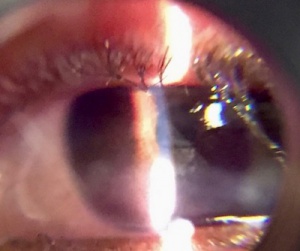
Exposure Keratopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Exposure keratopathy (EK) is damage to the cornea that occurs primarily from prolonged exposure of the ocular surface to the outside environment. EK can lead to ulceration, microbial keratitis, and permanent vision loss from scarring.
Etiology, Pathophysiology
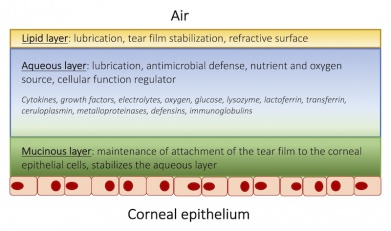
Under normal circumstances, the eyelids and tear film protect the cornea, an avascular, nonkeratinized epithelium, from trauma, desiccation, and microbial attack.[7][8] The tear film, a composite fluid of three layers, has several important roles: it nourishes and lubricates the cornea, aids in crisp visual acuity, and protects the cornea from bacterial invasion (Figure 1).[8] Several factors help maintain an adequate distribution of the tear film; these include an intact blink reflex, normal blink rate, and complete eyelid closure during sleep and blinking.[8] Disruption to this system may lead to an epithelial defect. The corneal epithelium serves as a barrier to the outside world through the use of tight junctions and damage to the integrity of this structure can facilitate penetration of microbes and external debris.[8]
Risk Factors
Patients at risk for EK include those who suffer from conditions that interfere with the ability to protect the cornea such asincomplete eyelid closure, inadequate blink reflex, inadequate blink rate and/or decreased protective lubrication of the cornea. A summary of risk factors and conditions is listed below.
Lagophthalmos
Lagophthalmos is the inability to close the eyelids completely. Because of this, a portion of the eye remains open during a blink and during sleep, and is subject to damage from exposure. The etiologies of lagophthalmos can be divided into the following subcategories:
- Facial nerve dysfunction (paralytic lagophthalmos)
- Eyelid dysfunction
- Excessive scar tissue or excessive eyelid removal during surgery (cicatricial lagophthalmos)
- Physiologic lagophthalmos (as in the case of nocturnal lagophthalmos).
- Medication effect (particularly sedatives or neuromuscular blockers)
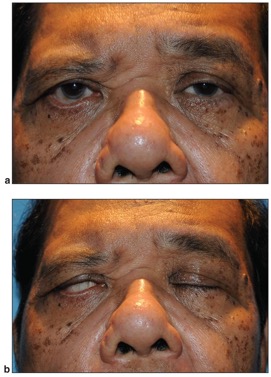
Paralytic lagophthalmos is most commonly due to facial nerve dysfunction. The facial nerve (CN VII) controls most of the eyelid muscles, and facial nerve dysfunction will therefore lead to weakened eyelid closure (Figure 2). It can be damaged by trauma, cerebrovascular accidents, Bell’s palsy, tumors, infections, immune-mediated causes, and congenital cranial dysinnervation syndromes.[9] Trauma, can be secondary to fractures to the skull base or mandible, or from surgery (particularly neurosurgery). Additionally, the facial nerve is supplied by the anterior inferior cerebellar artery and as such may be susceptible to cerebrovascular accidents. Bell’s palsy is a type of facial paralysis that results in a weakness of facial muscles on one side of the face. It holds its own host of possible causes, most notably, reactivation of herpes simplex virus. Other infectious causes of Bell’s palsy include Lyme disease, varicella, mumps, poliomyelitis, Guillaine-Barré syndrome, leprosy, diphtheria, and botulism.[9] Tumors that have the potential to cause facial nerve dysfunction include acoustic neuromas in the cerebellopontine angle as well as metastases.[9] Lastly, cranial dysinnervation syndromes, such as Mobius’ syndrome and congenital facial palsy, are characterized by facial nerve dysfunction and subsequent lagophthalmos.[9]
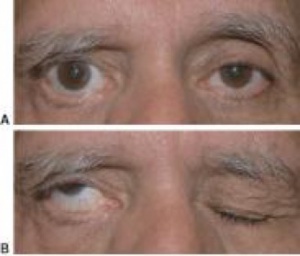
Post-operative lagophthalmos can commonly be seen following blepharoplasty, with one study citing post-op lagophthalmos numbers as high as 47%.[10] Lagophthalmos in this setting may be due to upper eyelid edema, orbital septal elevation, or excessive skin removal.[11] When due to edema, the lagophthalmos usually resolves within seven days.[11] However, if lagophthalmos persists, additional surgical treatment may be needed. In the case of upper-eyelid malposition, scar tissue release with full-thickness interposition skin grafting may be needed.[11] Additionally, trauma procedures and orbital tumor debulking and removal have the potential to be complicated by post-operative lagophthalmos.
Nocturnal lagophthalmos, which is incomplete eyelid closure during sleep, is relatively common in the general population, with one report citing up to 23% of individuals demonstrating some degree of nocturnal lagophthalmos.[12] The presence of Bell’s phenomenon, whereby the eyeball (and thus the cornea) rotates upward upon eyelid closure, particularly during sleep, may serve to protect the cornea in cases of nocturnal lagophthalmos. However, not all sleeping patients exhibit Bell’s phenomenon, with one study citing only 42% of 234 patients as having a positive Bell’s during sleep.[13]
Certain medications may cause iatrogenic lagophthalmos, particularly medications used for anesthesia, such as sedatives and neuromuscular blocking agents. Additionally, periocular anesthesia used in aesthetic surgery may also lead to lagophthalmos given the potential muscle toxicity associated with lidocaine, mepivacaine, procaine, and tetracaine.[14] Such toxicity, although rare, can lead to damage of the orbicularis oculi muscle and lagophthalmos. Steps to avoid orbicularis oculi toxicity include placement of the anesthetic injection high in the upper eyelid and low in the lower eyelid, subcutaneous injection to avoid muscle tissue, and use of the lowest volume and concentration of local anesthetic necessary.[14]
Of note, it’s been demonstrated that patients in intensive care units (ICU) and critical care settings have an increased risk of developing EK.[15] The incidence of lagophthalmos in sedated patients in the ICU is quite high (reports range between 21-75%), and has partly been attributed to the sedative and neuromuscular blocking agents commonly used in this setting.[7][16][17] In addition to their negative effects on eyelid closure, sedatives and neuromuscular blockers interfere with the blink reflex that is responsible for adequate distribution of the tear film over the cornea.[7] They also negatively affect Bell’s phenomenon.[7] Other factors that contribute to EK in the ICU setting include positive pressure ventilation, which raises the patient’s venous pressure and indirectly causes conjunctival edema and lagophthalmos, high flow oxygen rates through nebulizers or face masks, which may desiccate the cornea, and fluid imbalances and increased vascular permeability, which can lead to conjunctival edema and subsequent lagophthamos.[18]
Proptosis
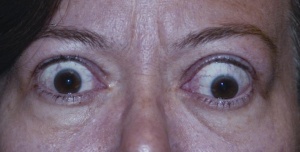
Proptosis, a term commonly interchanged with exophthalmos, is anterior displacement of the eyeball with respect to the orbit. This protrusion of the eyeball may be so great as to disrupt normal eyelid closure. Conditions that are associated with proptosis include craniosynostosis syndromes, thyroid disease, orbital tumors and Cushing’s syndrome. Patients with pulsatile exophthalmos syndromes may also present with EK; such patients may include those with aortic regurgitation, neurofibromatosis 1, carotid-cavernous sinus fistulae, arachnoid cysts, and trauma.
Thyroid eye disease warrants special mention as it is one of the leading causes of EK (Figure 3). Thyroid eye disease can cause proptosis of the orbit, eyelid retraction, and chemosis. Proptosis occurs secondary to increased volume behind the orbit due to cellular proliferation, inflammation, and accumulation of glycosaminoglycans in the eye muscles and retroorbital adipose and connective tissues.[19] Additionally, patients with thyroid disease may have eyelid retraction as well as periorbital edema secondary to decreased venous drainage from the congested orbital space.[19] Both of these factors contribute to incomplete eyelid closure and increase the risk for EK.[19] Additionally, patients with thyroid dysfunction may suffer from corneal nerve dysfunction and subsequent malfunctioning of the blink reflex, further increasing the risk of EK.[20] Grave’s disease is an autoimmune disease that results in the overproduction of thyroid hormones (hyperthyroidism). Although thyroid eye disease is traditionally associated with Graves’ disease, it is also occasionally seen in euthyroid patients and in patients with hypothyroid chronic autoimmune thyroiditis.[19]
Neurologic and neurotrophic disease
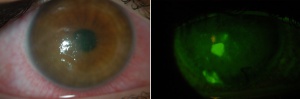
Patients with either neurologic or neurotrophic diseases may have an impaired blink reflex that could predispose them to EK (Figure 4). Examples of neurologic disease include Parkinson disease or neuromuscular disease. EK results from decreased blink rate and blink force.[21]
Neurotrophic diseases are the result of decreased corneal sensation. They are characterized by corneal nerve dysfunction, which interferes with the normal blink reflex that is needed to lubricate the eye and protect it from the external environment. Neurotrophic disease can be either acquired or hereditary and infectious or non-infectious. Non-infectious non-hereditary forms of neurotrophic keratitis can be secondary to a pathological change, due to autoimmune disease, iatrogenic, substance - induced, or as part of a polyneuropathy syndrome. Examples of decreased corneal nerve function secondary to the effect of ophthalmic pathological changes can be seen in keratoconus, bullous keratopathy, and atopic keratoconjuctivitis.[20] Autoimmune causes of decreased corneal nerve function include Graves’ disease and Sjogren’s syndrome.[20] Iatrogenic corneal nerve dysfunction may occur in refractive vision corrective procedures.[20] Intranasal cocaine abuse may also impair corneal nerve sensitivity and may predispose to EK.[22] Lastly, systemic polyneuropathies may lead to impaired blink reflex secondary to decreased corneal nerve sensitivity and include peripheral diabetic neuropathy, amyloidosis, sarcoidosis, vitamin B12 deficiency, and alcoholism.[20]
Hereditary forms of sensory and autonomic neuropathies that have the potential to compromise corneal nerve integrity include Riley-Day syndrome, congenital aniridia, ocular-auriculo-vertebral dysplasia, and multiple endocrine neoplasia 2A and 2B.[20] Lastly, infectious agents that can affect the corneal nerves include herpesviridae (herpes-simplex virus type 1 and varicella-zoster virus, the latter of which can present as Ramsay-Hunt syndrome), Mycobacterium leprae, Acanthamoeba, and fungi.[20]
Lid malposition
Lid malposition can prevent adequate closure of the eyelids, leading to EK. Causes of lid malposition include cicatricial retraction, as previously mentioned, ectropion, entropion, and eyelid coloboma.
Ectropion is an outward rotation of the eyelid margin, whereas entropion is an inward rotation of the eyelid margin. Ectropion can be either congenital, involutional, paralytic, cicatricial, or mechanical.[23] Congenital ectropion of the upper lid may be associated with Down syndrome, whereas congenital ectropion of lower lid may be associated with blepharophimosis syndrome.[23] Involutional lower lid ectropion may be caused by disinsertion of the lower lid retractors, laxity of the medial and lateral canthal tendons, and atrophy or degeneration of the orbicularis oculi.[23] This can occur as part of the natural aging process. Paralytic ectropion may be caused by facial nerve palsy leading to laxity of the orbicularis oculi (Figure 5).[23] Cicatricial ectropion can be caused by shortening of the anterior lamella or skin of the orbicularis oculi muscle from actinic skin changes, resection of skin lesions, trauma, burns, toxic reactions to topical eye medications, skin diseases, and aggressive blepharoplasty.[23] A rare cause of cicatricial ectropion is cutaneous anthrax infection, whereby the Bacillus anthracis organisms proliferate at the site of inoculation and form a papule that progresses to form a vesicle, pustule, and ultimately a necrotic black ulcer.[24] When the eyelid is the primary site of entry, progressive lid edema may lead to cicatricial ectropion.[24] Lastly, mechanical pulling of the eyelid may cause ectropion such as in the setting of a tumor.[23]
The causes of entropion may be congenital, acute spastic, involutional or cicatricial. Congenital entropion can occur in the setting of retractor dysgenesis and shortened posterior lamella.[23] Acute spastic entropion may be caused by ocular inflammation or irritation as can be seen with trichiasis.[23] Involutional entropion is associated with medial and lateral canthal laxity or dehiscence, disinsertion or stretching of the lower lid retractor complex, override of the preseptal orbicularis, and extension of orbital fat anterior to the orbital rim.[23] Lastly, cicatricial entropion may be caused by shortening of the posterior lamella from inflammation which may be caused by trachoma, Stevens-Johnson syndrome, ocular cicatricial pemphigoid, herpes zoster, chemical injuries, and toxic reactions to certain topical medications.[23]
Eyelid coloboma is another cause of eyelid malposition that may lead to EK. Eyelid coloboma may be isolated or as part of a syndrome. Isolated eyelid colobomas may present with corneopalpebral adhesions and may be complete, incomplete, or of the abortive type which has variable size and a diverse range of corneopalpebral adhesions.[25] Syndromic variants of eyelid coloboma include Fraser syndrome, Goldenhar syndrome, CHARGE syndrome (coloboma of the eye, heart defects, atresia of the nasal choanae, retardation of growth and/or development, genital and/or urinary abnormalities, ear abnormalities and deafness), or several other more rare associated syndromes.[25]
Primary Prevention
Primary prevention of EK involves the use of artificial tears and lubricating gel in at risk populations.
Diagnosis
Diagnosis is made based on classic history and symptoms as well as physical exam signs that are outlined below.
History
Assess for a history of EK and the aforementioned risk factors above. Specific inquiry should be made regarding any history of surgery, medical comorbidities (with particular emphasis on thyroid disease and diabetes), current medication use (emphasis on sedatives or muscle relaxants), and history of trauma.
Physical Examination
- Evaluate corneal sensation for possible neurotrophic keratopathy prior to instillation of any topical anesthetic. Corneal sensation can be tested with either a cotton swab or with a graded Cochet Bonnet esthesiometer (Figure 6).
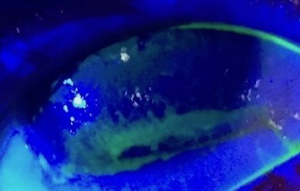
- Slit lamp examination with and without fluorescein dye staining (Rose Bengal and Lissamine green may also be used). Prior to staining assess for external signs as outlined below. Early EK is characterized by corneal desiccation and upon administration of fluorescein dye superficial punctate epithelial staining, most commonly localized to the inferior one third of the cornea becomes apparent (Figure 6).[7] These microepithelial defects may coalesce into an abrasion or progress to form an ulcer or infiltrate.[7] Assess for external signs as outlined below.
Signs
External signs to note include incomplete blink, lagophthalmos, exophthalmos, eyelid deformity or malposition, and presence or absence of Bell’s phenomenon.[21] Assess for conjunctival injection or chemosis.
Symptoms
Patients may complain of foreign body sensation, burning, increased tearing, and intermittent blurry vision (from an unstable tear film).[21] Symptoms may be worse in the morning if they are due to nocturnal lagophthalmos.[21] Other possible symptoms include pain and photophobia.[26]
Management
The cornerstone of treatment involves treating the underlying condition that led to exposure keratopathy. In the interim, medical and surgical treatments can be applied.
Medical therapy
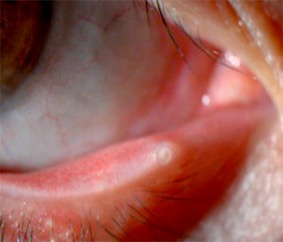
Standard treatments include the use of frequent artificial tears with nightly lubricating ointment.[21] Preservative free artificial tears can be used hourly. Ideally, the artificial tears and lubricating drops should be preservative-free as frequent use of eye drops with preservatives can promote inflammation and may disrupt the tear film.[27] Punctal plugs may also be considered (Figure 7).[26]
Other treatment strategies promote eyelid closure when the primary cause of EK is lagophthalmos. Methods of eyelid closure include passive closure, eyelid taping (MicroporeTM [3M]), eye patching, Geliperm dressings, saline-soaked gauzes, paraffin gauze and suturing (Frost sutures or temporary tarsorrhaphy).[7] Botulinum toxin may be injected to the upper lid to promote a temporary chemical ptosis lasting approximately 3 months. Lastly, another strategy includes the use of a moisture chamber or polyethylene film cover.[28][29]
A literature review indicated that the use of ocular lubrication was significantly more effective than passive eyelid closure, basic eye toilet or Geliperm but equally effective to the use of lid taping in the critical care setting.[7] The use of moisture chambers or polyethylene films provided the greatest amount of protection.[7][29] Recent reports have documented the utility of using Glad Press’n Seal as a simple method of creating a moisture chamber.[30]
Bandage and scleral contact lenses are another method of protecting the cornea.[26] However, infection is always a risk with bandage contact lenses. Patients should be followed closely and antibiotic prophylaxis should be considered, especially if the lens is worn overnight. Specialty scleral contact lenses like the PROSE lens are especially helpful, but are not always covered by insurance companies.[26]
Surgery
Surgical treatments are often necessary to address the underlying pathophysiology. Usually medical treatment is initially employed and surgical treatments are reserved for refractory cases of EK. In the case of lagophthalmos temporary or permanent tarsorraphy has been used as well as gold weight implantation (also for facial nerve palsy).[31][21] If the exposure is secondary to lid malposition, canthoplasty, lid tightening procedures, and lid suspension may be considered.[31] Eyelid reconstruction (for ectropion) and orbital decompression (for proptosis) can also be considered when applicable.[21] Amniotic membrane has also been used in settings with persistent epithelial defect. The membrane can be attached surgically via fibrin glue or sutures, or through the use of a suspension ring/specialty lens, as in the case of the Prokera lens.[26]
Follow up
Follow up depends on severity but is generally within 1-2 weeks for less severe cases and every 1-2 days for severe cases that are complicated by the presence of a corneal abrasion, ulceration or infiltrate.[21]
Complications
Complications include corneal abrasion, ulceration, microbial keratitis, perforation and corneal scar leading to vision loss (Figure 8).[32] Additionally, band keratopathy may present as a complication of chronic EK.[33]
Of special consideration are patients with pre-existing dry-eye disease, which may make EK more resistant to treatments and can worsen the prognosis.
Prognosis
Mild EK has an excellent prognosis, with most if not all patients making a full recovery if the cornea remains intact without erosion, ulceration, or microbial infection.[18] Severe cases can lead to scarring, perforation and permanent vision loss. Ultimate prognosis depends on the ability to reverse the underlying etiology.
Additional Resources
- Boyd K, Pagan-Duran B, Hazanchuk V. Artificial Tears. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/treatments/artificial-tears-list. Accessed November 17, 2022.
References
- ↑ McCulley, J. P. & Shine, W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc 95, 79-88; discussion 88-93 (1997).
- ↑ Holland, E. J., Mannis, M. J. & Lee, W. B. Ocular surface disease : cornea, conjunctiva and tear film. (Elsevier/Saunders, 2013).
- ↑ Holland, E. J., Mannis, M. J. & Lee, W. B. Ocular surface disease : cornea, conjunctiva and tear film. (Elsevier/Saunders, 2013).
- ↑ Krachmer, J., Mannis, M. J. & Holland, E. J. Cornea. Third edn, Vol. 1 (Mosby, 2011).
- ↑ Krachmer, J., Mannis, M. J. & Holland, E. J. Cornea. Third edn, Vol. 1 (Mosby, 2011).
- ↑ Krachmer, J., Mannis, M. J. & Holland, E. J. Cornea. Third edn, Vol. 1 (Mosby, 2011).
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Grixti, A., Sadri, M., Edgar, J. & Datta, A. V. Common ocular surface disorders in patients in intensive care units. Ocul Surf 10, 26-42, doi:10.1016/j.jtos.2011.10.001 (2012).
- ↑ 8.0 8.1 8.2 8.3 Krachmer, J., Mannis, M. J. & Holland, E. J. Cornea. Third edn, Vol. 1 (Mosby, 2011).
- ↑ 9.0 9.1 9.2 9.3 Lawrence, S. and C. Morris (2008). Lagophthalmos Evaluation and Treatment. EyeNet. Accessed 2017.
- ↑ Mokhtarzadeh, A. & Bradley, E. A. Safety and Long-term Outcomes of Congenital Ptosis Surgery: A Population-Based Study. J Pediatr Ophthalmol Strabismus 53, 212-217, doi:10.3928/01913913-20160511-02 (2016).
- ↑ 11.0 11.1 11.2 Trussler, A. P. & Rohrich, R. J. MOC-PSSM CME article: Blepharoplasty. Plast Reconstr Surg 121, 1-10, doi:10.1097/01.prs.0000294667.93660.8b (2008)
- ↑ Howitt, D. A. & Goldstein, J. H. Physiologic lagophthalmos. Am J Ophthalmol 68, 355 (1969).
- ↑ Hall, A. J. Some Observations on the Acts of Closing and Opening the Eyes. Br J Ophthalmol 20, 257-295 (1936).
- ↑ 14.0 14.1 Skibell, B. C., Soparkar, C.N., Tower, R. N. & Patrinely, J. R. Periocular anesthesia in aesthetic surgery. Semin Plast Surg 21, 37-40, doi:10.1055/s-2007-967746 (2007).
- ↑ Kuruvilla, S. et al. Incidence and risk factor evaluation of exposure keratopathy in critically ill patients: a cohort study. J Crit Care 30, 400-404, doi:10.1016/j.jcrc.2014.10.009 (2015).
- ↑ Dawson, D. Development of a new eye care guideline for critically ill patients. Intensive Crit Care Nurs 21,119-122, doi:10.1016/j.iccn.2005.01.004 (2005)
- ↑ Mercieca, F., Suresh, P.,Morton, A. & Tullo, A. Ocular surface disease in intensive care unit patients. Eye (London, England) 13 ( Pt 2), 231-236, doi:10.1038/eye.1999.57 (1999).
- ↑ 18.0 18.1 Imanaka, H., Taenaka, N.,Nakamura, J., Aoyama, K. & Hosotani, H. Ocular surface disorders in the critically ill. Anesth Analg 85, 343-346 (1997).
- ↑ 19.0 19.1 19.2 19.3 Bahn, R. S. Graves' ophthalmopathy. N Engl J Med 362, 726-738, doi:10.1056/NEJMra0905750 (2010).
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 Shaheen, B. S., Bakir, M. & Jain, S. Corneal nerves in health and disease. Surv Ophthalmol 59, 263-285, doi:10.1016/j.survophthal.2013.09.002 (2014).
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 21.7 Gerstenblith, A. T. & Rabinowitz, M. P. The Wills eye manual :office and emergency room diagnosis and treatment of eye disease. 6th edn, (Wolters Kluwer/Lippincott Williams & Wilkins, 2012).
- ↑ Mantelli, F. et al. Cocaine snorting may induce ocular surface damage through corneal sensitivity impairment. Graefes Arch Clin Exp Ophthalmol 253, 765-772, doi:10.1007/s00417-015-2938-x (2015).
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 23.9 Vallabhanath, P. & Carter, S. R. Ectropion and entropion. Curr Opin Ophthalmol 11, 345-351 (2000).
- ↑ 24.0 24.1 Albert, D. M. & Jakobiec, F. A. Principles and practice of ophthalmology. 2nd edn, (W.B. Saunders Co., 2000).
- ↑ 25.0 25.1 Tawfik, H. A., Abdulhafez, M. H. & Fouad, Y. A.Congenital upper eyelid coloboma: embryologic, nomenclatorial, nosologic, etiologic, pathogenetic, epidemiologic, clinical, and management perspectives. Ophthal Plast Reconstr Surg 31, 1-12, doi:10.1097/IOP.0000000000000347 (2015).
- ↑ 26.0 26.1 26.2 26.3 26.4 Rajaii, F. and C. Prescott (2014). Management of Exposure Keratopathy. EyeNet Magazine. Accessed 2017.
- ↑ Vaede, D., Baudouin, C., Warnet, J. M. & Brignole-Baudouin, F. [Preservatives in eye drops: toward awareness of their toxicity]. J Fr Ophtalmol 33, 505-524, doi:10.1016/j.jfo.2010.06.018 (2010).
- ↑ Cortese, D., Capp, L. & McKinley, S. Moisture chamber versus lubrication for the prevention of corneal epithelial breakdown. Am J Crit Care 4, 425-428 (1995).
- ↑ 29.0 29.1 Zhou, Y., Liu, J., Cui, Y., Zhu, H. & Lu, Z. Moisture chamber versus lubrication for corneal protection in critically ill patients: a meta-analysis. Cornea 33, 1179-1185, doi:10.1097/ICO.0000000000000224 (2014).
- ↑ Scofield-Kaplan, S., Dunbar, K. & Kazim, M. Glad Press'n Seal for the Treatment of Chronic Exposure Keratopathy. Ophthal Plast Reconstr Surg 33, 152-153, doi:10.1097/IOP.0000000000000836 (2017).
- ↑ 31.0 31.1 Pereira, M. V. & Gloria, A. L. Lagophthalmos. Semin Ophthalmol 25, 72-78, doi:10.3109/08820538.2010.488578 (2010).
- ↑ Rosenberg, J. B. & Eisen, L. A. Eye care in the intensive care unit: narrative review and meta-analysis. Crit Care Med 36, 3151-3155, doi:10.1097/CCM.0b013e31818f0ee7 (2008).
- ↑ Jhanji, V., Rapuano, C. J.& Vajpayee, R. B. Corneal calcific band keratopathy. Curr Opin Ophthalmol 22, 283-289, doi:10.1097/ICU.0b013e3283477d36 (2011).