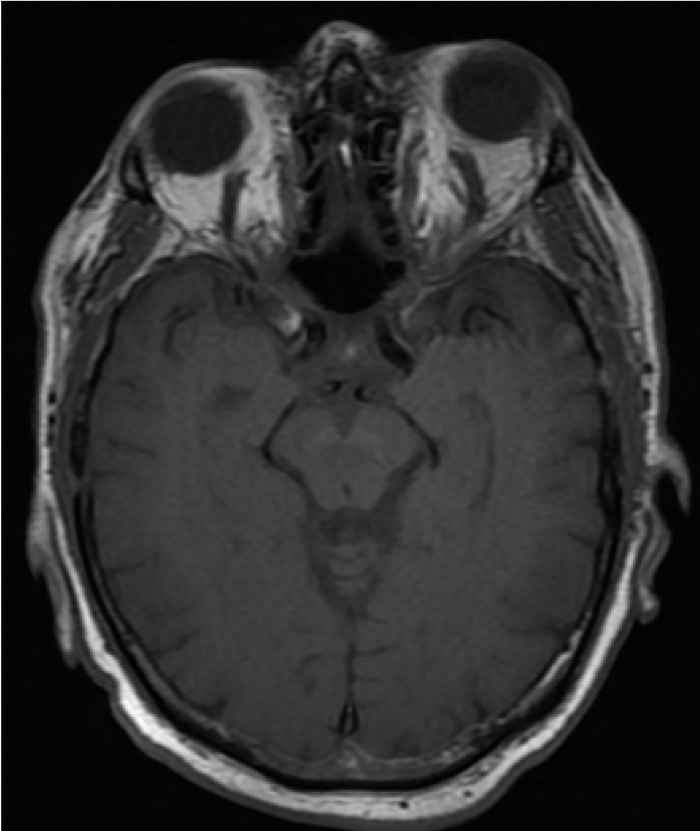
Carotid Cavernous Fistula
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
A carotid-cavernous fistula (CCF) is the result of an abnormal vascular connection between the internal carotid artery (ICA) or external carotid artery (ECA) and the venous channels of the cavernous sinus. CCFs are classified based on the arterial system involved, hemodynamics, and etiology. The presentation can be varied and nonspecific; however, patients with CCFs commonly present with ophthalmic manifestations due to venous drainage of the orbit from the cavernous sinus. Early diagnosis and appropriate management is essential to avoid vision and life-threatening complications.
Disease
ICD-10-CM I77.0 Arteriovenous fistula, acquired
Epidemiology
CCFs are a rare entity that can occur spontaneously or secondary to trauma. Traumatic CCFs account for the majority of CCFs. They can occur following closed head injury, skull base fractures, penetrating head trauma, or iatrogenically after intracranial surgery, endoscopic transsphenoidal or sinus surgery, and endovascular procedures.[1][2] These CCFs typically correspond to Type A CCFs (see Classification section). The true incidence is not well-documented; however, studies have reported an incidence of 0.2% in patients with traumatic brain injury and up to 4% with skull base fractures.[3][4] Bilateral CCFs are rare but more commonly reported in traumatic CCFs, occurring in up to 1% of traumatic cases.[5]
Spontaneous CCFs account for up to 30% of all CCFs reported in the literature and are more common in the postmenopausal patients.[6][7] Spontaneous CCFs may result from the rupture of an aneurysm of the cavernous segment of the internal carotid artery (ICA). This can occur in up to 24% of individuals with such aneurysms, dependent on size and morphology.[6] Musculoskeletal and collagen related disorders including Ehler’s-Danlos syndrome, pseudoxanthoma elasticum, osteogenesis imperfecta, and fibromuscular dysplasia are also thought to predispose to CCF formation due to presumed arterial wall defects and risk of dissection.[8][9][10] CCF from an cavernous ICA aneurysm rupture or cavernous ICA dissection would commonly result in a Type A CCF (see Classification section).
Anatomy
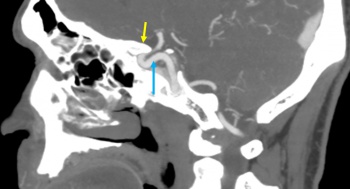
The cavernous sinus (CS) is a paired dural venous sinus centered around the sella turcica.[11] It contains multiple venous channels extending from the endosteal dural layer of the sphenoid bone inferiorly and medially to the meningeal dural layer of the floor of the middle cranial fossa. The CS extends from the superior orbital fissure (SOF) anteriorly to the petrous apex posteriorly, between the dorsum sellae medially and Meckel’s cave laterally. The CS serves as a confluence of venous systems draining the orbit (via superior and inferior ophthalmic veins, SOV, IOV), Sylvian fissure, anterior and middle cranial fossae (via sphenoparietal sinus) and posterior cranial fossa (via the basilar plexus and superior and inferior petrosal sinuses), and they are connected by intercavernous sinuses existing within the sella. The CS also drains inferiorly via emissary veins through the foramen ovale into the pterygoid venous plexus. The venous drainage patterns of the cavernous sinus are pertinent for the pathophysiology of CCFs.
The cavernous segment of the ICA courses through the cavernous sinus (Figure 1). This segment has also been referred to as both the intercavernous and intracavernous ICA. The ICA enters the cavernous sinus as it passes the petrolingual ligament and exits at the proximal dural ring becoming the clinoidal segment.[11] Although the course of the cavernous ICA is variable and dependent of patient specific factors, the typical segments include the proximal ascending, posterior genu, horizontal and anterior genu (Figure 2).[12][13] The anterior genu is at the posterior border of the optic strut, a region of bone between the anterior clinoid process and body of the sphenoid between the optic canal and SOF.[11] Branches of the cavernous ICA include the meningohypophyseal trunk (MHT), inferolateral trunk (ILT) and capsular arteries of McConnell.
The oculomotor (CN III), trochlear (CN IV), trigeminal (CN V) and abducens (CN VI) nerves are associated with the CS.[11] From superior to inferior, CNs III, IV, V1 (ophthalmic division) and V2 (maxillary division) course within the lateral wall of the CS. CNs III (superior and inferior divisions), IV, V1 (lacrimal nerve, frontal nerve, nasociliary branch) and VI exit the CS via the SOF along with the SOV and IOV.
Classification
CCFs are primarily classified into 4 types based on the arterial system involved as described by Barrow et al (Table 1).[14] Type A are direct fistulas, whereas types B, C, and D are indirect fistulas. CCFs are further classified into traumatic or spontaneous etiology and high or low flow based on hemodynamic properties (Table 2). The flow rate is determined by angiography and largely influences clinical presentation.
Table 1: Barrow Classification[14]
| Type | Description |
|---|---|
| A | Direct connection between the ICA and cavernous sinus |
| B | Connection between meningeal branches of ICA and cavernous sinus |
| C | Connection between meningeal branches of ECA and cavernous sinus |
| D | Connection between meningeal branches of both ICA and ECA and the cavernous sinus |
Table 2: CCF Classification[6]
| Type | Classification |
|---|---|
| Anatomical | Direct vs Indirect |
| Hemodynamic | High vs Low Flow |
| Etiology | Traumatic vs Spontaneous |
An updated classification system proposed by Thomas et al. is based on venous drainage and demonstrates a significant correlation with clinical symptoms, treatment planning, and outcome (Table 3).[15]
Table 3: Classification of CCF by Venous Drainage[15]
| Type | Description |
|---|---|
| 1 | Posterior/inferior venous drainage only |
| 2 | Posterior/inferior and anterior venous drainage |
| 3 | Anterior venous drainage only |
| 4 | Retrograde cortical venous drainage |
| 5 | Direct ICA-cavernous sinus fistulae corresponding to the type A Barrow classification |
Pathophysiology
All types of CCFs lead to shunting of blood from a high-flow arterial system (ICA or ECA) into a low-flow venous system (cavernous sinus) without an intervening capillary bed. This produces increased vascular pressure and resistance which impedes venous drainage and leads to vascular congestion in areas that are drained by the cavernous sinus. Impaired drainage of the orbit in an anterior draining CCF is what leads to the common ophthalmic manifestations as a result of congestion, ischemia, and mass effect.[16]
Direct or Type A CCFs are the most common type and form a direct connection between the cavernous segment of the ICA and the cavernous sinus.[14] This high-flow variant of CCFs causes retrograde blood flow from the cavernous sinus into the superior ophthalmic vein (SOV) leading to dilation of the SOV and ophthalmic clinical manifestations. The majority of direct CCFs are caused by trauma, frequently involving a tear in the muscular wall of the ICA, a laceration of one of its branches, or complete transection of the ICA by shearing forces.[16] The cavernous segment of the ICA is particularly susceptible to injury in basilar skull fractures.[3][17] In a review of 91 cases with a direct CCF, Gupta et al. identified 85 trauma-related causes, while 6 were secondary to aneurysm rupture.[18] Less commonly, direct CCFs can occur spontaneously; particularly in patients with predisposing muscular and collagen disorders or cavernous ICA aneurysms.[8][9]
Type B, C, and D CCFs are dural, low-flow fistulae that result from an indirect connection between meningeal branches of the internal carotid artery (ICA) and/or external carotid artery (ECA) and the cavernous sinus.[14] Indirect CCFs are considered low-flow shunts because the originate from these dural arterial branches rather than the high-flow ICA. The majority of indirect CCFs occur spontaneously and are thought to be caused by a dural rupture in the arterial wall; however, the pathophysioloogy is poorly understood. Associations with hypertension, female gender, and older age have been reported.[14] An increased incidence is seen in pregnancy and is postulated to be related to a hormonally induced hypercoagulable state.[19] Indirect CCFs can also be seen in patients with sinusitis, recent trauma or surgery, hypercoagulable states, and cavernous sinus thrombosis.[20][21]
Diagnosis
The diagnosis of CCF is based on the combination of clinical presentation, physical and ophthalmic examination, and diagnostic procedures.
Clinical Presentation
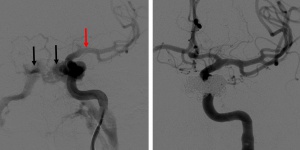
The clinical presentation is influenced by the etiology, classification, size, location within the cavernous sinus, the blood flow rate, and drainage route of the CFF.[22] Direct CCFs tend to have a more severe, acute onset presentation while indirect CCFs are associated with a more gradual onset and chronic course. The earliest clinical features typically involve the orbit, due to venous congestion and venous drainage through the superior and inferior ophthalmic vein into the cavernous sinus.
Direct CCFs often present with a classic triad of pulsatile exophthalmos, orbital bruit, and chemosis. Patients may report diplopia, ocular redness, orbital/retro-orbital pain, swelling, swishing or buzzing sounds, headache, or vision loss. Patients can also have pulsatile tinnitus as a result of turbulent flow through the venous system (Figure 3).[23] Symptoms are typically ipsilateral to the fistula but can occur bilaterally depending on the severity and chronicity of venous congestion.
Ophthalmic signs that can be seen in both direct and indirect CCFs include proptosis, eyelid edema, ptosis, conjunctival arterialization, elevated intraocular pressure, pulsatile exophthalmos, pupillary abnormalities, ocular misalignment, papilledema, dilated retinal veins, ischemic optic neuropathy, central retinal vein occlusion, or choroidal detachment.[14][24] The conjunctival hyperemia associated with CCFs is a result of the arterialization of conjunctival and episcleral veins, consisting of distinct tortuous corkscrew blood vessels that converge at the limbus.[22]
Cranial nerve deficits from ischemia to cranial nerves III, IV, and/or VI can occur and change over time. Ophthalmoplegia has been reported in up to 63% of patients.[25][26] A Horner's syndrome can also be seen with a CN VI palsy due to compression within the cavernous sinus. Compression of V1 and V2 can produce ipsilateral decreased facial sensation. CCFs can also present as an isolated oculomotor palsy without anterior orbital congestive signs and is referred to as a “white-eyed shunt.”[27] A history of trauma should always be elicited in young patients presenting with an isolated CN VI palsy.
In contrast to direct high-flow CCFs, indirect low-flow CCFs may be asymptomatic and insidious in onset. The most common presentation is conjunctival injection which may be initially treated as conjunctivitis causing a delay in diagnosis. With advanced indirect CCFs, increased arterial flow into the cavernous sinus creates venous congestion and retrograde drainage into the orbital veins. The most common findings include arterialization of the conjunctival veins, chemosis, proptosis, diplopia, bruit, retro-orbital headache, elevated intraocular pressure, and a decrease in vision.[28] The classic triad of ocular symptoms seen in direct CCFs are less commonly seen in indirect CCFs.
Diagnosis
A complete history and a detailed clinical exam with appropriate diagnostic testing can help identify and classify CCFs. In office modalities that can be performed include auscultation for an ocular bruit and pneumotonometry to assess for increased ocular pulse amplitude.[29] An ocular doppler ultrasound can identify SOV dilation, arterial flow within the SOV, or enlargement of the extraocular muscles.[24] While a specific diagnosis of direct CCFs is often made quickly, the majority of dural CCFs present insidiously and often go months without a diagnosis.[16] The workup may vary significantly given the clinical symptoms, potential etiologies and pathological states associated with CCFs.
Diagnostic Imaging
The goal of diagnostic procedures is for initial diagnosis of the CCF and then to evaluate the anatomy and physiology of the CCF as it relates to potential treatment options. This workup includes identifying the arterial supply, venous drainage, presence of intracranial or ophthalmic artery collaterals, and assessment of the carotid bifurcation before initiating treatment.[28]
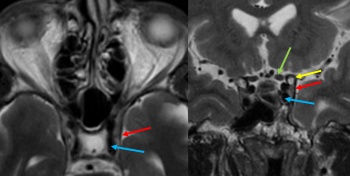
Most patients initially undergo noninvasive imaging with computed tomography (CT), magnetic resonance imaging (MRI), CT angiography (CTA), or magnetic resonance angiography (MRA). The most common neuroimaging signs of anterior draining CCFs include proptosis, enlargement of the SOV, CS, and extraocular muscle enlargement.[30] Enlargement of the IOV and cerebral venous congestion can also seen. If there is a high degree of clinical suspicion or suggestion of a CCF on noninvasive imaging, diagnostic cerebral angiography (digital subtraction angiography, DSA) can confirm the diagnosis and guide treatment.
CTA/MRA: CTA and MRA are often used as first-line noninvasive imaging modalities to evaluate CCFs (Figure 4).[31] CTA and MRA will identify the majority of arterial dissections and cavernous ICA aneurysms causing a CCF as well as enlargement of the affected CS. These modalities can also detect abnormal flow voids, proptosis, enlargement of the SOV/IOV and enlargement of extraocular muscles.[32]
CT: CT without contrast is a useful screening modality to detect skull base fractures in the setting of trauma. Performed with contrast without CTA, a CT may reveal proptosis and engorgement of the SOV.
MRI: Compared to CT, MRI can better demonstrate fat stranding reflecting orbital edema and abnormal flow voids.[33]
Although non-invasive studies can demonstrate findings suggestive of CCF, they cannot definitively diagnosis a CCF which requires the presence of abnormal venous drainage due to the fistulous connection. Further, the absence of findings on non-invasive imaging does not exclude a diagnosis of CCF.
DSA: DSA remains the gold standard imaging modality in the diagnosis of direct and indirect CCFs. DSA is necessary to identify CCF location, arterial supply, flow rate, and venous drainage to classify the CCF and help plan for potential endovascular treatment strategies.[24][28]
Differential Diagnosis
Due to the significant variability in presentation of CCFs, the differential diagnosis will depend on clinical signs and symptoms. It is important to consider the other diagnoses below.
- Cavernous sinus thrombosis
- Superior orbital fissure syndrome
- Orbital apex syndrome
- Retrobulbar hemorrhage
- Thyroid Eye Disease
Management
General treatment
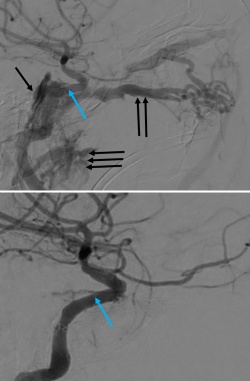
The management of CCFs depends on the etiology, clinical manifestations, radiographic findings, and risk for ophthalmic and neurological complications. Patients with asymptomatic, incidentally discovered indirect CCFs can often be safely observed with routine follow-up to assess for ophthalmologic symptoms and exam changes. Patients with mild ocular symptoms can be treated with appropriate topical medications and observed for visual acuity, intraocular pressure, or ophthalmoscopic changes. Indirect CCFs may also spontaneously resolve in some cases. Direct CCFs are rarely asymptomatic and are treated urgently.[34] For symptomatic CCFs, endovascular treatment is first line therapy with the approach to obliterate the fistulous connection (i.e. transarterial, transvenous or transorbital) dependent on arterial supply, venous drainage and pathophysiology. Despite treatment, symptoms may persist depending on severity and chronicity especially if presenting with blindness.
Direct CCFs
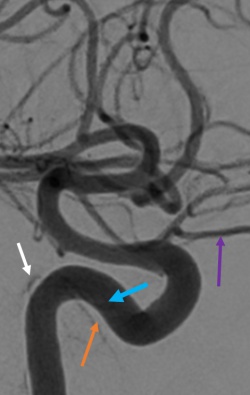
Direct CCFs are unlikely to close spontaneously, and upfront treatment is indicated due to the risk of neurological deficits and worsening of venous congestion. Signs for urgent treatment of direct CCFs include visual impairment, progressive paresis of extraocular muscles, intractable orbital pain, bruit, and progressive exophthalmos.[14][18] Because direct CCFs originate from a defect in the cavernous ICA either from an aneurysm rupture or dissection, the goal of treatment is to cease flow into the CS and reconstruct the cavernous ICA. There are numerous endovascular techniques utilized to achieve this end including coil embolization (Figure 5), coil embolization with balloon remodeling of the ICA and coil embolization with stent-assistance.[35][36][37] Combined transvenous and transarterial approaches can be used to preserve the ICA and pack the CS with coils to stop flow. Liquid embolics such as Onyx (ethylene vinyl alcohol copolymer, Medtronic, USA) and n-butyl cyanoacrylate glue (n-BCA, Trufill, Cerenovus, USA) are less commonly used for direct CCF due to the risk of distal embolization into cerebral arteries and stroke. A large cavernous ICA defect such as that from a traumatic transection may require ICA sacrifice as a life-sustaining treatment. Parent vessel sacrifice (endovascular occlusion) may also be option in cases of recurrence if the patient passes a balloon test occlusion. Less commonly used approaches include covered stents, packing the cavernous sinus via open microsurgery, and cavernous ICA trapping with bypass.
Indirect CCFs
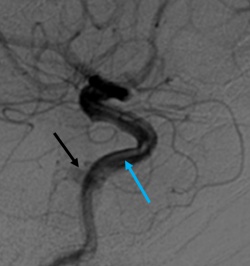
Indirect CCFs that fail conservative therapy or demonstrate progression of symptoms can be considered for endovascular treatment. Symptoms may include decreased vision, diplopia, intractable headache or bruit, or worsening proptosis with exposure keratitis can. Further, neurological deficits, intradural hemorrhage, and venous thrombosis indicate a need for more urgent treatment. While endovascular treatment is the mainstay of therapy, manual compression therapy via contralateral hand compresses has been reported to occlude indirect CCFs in up to 30% of cases.[38] Indirect CCFs may also spontaneously thrombose in up to 60% cases.[6][14]
Endovascular treatment of indirect CCFs is primarily via a transvenous route unlike treatment of direct CCF.[35][39] Transarterial embolization is often primarily attempted via the femoral artery. Occasionally fistulous connection from dural branches the ICA limits the transarterial approach due to the risk of stroke and arterial dissection with treatment. When anatomic variations limit transarterial access, transvenous embolization may be attempted via the petrosal sinuses and pterygoid plexus. Additionally, an orbital surgeon may be asked to expose the superior ophthalmic vein via an anterior orbitotomy for direct canulation.[40][41] Embolization proceeds with detachable coils or liquid embolics (Onyx, n-BCA) to obliterate the fistula, restore normal orbital venous drainage and preserve intradural arterial flow. During transvenous embolization, the arterial circulation is monitored with intermittent angiography via the ipsilateral ICA to ensure the patency of normal cerebral vasculature.
Radiosurgery may be considered in patients with low-flow, indirect CCFs who cannot tolerate endovascular treatment or as salvage therapy for recurrent CCF with limited endovascular options.
Follow Up
A multi-disciplinary approach is often necessary under the care of neurosurgery, oculoplastic surgery and ophthalmology.
Complications
Cranial neuropathies can be seen secondary to compression secondary to mass or thrombogenic effect with coil and less commonly liquid embolization of CCFs.[42] These are typically transient with resolution within several months but can sometimes be permanent.[43] Cerebellar hemorrhage and venous infarction can also occur when liquid embolization is incomplete causing redirected flow to deeper cerebellar veins. Complications of the SOV approach for embolization include hemorrhage, superior oblique damage, CNIV damage, and infection.[44]
Prognosis
The results of embolization for direct CCFs and indirect CCFs are favorable with successful angiographic closure in up to 93% and 92%, respectively.[28][45] Direct or indirect CCFs carry a favorable visual prognosis unless there is evidence of retinal or optic nerve ischemia prior to treatment.[24] Mortality is possible with CCFs if retrograde cortical drainage is present due to the risk of intracerebral hemorrhage. Immediate improvement of intraocular pressure and resolution of an ocular bruit is often observed after successful embolization.[22] Improvement of proptosis, chemosis, and extraocular muscle enlargement is typically seen within weeks of treatment.[22][46][47] A transient “paradoxical worsening” after treatment due to an extension of cavernous sinus thrombosis to the SOV may occur and can be mitigated with corticosteroids prior to spontaneous resolution.[22] Recurrence is rare but more common in younger patients and should be suspected in a patient with recurrence of diplopia.[48]
Additional Resources
https://webeye.ophth.uiowa.edu/eyeforum/cases/111-Carotid-Cavernous-Fistula.htm
References
- ↑ Ono K, Oishi H, Tanoue S, Hasegawa H, Yoshida K, Yamamoto M et al. Direct carotid-cavernous fistulas occurring during neurointerventional procedures. Interv Neuroradiol. 2016; 22(1): 91–96.
- ↑ Chang C.M., and Cheng C.S.: Late intracranial haemorrhage and subsequent carotid-cavernous sinus fistula after fracture of the facial bones. Br J Oral Maxillofac Surg 2013; 51: pp. e296
- ↑ Jump up to: 3.0 3.1 Liang W, Xiaofeng Y, Weiguo L, Wusi Q, Gang S, Xuesheng Z. Traumatic Carotid Cavernous Fistula Accompanying Basilar Skull Fracture: a Study on the Incidence of Traumatic Carotid Cavernous Fistula in the Patients With Basilar Skull Fracture and the Prognostic Analysis About Traumatic Carotid Cavernous Fistula. The Journal of Trauma: Injury, Infection, and Critical Care. 2007;63(5):1014-1020.
- ↑ Helmke K, Krüger O, Laas R. The direct carotid cavernous fistula: A clinical, pathoanatomical, and physical study. Acta Neurochirurgica. 1994;127(1-2):1-5.
- ↑ Luo CB, Teng MMH, Chang FC, Sheu MH, Guo WY, Chang CY. Bilateral traumatic carotid-cavernous fistulae: Strategies for endovascular treatment. Acta Neurochirurgica. 2007;149(7):675-680.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 Ellis JA, Goldstein H, Connolly ES, Meyers PM. Carotid-cavernous fistulas. Neurosurgical Focus. 2012;32(5).
- ↑ Keizer R. Carotid-cavernous and orbital arteriovenous fistulas: ocular features, diagnostic and hemodynamic considerations in relation to visual impairment and morbidity. Orbit. 2003;22(2):121-142.
- ↑ Jump up to: 8.0 8.1 Chuman H, Trobe JD, Petty EM, et al. Spontaneous Direct Carotid-Cavernous Fistula in Ehlers-Danlos Syndrome Type IV: Two Case Reports and a Review of the Literature. Journal of Neuro-Ophthalmology. 2002;22(2):75-81
- ↑ Jump up to: 9.0 9.1 Rios-Montenegro EN. Pseudoxanthoma Elasticum. Archives of Neurology. 1972;26(2):151.
- ↑ Masson-Roy J, Savard M, Mackey A. Carotid cavernous fistula in a patient with type IV Ehlers-Danlos syndrome. Can J Neurol Sci 2017; 44(4): 1–2.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 Rhoton Jr AL. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002;51(suppl_4):S1-375-S371-410.
- ↑ Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38(3):425-432; discussion 432-423.
- ↑ Shapiro M. Internal carotid artery and its aneurysms. Available at http://neuroangio.org/anatomy-and-variants/internal-carotid-artery-and-its-aneurysms/. Accessed March 24, 2020.
- ↑ Jump up to: 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62(2):248-256.
- ↑ Jump up to: 15.0 15.1 Thomas AJ, Chua M, Fusco M, et al. Proposal of venous drainage-based classification system for carotid cavernous fistulae with validity assessment in a multicenter cohort. Neurosurgery. 2015;77:380–385.
- ↑ Jump up to: 16.0 16.1 16.2 Fattahi TT, Brandt MT, Jenkins WS, Steinberg B. Traumatic Carotid-Cavernous Fistula: Pathophysiology and Treatment. J Craniofac Surg. 2003;14(2):240.
- ↑ Tseng CH, Wu KL, Tsai SJ, et al. Development of carotid cavernous fistula after traumatic brain injury. Am J Phys Med Rehabil. 2013;92:187–188.
- ↑ Jump up to: 18.0 18.1 Gupta AK, Purkayastha S, Krishnamoorthy T, et al. Endovascular treatment of direct carotid cavernous fistulae: A pictorial review. Neuroradiology. 2006;48(11):831-839.
- ↑ Yeung SW, Suen SS, Yu SC, et al. Spontaneous carotid cavernous fistula complicating pregnancy. Hong Kong Med J. 2013;19:258–261.
- ↑ Halbach V, Hieshima G, Higashida R, Reicher M. Carotid cavernous fistulae: Indications for urgent treatment. Am J Roentgenol. 1987;149(3):587-593.
- ↑ Gao BL, Zhao W, Xu GP. The development of a de novo indirect carotid-cavernous fistula after successful occlusion of bilateral direct carotid-cavernous fistulas. J Trauma. 2009;66:E28–E31.
- ↑ Jump up to: 22.0 22.1 22.2 22.3 22.4 Miller NR. Diagnosis and management of dural carotid-cavernous sinus fistulas. Neurosurg Focus. 2007;23:E13.
- ↑ Lerut B, De Vuyst C, Ghekiere J, et al. Post-traumatic pulsatile tinnitus: the hallmark of a direct carotico-cavernous fistula. J Laryngol Otol. 2007;121:1103–1107.
- ↑ Jump up to: 24.0 24.1 24.2 24.3 Williams ZR. Carotid-Cavernous Fistulae. International Ophthalmology Clinics. 2018;58(2):271-294.
- ↑ Lewis AI, Tomsick TA, Tew JM. Management of 100 Consecutive Direct Carotid-Cavernous Fistulas. Neurosurgery. 1995; 36(2):239-245.
- ↑ Wang W, Li YD, Li MH, Tan HQ, Gu BX, Wang J, et al: Endovascular treatment of post-traumatic direct carotid-cavernous fistulas: A single-center experience. J Clin Neurosci. 2011; 18:24–28, 2011.
- ↑ Holmes JD, Dierks EJ. Carotid-cavernous fistula after partial maxillectomy: Case report. Journal of Oral and Maxillofacial Surgery. 2001;59(1):102-105.
- ↑ Jump up to: 28.0 28.1 28.2 28.3 Meyers PM, Halbach VV, Dowd CF, Lempert TE, Malek AM, Phatouros CC, et al: Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002; 134:85–92.
- ↑ Henderson AD, Miller NR. Carotid-cavernous fistula: current concepts in aetiology, investigation, and management. Eye. 2017;32(2):164-172.
- ↑ Jindal G, Miller T, Raghavan P, et al. Imaging evaluation and treatment of vascular lesions at the skull base. Radiol Clin North Am. 2017;55:151–166.
- ↑ Chen CC-C, Chang PC-T, Shy C-G, Chen W-S, Hung H-C. CT Angiography and MR Angiography in the Evaluation of Carotid Cavernous Sinus Fistula Prior to Embolization: A Comparison of Techniques. Am J Neuroradiol. 2005;26(9):2349-2356.
- ↑ Rucker JC, Biousse V, Newman NJ. Magnetic resonance angiography source images in carotid cavernous fistulas. Br J Ophthalmol. 2004;88(2):311-311.
- ↑ Rahman WT, Griauzde J, Chaudhary N, et al. Neurovascular emergencies: imaging diagnosis and neurointerventional treatment. Emerg Radiol. 2017;24:183–193.
- ↑ Gemmete JJ, Chaudhary N, Pandey A, Ansari S. Treatment of Carotid Cavernous Fistulas. Curr Treat Options Neurol. 2010;12(1):43-53.
- ↑ Jump up to: 35.0 35.1 Ducruet AF, Albuquerque FC, Crowley RW, McDougall CG. The evolution of endovascular treatment of carotid cavernous fistulas: a single-center experience. World Neurosurgery. 2013;80(5):538-548.
- ↑ De Renzis A, Nappini S, Consoli A, et al. Balloon-Assisted Coiling of the Cavernous Sinus to Treat Direct Carotid Cavernous Fistula: A Single Center Experience of 13 Consecutive Patients. Interventional Neuroradiology. 2013;19(3):344-352.
- ↑ Nossek E, Zumofen D, Nelson E, et al. Use of pipeline embolization devices for treatment of a direct carotid-cavernous fistula. Acta neurochirurgica. 2015;157(7):1125-1130.
- ↑ Kai Y, Hamada J, Morioka M, Yano S, Kuratsu J. Treatment of cavernous sinus dural arteriovenous fistulaeby external manual carotid compression. Neurosurgery. 2007;60(2):253–257.
- ↑ Morton RP, Tariq F, Levitt MR, et al. Radiographic and clinical outcomes in cavernous carotid fistula with special focus on alternative transvenous access techniques. Journal of Clinical Neuroscience. 2015;22(5):859-864.
- ↑ Workman MJ, Dion JE, Tong FC, et al. Treatment of Trapped CCF by Direct Puncture of the Cavernous Sinus by Infraocular Trans-SOF Approach. Case Report and Anatomical Basis. Interv Neuroradiol 2002;8:299–304.
- ↑ Chalouhi N, Dumont AS, Tjoumakaris S, et al. The superior ophthalmic vein approach for the treatment of carotid-cavernous fistulas: a novel technique using Onyx. 2012;32(5):E13.
- ↑ Korkmazer B, Kocak B, Tureci E, et al. Endovascular treatment of carotid cavernous sinus fistula: a systematic review. World J Radiol. 2013;5:143–155.
- ↑ Elhammady MS, Wolfe SQ, Farhat H, et al. Onyx embolization of carotid-cavernous fistulas. J Neurosurg. 2010;112:589–594.
- ↑ Phan K, Xu J, Leung V, et al. Orbital approaches for treatment of carotid cavernous fistulas: a systematic review. World Neurosurg. 2016;96:243–251.
- ↑ Holland LJ, Ranzcr KM, Harrison JD, Brauchli D, Wong Y, Sullivan TJ. Endovascular treatment of carotid–cavernous sinus fistulas: ophthalmic and visual outcomes. Orbit. 2018;38(4):290-299.
- ↑ Alderazi YJ, Dharmadhikari S, Haussen DC, Yavagal DR. Teaching NeuroImages: Reversible pontomesencephalic edema caused by traumatic carotid cavernous fistula. Neurology. 2014;83(2).
- ↑ Huang JF, Toledano M, Katz BS, Lanzino G, Moseley BD. Teaching NeuroImages: Seeing double: Intercavernous sinus dural arteriovenous fistula causing bilateral abducens palsy. Neurology. 2012;78(15).
- ↑ Jung K-H, Kwon BJ, Chu K, et al. Clinical and angiographic factors related to the prognosis of cavernous sinus dural arteriovenous fistula. Neuroradiology. 2010;53(12):983-992.