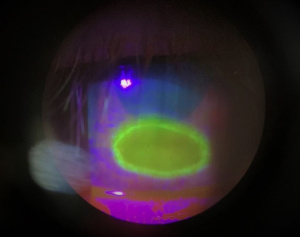
Neurotrophic Keratitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
ICD-10 H16.239
Disease
Neurotrophic keratitis (or keratopathy) (NK) is a corneal degenerative disease characterized by a reduction or absence of corneal sensitivity. In NK, corneal innervation by the trigeminal nerve is impaired. Partial or complete loss of corneal sensation may result in epithelial keratopathy, epithelial defect, stromal ulceration, and eventually corneal perforation.
Epidemiology
The epidemiology of NK is still uncertain. As a rare disease, its estimated prevalence is less than 50/100,000 individuals.[1]
Etiology
Every ocular or systemic condition altering corneal sensory innervation—which runs from the cornea itself to the pontine trigeminal nucleus—can result in NK.
- Most common ocular conditions associated with NK are herpetic keratitis (zoster and simplex), topical anesthetic abuse, chemical and thermal burns, contact lens abuse, topical drug toxicity, irradiation to eye or adnexa, and corneal surgery.
- Chronic use of topical medications containing benzalkonium chloride (BAK) may reduce corneal sensation via nerve damage and impair corneal epithelial healing.[2]
- With regards to corneal procedures, corneal refractive procedures, such as laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK), have been linked to NK.[3] [4][5] The incidence of transient nerve damage, however, seems to be significantly higher in LASIK than in PRK.[6] Corneal transplantation surgery, and specifically penetrating keratoplasty (PK) and deep anterior lamellar keratoplasty (DALK), can result in reduced central corneal sensitivity up to 12 months after either surgical approach.[7] Endothelial keratoplasty (DSAEK or DMEK) does not significantly affect central corneal sensitivity, and thus, may not be associated with NK.[8] A reduction of corneal sensation was also observed in keratoconus eyes following collagen crosslinking.[9]
- Non-corneal ocular surgeries or procedures can also induce NK. Vitrectomy for retinal detachment and photocoagulation to treat diabetic retinopathy have been associated with development or worsening of NK.[10] Routine, single session, indirect laser for proliferative diabetic retinopathy has been reported as a possible cause of NK via damaging ciliary nerves.[11]
- Non-ocular causes include neurosurgical procedures or trauma damaging the fifth cranial nerve, stroke, aneurysms, multiple sclerosis, intracranial masses (e.g. acoustic neuroma), diabetes (NK has been described as the sole sign of diabetes in a patient),[12] leprosy, vitamin A deficiency, and drugs (narcoleptics and antipsychotics). Congenital hypoplasia of the trigeminal nerve has also been described in association with NK.[13]
Risk Factors
Risk factors for NK are those related to the underlying etiology.
General Pathology
A number of histological alterations are seen in corneas of patients with NK, including thinning/disruption of the epithelial layer, cytoplasmic swelling of epithelial cells, loss of microvilli, disorganization of Bowman’s membrane, stromal melting/scarring, and corneal neovascularization. The conjunctiva is also involved with a reduction in goblet cell density and in cell-surface microplicae. Evidence in animal models suggests that NK may also affect corneal neovascularization[14] and stem cell populations.[15]
Diagnosis
History
Medical and surgical history should be accurately reviewed, with special stress on ocular and systemic conditions discussed in the Etiology section.
Symptoms
Since corneal sensory innervation is impaired in NK, patients do not commonly complain of ocular surface symptoms. This makes NK particularly challenging, as patients may seek medical advice months or years after the disease has started. Sometimes, however, blurred vision can be reported due to punctate keratopathy, irregular epithelium, epithelial defects, scarring, or edema.
Babies and young children with impaired corneal sensation may only blink to menacing gestures and have self-inflicted corneal injuries.[16]
Physical Examination
Clinical presentation of NK ranges from subtle corneal surface irregularities to corneal melting and perforation. NK is usually graded in three different stages according to the “Mackie classification”.[17]
- Stage I is characterized by epithelial irregularity most commonly in the form of punctate keratopathy without epithelial defect.
- Stage II is defined by recurrent or persistent epithelial defects (PED) without stromal involvement. The PED is usually oval in shape and its margins are characteristically smooth and rolled due to impaired epithelial healing. Descemet's folds and anterior chamber inflammation may be observed.
- Stage III is characterized by stromal involvement leading to corneal ulcer, melting, and perforation.
Diagnostic Procedures
- Eyelids should be assessed to detect any closure abnormality/lagophthalmos, such as exophthalmos, facial nerve palsy, ectropion, or lid scarring/coloboma. Patients' family members may report nocturnal lagophthalmos. Additionally, the blink rate should be assessed as it is usually reduced in NK.
- Corneal sensitivity should be evaluated with esthesiometry, either qualitatively with a cotton swab or quantitatively with a handheld esthesiometer (e.g. Cochet-Bonnet contact aesthesiometer, CRCERT-Belmonte non-contact aesthesiometer).[18] It should be noted that any eye drops should be applied AFTER having tested corneal sensitivity as they could otherwise alter this measurement.
- Corneal staining with fluorescein is performed in order to highlight corneal epithelial changes. Other vital dyes (i.e. lissamine green or rose bengal) can be used to evaluate corneal/conjunctival integrity. These vital dyes can also be used to evaluate tear meniscus height and tear break-up time.
- Schirmer test is performed to evaluate tear production, which can be impaired as a result of a reduction in corneal sensitivity.
- Slit-lamp exam may reveal features that can be associated with underlying disease. Corneal scarring and neovascularization along with sectoral iris atrophy may suggest herpetic eye disease.
- Fundus exam may reveal diabetic retinopathy and/or panretinal photocoagulation scars that may be associated with NK.
- In vivo confocal microscopy can be used to visualize the affected structure of the sub-basal nerves.
Differential Diagnosis
The finding of severe corneal staining or epithelial defect in absence of ocular discomfort symptoms (due to corneal anesthesia) is highly suspicious of NK. However, dry eye, topical drug toxicity, exposure keratopathy, contact lens-related disorders, chemical injury, and limbal stem cell deficiency may also be associated with some degree of corneal anesthesia but may remain as separate entities until the corneal sensation is affected. The fact that active herpetic corneal disease also reduces corneal sensitivity should be kept in mind since pure NK is sterile. Acanthamoeba keratitis often causes intense ocular pain, but it should be noted that it can also be associated with some degree of corneal anesthesia.[19]
Management
General Management
The management of NK aims to promote corneal healing and avoid complications. Patients with NK should use preservative-free eye medications, as epithelial drug toxicity could complicate the disease. Ocular surface diseases other than/associated with NK (i.e. dry eye, blepharitis, exposure keratopathy, limbal stem cell deficiency) should be properly treated. If it is determined nocturnal lagophthalmos is occurring, patients should patch or tape their eyelids closed prior to sleeping.
Topical NSAIDs should be avoided in patients with NK because they do not show any benefit in healing and they can further decrease corneal sensitivity.[20] If patients have concurrent glaucoma, beta-blockers (e.g. Timolol) should be replaced with eye drops that do not affect esthesia, or earlier laser or surgical intervention for glaucoma should be considered.
Therapy of NK depends on the disease stage:
- Stage I: The therapeutic goal is to improve the quality and transparency of epithelium and to avoid epithelial breakdown. At this stage, frequent application of preservative-free artificial tear eye drops and lubricant ointments is suggested. Punctal occlusion may be beneficial at this stage. In cases of persistent keratopathy, autologous serum tears could be considered. Also, therapeutic soft contact lens application could improve the quality of vision in some cases. In cases with no response using these therapeutic options, recombinant human nerve growth factor (rhNGF) drops[21] [22][23]and a self-retaining cryopreserved amniotic membrane (PROKERA®, Bio-Tissue, Miami, FL) can be considered for this stage.
- Stage II: The aim of the therapy is to promote PED healing and prevent the development of a corneal ulcer. Treatment includes (i) the use of unpreserved artificial tears, lubricant ointments, (ii) therapeutic soft contact lenses or patching, (iii) topical autologous serum application,[24][25] (iv) amniotic membrane grafting either in-office using a self-retaining cryopreserved amniotic membrane (PROKERA®, Bio-Tissue, Miami, FL) or in the operating room,[25] (v) tarsorrhaphy or botulinum induced ptosis, and (vi) topical rhNGF treatment. Antibiotic eye drops can be prescribed to prevent bacterial infections.[1] Topical corticosteroids can be administered to control inflammation cautiously, as they could induce stromal melting.[26]
- Stage III: The therapy focuses on ulcer healing and prevention of corneal perforation. In addition to the therapy suggested for stages I and II, N-acetylcysteine, oral tetracycline, and medroxyprogesterone can be prescribed in case of stromal melting. Vitamin C supplementation can be helpful to prevent collagen degradation.
Insulin
- History
The use of insulin in corneal diseases began in 1945 when it was used in five patients with corneal ulcers – topical (one patient) and systemic (four patients) administration. [27] Then, in 2007, the effect of topical insulin was studied in rodent models and it was noted that it prevented the delay in ocular surface epithelial wound healing observed in poorly controlled diabetic animals, although no promising results were obtained in non-diabetic rats. [28]
- Mechanism of Action
It is still not fully understood how topical insulin works in corneal re-epithelialization. In 2002, it was demonstrated the presence of insulin in the human tear film and the presence of insulin and IGF-1 receptors in the human ocular surface. [29] In vitro, insulin promotes cell proliferation, inhibits apoptosis, and facilitates wound closure through the activation of ERK and PI3K in corneal epithelial cells. Moreover, its wound healing-promoting property was reported to be mediated via EGFR transactivation. In cultured keratocytes, insulin promotes cell proliferation, maintains their phenotype, and prevents proteoglycan degradation. [30] Zagon et al. proposed cell proliferation to be one of the mechanisms of action - Treatment of epithelial defects in diabetic animals with topical insulin restored decreased DNA synthesis in basal epithelial cells to normal levels when measured 48 hours after wounding. [31] Additionally, a study with confocal microscopy showed that topical delivery of insulin to the eye of diabetic mice prevented reduced nerve occupancy in the sub-basal plexus, without any effects on systemic glycemic control. [32]
- Preparation
In Bastion ML et al. 1 unit (U) of topical insulin was prepared using an aseptic technique with 2.5 mL of insulin (Actrapid HM®, novo nordisk) containing 250U insulin added to 2.5 mL normal saline in a commercial applicator bottle to give 50 U/mL of insulin. Given that commercial eye drop bottles of 5 ml size result in 20 uL per drop, then insulin at 50U/mL from a conventional bottle delivers approximately 1U per drop. A new preparation was made every 3 days. [33]
The insulin used in Fai S. et al. was also Actrapid HM® 100 U/mL from novo nordisk. The insulin eye drops were prepared and diluted with 0.9% normal saline. The final solution became 125U of insulin in 5 mL solution and so 1 drop (20 μL) would contain 0.5U of topical insulin. One unit of topical insulin was prepared by adding 2.5 mL of Actrapid HM (250 U) to 2.5 mL of normal saline (total 5 mL), whereas 2 units of topical insulin consisted of 5 mL of Actrapid HM (500 U) without normal saline. [34]
In Diaz-Valle D. et al. the insulin eye drops were prepared at a concentration of 1 U/mL, using fast-acting insulin (Actrapid; Novo Nordisk A/S, Soborg, Denmark) in solution for subcutaneous injection. The insulin solution (100 IU/ml) was diluted in polyethylene glycol and polypropylene glycol base. A sterile filtration was performed, and the final solution was packaged in sterile amber glass eye drop bottles. The drops were refrigerated and used up to one month after preparation. [35]
In Soares RJ. et al. topical insulin drops were prepared by diluting 1 unit of fast-acting insulin per 1 mL of an artificial tear with a propylene glycol base and were preserved at a low temperature (2°C). A therapeutic corneal contact lens (CL) was placed in every patient, and fluoroquinolone drops were applied to prevent possible CL side effects. [36]
- Prescription
Currently, there is still no consensus on the dosage of treatment, but generally, topical insulin eye drops are installed four times a day. [33][34][35][36]
- Safety and side effect
In Zagon et al it was shown that insulin use was not toxic to the cornea, as determined by ocular surface morphological features, intraocular pressure, or corneal thickness. [28] Topical insulin has been proven to be a safe and well-tolerated treatment. [33][34][36] In one study, one patient developed crystalline keratopathy, but it was attributed to corticoids prolonged use. [35]
Cenegermin (Oxervate™) that contains recombinant human nerve growth factor (rhNGF) is the first approved topical medication for NK for the treatment of neurotrophic keratitis in the United States on August 22, 2018, and for the treatment of moderate to severe neurotrophic keratitis in the European Union on July 20, 2017.[37][38][39]It is important to note that cenegermin is no longer available through the National Health Service in the United Kingdom as of 2020; it was classified as not cost-effective.[40] Two randomized clinical trials demonstrated that a greater proportion of patients with stage II or III NK achieved complete corneal healing with topical cenegermin treatment compared to vehicle.[41][42] Recent clinical trials are studying the safety and efficacy of cenegermin in stage I NK (https://clinicaltrials.gov/ct2/show/NCT04485546). In a small size case series, the use of cenegermin was found effective and well-tolerated when used in patients with stage I NK. [21][22] Cenergermin has not been shown to result in significant improvement in corneal sensation, and upon discontinuations of treatment, some patients have recurrence of their neurotrophic keratitis.
ReGeneraTing Agent (RGTA) - matrix therapy agent (Cacicol®) administered topically is also suggested to promote and speed up corneal healing in patients with NK based on observational, non-controlled clinical studies.[43] [44]Currently, Cacicol matrix therapy is available only in Europe.
Finally, if corneal perforation occurs, the treatment varies. In case of small size of perforation, the application of cyanoacrylate glue and soft bandage contact lens or amniotic membrane is performed. In case of a larger defect, a tectonic penetrating or lamellar keratoplasty can be performed.
Surgical Management
In recent years, corneal neurotization has become an increasingly explored topic in the management of neurotrophic keratitis. Neurotization of the cornea typically transfers the supraorbital, supratrochlear, or infraorbital nerve either directly or indirectly with a nerve graft (i.e. sural nerve) to the neurotrophic cornea. Neurotization and nerve reconstruction are well established for use in the reinnervation of peripheral nerve injuries.[45] Corneal neurotization was first investigated in 2009[46] with promising results and many subsequent studies have reported significant improvement in corneal sensation.
Results thus far reveal neurotization leads to improvement in corneal sensation, improvement in visual acuity, and reduction of symptoms for months to years after surgery.[47][46][48][49][50][51][52][53] It is worth noting that neurotization of the cornea is an extensive surgery that requires a high level of experience . Patients should be advised that treatment involving regeneration of corneal nerve fibers, such as with corneal neurotization or cenegermin, might initially result in pain due to recovery of sensation.
Follow-up
The follow-up of NK depends on the disease stage. Patients with stage I disease should be closely monitored due to the risk of asymptomatic disease progression. In stages II and III, patients should be evaluated more frequently, ideally every 1-2 days, until the risk of perforation is reduced and improvement occurs.[54]
Patients with history of NK or impaired corneal sensation should be instructed to pay close attention to new onset ocular redness or blurry vision in order to minimize complications from potential corneal ulcers, foreign bodies, and infections that they may not notice.
Prognosis
Prognosis of patients affected by NK depends on disease stage, degree of anesthesia, and association with other ocular surface diseases. The surgical outcome of keratoplasty in patients affected by NK is generally poor due to impairment in wound healing and the risk of PED recurrence.
Additional Resources
- AAO, Basic and Clinical Science Course. Section 8: External Disease and Cornea, 2019-2020.
- AAO, Focal Points: Neurotrophic Keratitis, Module #2, 2003.
- AAO, Focal Points: Persistent Epithelial Defects, 2016.
References
- ↑ Jump up to: 1.0 1.1 Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clinical ophthalmology 2014;8:571-9.
- ↑ Labbe A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci 2012;53(8):4926-31.
- ↑ Kauffmann T, Bodanowitz S, Hesse L, Kroll P. Corneal reinnervation after photorefractive keratectomy and laser in situ keratomileusis: an in vivo study with a confocal videomicroscope. German journal of ophthalmology 1996;5:508-12.
- ↑ Wilson SE. Laser in situ keratomileusis-induced (presumed) neurotrophic epitheliopathy. Ophthalmology 2001;108:1082-7.
- ↑ Wilson SE, Ambrosio R. Laser in situ keratomileusis-induced neurotrophic epitheliopathy. American journal of ophthalmology 2001;132:405-6.
- ↑ Netto MV, Mohan RR, Ambrosio R, Jr., Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea 2005;24:509-22.
- ↑ Lin X, Xu B, Sun Y, Zhong J, Huang W, Yuan J. Comparison of deep anterior lamellar keratoplasty and penetrating keratoplasty with respect to postoperative corneal sensitivity and tear film function. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 2014;252:1779-87.
- ↑ Kumar RL, Koenig SB, Covert DJ. Corneal sensation after descemet stripping and automated endothelial keratoplasty. Cornea 2010;29:13-8.
- ↑ Wasilewski D, Mello GH, Moreira H. Impact of collagen crosslinking on corneal sensitivity in keratoconus patients. Cornea 2013;32:899-902.
- ↑ Banerjee PJ, Chandra A, Sullivan PM, Charteris DG. Neurotrophic corneal ulceration after retinal detachment surgery with retinectomy and endolaser: a case series. JAMA ophthalmology 2014;132:750-2.
- ↑ Tinley CG, Gray RH. Routine, single session, indirect laser for proliferative diabetic retinopathy. Eye 2009;23:1819-23.
- ↑ Lockwood A, Hope-Ross M, Chell P. Neurotrophic keratopathy and diabetes mellitus. Eye 2006;20:837-9.
- ↑ Morishige N, Morita Y, Yamada N, Nishida T, Sonoda KH. Congenital hypoplastic trigeminal nerve revealed by manifestation of corneal disorders likely caused by neural factor deficiency. Case reports in ophthalmology 2014;5:181-5.
- ↑ Ferrari G, Hajrasouliha AR, Sadrai Z, Ueno H, Chauhan SK, Dana R. Nerves and neovessels inhibit each other in the cornea. Investigative ophthalmology & visual science 2013;54:813-20.
- ↑ Ueno H, Ferrari G, Hattori T, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investigative ophthalmology & visual science 2012;53:867-72
- ↑ Wong VA, Cline RA, Dubord PJ, & Rees M. Congenital trigeminal anesthesia in two siblings and their long-term follow-up. Am J Ophthalmol 2000;129(1):96-98. doi:10.1016/s0002-9394(99)00290-1.
- ↑ Mackie IA. Neuroparalytic keratitis. In: Fraunfelder F, Roy FH, Meyer SM, eds. Current Ocular Therapy. Philadelphia, PA: WB Saunders; 1995:452-4.
- ↑ Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: implications of the use of a non-contact air jet aesthesiometer versus the Cochet-Bonnet aesthesiometer. Experimental eye research 2011;92:408-13.
- ↑ Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. American journal of ophthalmology 2009;148:487-99 e2.
- ↑ Hersh PS, Rice BA, Baer JC, et al. Topical nonsteroidal agents and corneal wound healing. Archives of ophthalmology 1990;108:577-83.
- ↑ Jump up to: 21.0 21.1 Saricay LY, Bayraktutar B, Lilley J, Massaro-Giordano M, Hamrah P; Efficacy and Tolerability of Cenegermin for Stage 1 Neurotrophic Keratopathy. Invest. Ophthalmol. Vis. Sci. 2020;61(7):374. (ARVO Annual Meeting Abstract)
- ↑ Jump up to: 22.0 22.1 Dai X, Zhu, X, Karakus S. Topical Recombinant Human Nerve Growth Factor for Management of Refractory Epithelial Keratopathy. https://doi.org/10.21203/rs.3.rs-1852250/v1
- ↑ Dai X, Tunc U, Zhu X, Karakus S. Effect of Topical Recombinant Human Nerve Growth Factor on Corneal Epithelial Regeneration in Refractory Epithelial Keratopathy. Ocul Immunol Inflamm. 2024 Mar 1:1-7. doi: 10.1080/09273948.2024.2322012. Epub ahead of print. PMID: 38427335.
- ↑ Jeng BH, Dupps WJ, Jr. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea 2009;28:1104-8
- ↑ Jump up to: 25.0 25.1 Turkoglu E, Celik E, Alagoz G. A comparison of the efficacy of autologous serum eye drops with amniotic membrane transplantation in neurotrophic keratitis. Seminars in ophthalmology 2014;29:119-26.
- ↑ Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye 2003;17:989-95.
- ↑ Aynsley Bour TR, Mouth NE. THE USE OF INSULIN IN THE TREATMENT OF CORNEAL ULCERS. 1945.
- ↑ Jump up to: 28.0 28.1 Zagon IS, Klocek MS, Sassani JW, Mclaughlin PJ. Use of Topical Insulin to Normalize Corneal Epithelial Healing in Diabetes Mellitus. Vol. 125, Arch Ophthalmol. 2007.
- ↑ Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJA, Velloso LA. Identification of Insulin in the Tear Film and Insulin Receptor and IGF-I Receptor on the Human Ocular Surface Human Tissues and Tear Film Collection. Vol. 43, Investigative Ophthalmology & Visual Science. 2002.
- ↑ Yu FSX, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Vol. 81, Brain Research Bulletin. 2010. p. 229–35.
- ↑ Zagon IS, Klocek MS, Sassani JW, Mclaughlin PJ. Use of Topical Insulin to Normalize Corneal Epithelial Healing in Diabetes Mellitus. Vol. 125, Arch Ophthalmol. 2007.
- ↑ Chen DK, Frizzi KE, Guernsey LS, Ladt K, Mizisin AP, Calcutt NA. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. Journal of the Peripheral Nervous System. 2013 Dec;18(4):306–15.
- ↑ Jump up to: 33.0 33.1 33.2 MLC B, KP L. Topical insulin for healing of diabetic epithelial defects?: A retrospective review of corneal debridement during vitreoretinal surgery in Malaysian patients. 2013.
- ↑ Jump up to: 34.0 34.1 34.2 Fai S, Ahem A, Mustapha M, Mohd Noh UK, Catherine Bastion ML. Randomized controlled trial of topical insulin for healing corneal epithelial defects induced during vitreoretinal surgery in diabetics. Asia-Pacific Journal of Ophthalmology. 2017 Oct 1;6(5):418–24.
- ↑ Jump up to: 35.0 35.1 35.2 Diaz-Valle D, Burgos-Blasco B, Rego-Lorca D, Puebla-Garcia V, Perez-Garcia P, Benitez-del-Castillo JM, et al. Comparison of the efficacy of topical insulin with autologous serum eye drops in persistent epithelial defects of the cornea. Acta Ophthalmol. 2022 Jun 1;100(4):e912–9.
- ↑ Jump up to: 36.0 36.1 36.2 Soares R, Arêde C, Sequeira J. Topical Insulin-Utility and Results in Refractory Neurotrophic Keratopathy in Stages 2 and 3 [Internet]. 2021. Available from: www.corneajrnl.com.
- ↑ Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000;107:1347-51; discussion 51-2.
- ↑ Lambiase A, Bonini S, Aloe L, Rama P, Bonini S. Anti-inflammatory and healing properties of nerve growth factor in immune corneal ulcers with stromal melting. Archives of ophthalmology 2000;118:1446-9.
- ↑ Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. The New England journal of medicine 1998;338:1174-80.
- ↑ Scottish Medicines Consortium. National Health Service Scotland. https://www.scottishmedicines.org.uk/medicines-advice/cenegermin-oxervate-non-sub-smc2124. Accessed 19 OCT 2023.
- ↑ Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmol. 2018;125(9):1332–433.
- ↑ Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmol. 2020;127(1):14–6.
- ↑ Aifa A, Gueudry J, Portmann A, Delcampe A, Muraine M. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Investigative ophthalmology & visual science 2012;53:8181-5.
- ↑ Guerra M, Marques S, Gil JQ, et al. Neurotrophic keratopathy: therapeutic approach using a novel matrix regenerating agent. J Ocul Pharmacol Ther. 2017;33:662–669.
- ↑ Beris A, Gkiatas I, Gelalis I, Papadopoulos D, Kostas-Agnantis I. Current concepts in peripheral nerve surgery. Eur J Orthop Surg Traumatol. 2019 Feb;29(2):263-269.
- ↑ Jump up to: 46.0 46.1 Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009 Jan;123(1):112-20.
- ↑ Leyngold IM, Yen MT, Tian J, Leyngold MM, Vora GK, Weller C. Minimally Invasive Corneal Neurotization With Acellular Nerve Allograft: Surgical Technique and Clinical Outcomes. Ophthalmic Plast Reconstr Surg. 2019 Mar/Apr;35(2):133-140. doi: 10.1097/IOP.0000000000001181. PMID: 30059392.
- ↑ Ting DSJ, Figueiredo GS, Henein C, et al. Corneal neurotization for neurotrophic keratopathy: clinical outcomes and in vivo confocal microscopic and histopathological findings. Cornea 2018; 37:641–646.
- ↑ Jacinto F, Espana E, Padilla M, et al. Ipsilateral supraorbital nerve transfer in a case of recalcitrant neurotrophic keratopathy with an intact ipsilateral frontal nerve: a novel surgical technique. Am J Ophthalmol Case Rep 2016; 4:14–17.
- ↑ Leyngold I, Weller C, Leyngold M, Tabor M. Endoscopic corneal neurotization: technique and initial experience. Ophthalmic Plast Reconstr Surg 2018; 34:82–85.
- ↑ Elbaz U, Bains R, Zuker RM, et al. Restoration of corneal sensation with regional nerve transfers and nerve grafts. JAMA Ophthalmol 2014; 132:1289.
- ↑ Catapano J, Fung SSM, Halliday W, Jobst C, Cheyne D, Ho ES, Zuker RM, Borschel GH, Ali A. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: long-term clinical outcomes and evidence of corneal reinnervation. Br J Ophthalmol. 2019 Dec;103(12):1724-1731.
- ↑ Sweeney AR, Wang M, Weller CL, Burkat C, Kossler AL, Lee BW, Yen MT. Outcomes of corneal neurotisation using processed nerve allografts: a multicentre case series. Br J Ophthalmol. 2022 Mar;106(3):326-330. doi: 10.1136/bjophthalmol-2020-317361. Epub 2020 Nov 16. PMID: 33199302.
- ↑ Semeraro F, Forbice E, Romano V, et al. Neurotrophic keratitis. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde 2014;231:191-7.