012, 7:226
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 19.5 Chamberlain, M. C. The role of chemotherapy and targeted therapy in the treatment of intracranial meningioma. [Miscellaneous Article]. Current Opinion in Oncology 24, 666–671 (2012).
- ↑ Jump up to: 20.0 20.1 20.2 20.3 20.4 20.5 20.6 Fisher FL, Zamanipoor Najafabadi AH, Schoones JW, Genders SW, van Furth WR. Surgery as a safe and effective treatment option for spheno-orbital meningioma: a systematic review and meta-analysis of surgical techniques and outcomes. Acta Ophthalmol. 2021 Feb;99(1):26-36. doi: 10.1111/aos.14517. Epub 2020 Jun 29. PMID: 32602264; PMCID: PMC7891445.
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 Talacchi A, De Carlo A, D'Agostino A, Nocini P. Surgical management of ocular symptoms in spheno-orbital meningiomas. Is orbital reconstruction really necessary? Neurosurg Rev. 2014 Apr;37(2):301-9; discussion 309-10. doi: 10.1007/s10143-014-0517-y. Epub 2014 Jan 25. PMID: 24463913.
- ↑ Jump up to: 22.0 22.1 Gaillard S, Pellerin P, Dhellemmes P, Pertuzon B, Lejeune JP, Christiaens JL. Strategy of craniofacial reconstruction after resection of spheno-orbital "en plaque" meningiomas. Plast Reconstr Surg. 1997 Oct;100(5):1113-20. doi: 10.1097/00006534-199710000-00004. PMID: 9326771.
- ↑ Jump up to: 23.0 23.1 23.2 23.3 Honeybul S, Neil-Dwyer G, Lang DA, Evans BT, Ellison DW. Sphenoid wing meningioma en plaque: a clinical review. Acta Neurochir (Wien). 2001 Aug;143(8):749-57; discussion 758. doi: 10.1007/s007010170028. PMID: 11678395.
- ↑ Jump up to: 24.0 24.1 24.2 24.3 24.4 24.5 Pace ST, Koreen IV, Wilson JA, Yeatts RP. Orbital Reconstruction via Deformable Titanium Mesh Following Spheno-Orbital Meningioma Resection: Ophthalmic Presentation and Outcomes. Ophthalmic Plast Reconstr Surg. 2020 Jan/Feb;36(1):89-93. doi: 10.1097/IOP.0000000000001444. PMID: 31373988.
- ↑ Jump up to: 25.0 25.1 25.2 25.3 Pritz MB, Burgett RA. Spheno-orbital Reconstruction after Meningioma Resection. Skull Base. 2009 Mar;19(2):163-70. doi: 10.1055/s-0028-1096199. PMID: 19721773; PMCID: PMC2671298.
- ↑ Jump up to: 26.0 26.1 Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Hénaux PL, Amelot A, François P. Spheno-Orbital Meningiomas Surgery: Multicenter Management Study for Complex Extensive Tumors. World Neurosurg. 2018 Apr;112:e145-e156. doi: 10.1016/j.wneu.2017.12.182. Epub 2018 Jan 6. PMID: 29317363.
- ↑ DeMonte F, Tabrizi P, Culpepper SA, Suki D, Soparkar CN, Patrinely JR. Ophthalmological outcome after orbital entry during anterior and anterolateral skull base surgery. J Neurosurg. 2002 Oct;97(4):851-6. doi: 10.3171/jns.2002.97.4.0851. PMID: 12405373.
- ↑ Jump up to: 28.0 28.1 Dos Santos AG, Paiva WS, da Roz LM, Santo MPDE, Teixeira MJ, Figueiredo EG, da Silva VTG. Spheno-orbital meningiomas: Is orbit reconstruction mandatory? Long-term outcomes and exophthalmos improvement. Surg Neurol Int. 2022 Jul 22;13:318. doi: 10.25259/SNI_165_2022. PMID: 35928313; PMCID: PMC9345102.
- ↑ Jump up to: 29.0 29.1 29.2 29.3 Kim RB, Fredrickson VL, Couldwell WT. Visual Outcomes in Spheno-Orbital Meningioma: A 10-Year Experience. World Neurosurg. 2022 Feb;158:e726-e734. doi: 10.1016/j.wneu.2021.11.048. Epub 2021 Nov 17. PMID: 34800732.
- ↑ Jump up to: 30.0 30.1 30.2 Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994 Feb;80(2):202-8. doi: 10.3171/jns.1994.80.2.0202. PMID: 8283257.
- ↑ Schick U, Bleyen J, Bani A, Hassler W. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006 Feb;104(2):208-14. doi: 10.3171/jns.2006.104.2.208. PMID: 16509494.
- ↑ Castellano F, Guidetti B, Olivecrona H. Pterional meningiomas en plaque. J Neurosurg. 1952 Mar;9(2):188-96. doi: 10.3171/jns.1952.9.2.0188. PMID: 14908653.