Limbal Stem Cell Deficiency
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
A review of Limbal Stem Cell Deficiency, including its etiology, pathophysiology, diagnosis, and treatment.
Disease Entity
Limbal Stem Cell Deficiency
Disease
The corneal epithelium is a stratified squamous epithelium from which superficial terminal cells are naturally shed. Limbal stem cell deficiency (LSCD) is characterized by a loss or deficiency of the stem cells in the limbus that are vital for the re-population of the corneal epithelium and to the barrier function of the limbus.[1][2] When these stem cells are lost, the corneal epithelium is unable to repair and renew itself. This results in epithelial breakdown and persistent epithelial defects, corneal conjunctivalization and neovascularization, corneal scarring, and chronic inflammation. All of these contribute to loss of corneal clarity, potential vision loss, chronic pain, photophobia, and failure of keratoplasty if done before addressing this.[2][3]
Etiology
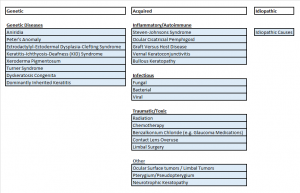
The etiologies can be genetic, acquired, or idiopathic.
Genetic:
LSCD has been associated with PAX6 gene mutations, which are also implicated in aniridia[4] and Peter’s Anomaly.[5] Other genetic disorders that have been reported with LSCD include ectrodactyly-ectodermal-dysplasia-clefting syndrome[6], keratitis-ichthyosis-deafness (KID) Syndrome[7], Xeroderma Pigmentosum[8], Dominantly Inherited Keratitis[9], Turner Syndrome[3] and Dyskeratosis Congenita.[10]
Acquired:
Inflammatory:
Other causes include inflammatory insults such as those seen in Steven-Johnsons Syndrome (SJS) [11], ocular cicatricial pemphigoid[12], and graft versus host disease.[13] Chronic ocular allergy, such as vernal keratoconjunctivitis, is another reported cause.[14] Neurotrophic keratopathy, whether neuronal or ischemic, can lead to this disease as well[2], as can bullous keratopathy .[15]
Infectious:
Any corneal infection, such as herpes keratitis[16] and trachoma, [17] can predispose to this condition.
Traumatic/Iatrogenic:
Acquired causes also include trauma from chemical or thermal burns and prior ocular surgeries or cryotherapies performed around the limbus.[16][18] Radiation and chemotherapy are other potential causes, and systemic[19] as well as topical chemotherapeutic medications may cause LSCD.[20] LSCD has also been seen with benzalkonium chloride toxicity with glaucoma medications.[21] Inappropriate contact lens use with consequent hypoxia and ocular irritation with the destruction of the limbus may also contribute to both focal and total LSCD.[22] [23]
Tumors/Overgrowth of Other Tissue:
Ocular surface tumors are a known cause of LSCD.[2] Pterygium may also cause a focal acquired absence of limbal stem cells.[24]
Risk Factors
Risk factors for LSCD vary according to the underlying cause, as above.
General Pathology
Pathology typically shows conjunctivalization of the cornea, which can be confirmed by the presence of goblet cells in the cornea. However, the goblet cells may not be identified in approximately one-third of patients.
Pathophysiology
LSCD is characterized by a loss or deficiency of stem cells that are vital for the re-population of the corneal epithelium.
Corneal transparency is essential for vision. The outer protective stratified corneal epithelium is continuously renewed with vigorous repair mechanisms. These mechanisms are essential as the cornea is constantly desquamating, and any trauma or loss of epithelial cells must be repaired quickly. Corneal epithelium completely regenerates every 3 to 10 days requiring constant renewal of cells.[9] The repair is essential to prevent infection and preserve vision.
Corneal stem cells are located peripherally at the limbus in the basal cell layer in pigmented crypts called the palisades of Vogt.[25] This pigmentation is thought to help protect the stem cells from ultraviolet light damage. In the normal cornea, renewal occurs from basal cells with centripetal migration of stem cells from the periphery.[26][27] This is a structure deeply related to the function of each cell. The stem cells and their progenitors require the vascular nutrition that is found in the stromal vasculature outside the cornea; thus, they must be at the periphery.[28]
Conversely, the cornea is an avascular tissue. It must remain avascular to prevent vascular structures from interfering with light transmission and, thus, vision. The limbus plays an important role in preventing vascularization of the cornea from the conjunctiva; thus, with loss of integrity of the limbus, conjunctival cells migrate to the cornea resulting in corneal neovascularization or conjunctivalization.[29][30]
Primary Prevention
Primary prevention for LSCD varies according to the underlying cause. Contact lens overwear can be treated with the cessation of lenses and frequent lubrication.[22] Traumatic causes, either mechanical or chemical, may be avoided with the use of eye protection. Treatment of systemic inflammatory disease is necessary to prevent ocular complications. Similarly, treating severe infections before they affect the limbal stem cells is critical to avoid damage in this area.
Diagnosis
The diagnosis of LSCD is largely made on clinical grounds. Patient history and clinical observation of corneal conjunctivalization associated with persistent epithelial defects hint strongly at LSCD.[31] Loss of the limbal anatomy and irregular staining with fluorescein, particularly increased late staining, may also be seen.[32]
History
Patients usually present with pain resulting from recurrent erosions and decreased vision. Other symptoms may include contact lens intolerance, photophobia, tearing, and blepharospasm.[16] The history will vary depending on the etiology. For example, a patient with LSCD from a chemical burn or trauma will give a history of such an event.
Physical Examination
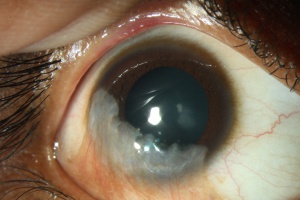
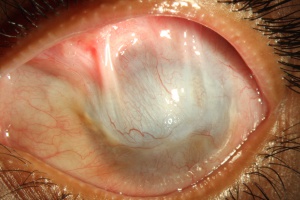
If left untreated, the patient with LSCD will present with recurrent epithelial erosions that lead to chronic keratitis, scarring, and calcification.[16] Delayed wound healing and corneal neovascularization occur with loss of limbal stem cells[33], and eventually, a process called conjunctivalization occurs. The corneal surface will be covered by conjunctiva-like epithelium that transforms into a cornea-like epithelium with loss of goblet cells, a process termed conjunctival transdifferentiation.[27] Patients usually suffer from recurrent erosions and decreased vision as a result of an irregular optical interface, weak tensile strength, and an incompetent barrier function.[27]
Signs
Patients present with progressive epitheliopathy with hazy, translucent epithelium extending centrally from the limbus, most commonly from the superior limbus. Epithelial staining, from punctate changes to more confluent staining, is broadest adjacent to the involved limbus and extends centripetally into the cornea to varying degrees in a whorl shape.[2] Patients often have evidence of mild to moderate tear film dysfunction, reduced tear film break-up time, or both.[21] Infectious keratitis is a common complication.[32] In late stages, superficial and deep vascularization, persistent epithelial defects leading to ulceration, melting and perforation, fibrovascular pannus, and finally, scarring, keratinization, and calcification can be seen.[34]
Symptoms
Eye pain and blurry vision are common complaints in this disease as the epithelial surface breaks down. Eye irritation, contact lens intolerance, and blurred or decreased vision were the most common symptoms in one study.[21]
Clinical Diagnosis
A diagnosis of LSCD requires both clinical signs and symptoms of the disease along with cytological evidence.[30] Typical findings of conjunctival changes to the cornea adjacent to the limbus are a hallmark of the disease.[21]
Diagnostic procedures
Impression cytology shows conjunctivalization of the cornea, and immunohistochemical markers of conjunctiva on impression cytology of the corneal surface (e.g., absence of keratin CK3) confirms the diagnosis.[35] On impression cytology, if the corneal impression is mainly acellular or contains normal corneal epithelial cells, then it is less likely that LSCD exists. However, if the impression consists of a mixture of corneal and conjunctival epithelial cells or mainly conjunctival epithelial cells, then it is highly confirmative of LSCD.[31]
On histopathology of the affected area, there is invasion and overgrowth of conjunctival epithelium, neovascularization, disruption of the basement membrane, and prominent inflammatory cell infiltrates.[36] Pathology typically shows conjunctivalization of the cornea, which can be indicated by the presence of goblet cells in the cornea. However, goblet cells may not be seen in approximately one-third of patients.
In vivo confocal microscopy has also been used to help diagnose LSCD. Changes may include the absence of the palisades of Vogt in the affected sector, metaplastic wing and basal epithelial cells with significantly decreased basal epithelial cell density and subbasal nerve density, and replacement of normal limbal epithelium by vascular fibrotic tissues in late stages.[37]
Differential diagnosis
- Early ulceration or peripheral infectious keratitis may resemble LSCD.
- Pterygium may resemble LSCD and would typically be nasal or temporal.
- Ocular surface squamous neoplasia may be mistaken for LSCD but can be differentiated by surface markers.
See the figure above for the potential causes of LSCD, though any injury or loss of limbal stem cells or their niche may lead to this disease.
Management
Management is typically symptom driven at the early stages of the disease. When limbal stem cell injury is transient, sometimes termed limbal stem cell disease or limbal stem cell distress, conservative medical measures as above may be sufficient.[21][31][38] However, total LSCD must be surgically managed.
Medical therapy
Medical management is aimed at restoring the limbal microenvironment with a stepwise approach based on both stopping traumatic or toxic insults to the limbus and optimizing the ocular surface by improving the tear film, controlling inflammation, and promoting differentiation of healthy epithelium.[21] This includes steps such as discontinuing contact lenses, aggressive lubrication with preservative-free artificial tears, and lid hygiene or warm compresses.[22] When the surface does not respond to such treatment, nightly topical Vitamin A ointment, short-term pulse topical corticosteroids such as methylprednisolone 1%, loteprednol etabonate 0.5% or 0.2%, or prednisolone acetate 1%, and cyclosporine 0.05% should be considered. Punctal occlusion may be performed in patients with significant aqueous tear film deficiency, and patients with rosacea may be treated with oral doxycycline.[21] Autologous serum eye drops may stimulate healing of the corneal surface.[39] A bandage contact lens, the PROSE device, or scleral lens is another option to optimize the health of the ocular surface.[40]
Medical follow up
Improvement in the ocular surface may manifest as decreased pain and increased visual acuity on follow-up examinations. Progressive epitheliopathy with hazy, translucent epithelium extending centrally from the limbus may begin to regress, as may the pattern of epithelial staining with fluorescein[21] As above if the signs and symptoms point to a true LSCD that is not improving, surgery is necessary.
Surgery
Before surgical intervention, effective assessment of tear film production and eye closure is an important prerequisite to ensure optimal surgical outcomes.[30] Resection of pannus tissue and subsequent amniotic membrane transplant may be helpful with partial or focal LSCD not responding to these treatments.[41][42]
Penetrating Keratoplasty (PK) alone is not a viable option in LSCD as the donor tissue does not include limbal stem cells in such a transplant. In addition, the pre-existing corneal vascularization and inflammation increase the risk of rejection and failure in these patients.[2] Thus, while the transplanted cornea will be temporarily clear, the same problems with its restoration and repair will eventually occur unless a viable source of stem cells to repair the lost cells is found.
Unilateral vs. Bilateral Disease:
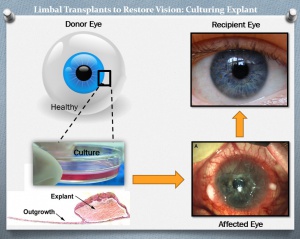
Unilateral LSCD can be treated with autologous limbal stem cell transplants from unaffected eyes, and the benefit is that systemic immunosuppression is unnecessary.[30] However, the removal of stem cells from the contralateral eye risks LSCD in the donor eye. The risk of epithelial problems in the donor eye is low when less than four to six clock hours of limbal tissue and a moderate amount of conjunctiva are removed.[16] Allogeneic transplants from donor eyes are used when the disease is bilateral.[43] Living donor tissue is preferred as cadaveric donor tissue has worse outcomes when transplanted.[44]
Ex Vivo Cultivation:
To minimize the loss of donor limbal tissue and the possibility of inducing LSCD in the donor eye, newer techniques use ex vivo cultivated limbal epithelial cells for transplantation. In this technique, a smaller area (generally 2mm x 2mm) of donor cells is grown in the laboratory on a fibroblast culture medium or graft tissue/amniotic membrane to expand the donor cell population in an attempt to increase success rates and decrease epithelialization time.[45][46] Because using animal feeder cells, such as fibroblasts, to grow explanted cells may represent an unknown risk for a potentially undetected viral transmission, xeno-free transplants on amniotic membrane have been investigated which only use human tissues and cells.[47]
An even newer technique for unilateral disease called Simple Limbal Epithelial Transplantation (SLET) seeds donor stem cells directly on an amniotic membrane placed on the ocular surface of the recipient, altogether bypassing the need for laboratory conditions of expansion.[48] These techniques may be combined with subsequent penetrating keratoplasty to further improve visual outcomes once the limbal stem cell niche has been restored.[49]
The newest techniques for transplanting limbal stem cells involve hydrogel lenses and plasma polymer-coated contact lenses for in vivo culture and transfer of transplanted cells.[50] These are still in the testing phase in animal studies and some small human studies.
Beyond Limbal Cells:
Other options aside from keratolimbal allograft transplantation include oral mucosal epithelial transplantation. The use of keratoprostheses, such as the modified osteo–odonto keratoprosthesis and the Boston Keratoprosthesis (KPro)[51] is generally a last resort for total LSCD with poor surface and tear quality. Human embryonic stem cells, hair follicles, umbilical cord, and dental pulp stem cells all show potential in recreating the corneal phenotype, but none has been perfected to date. [30] Each of these is an attempt to recreate the ocular surface to create a clear vision.
Surgical follow up
Postoperative treatment consists of preservative-free topical antibiotics, topical immunosuppressants, and frequent preservative-free artificial tears. Steroids are rapidly tapered in autologous limbal transplantation.[16] Transplantation of an allograft poses a high risk of rejection even in HLA matched recipients. Therefore, graft survival depends on systemic immunosuppression for a prolonged, if not indefinite, period.[49][52]
During the early postoperative period, the limbal explant is carefully monitored for any areas of epithelial loss. Conjunctival epithelium can cross the explant at these sites and gain access to the corneal surface. If conjunctival encroachment is observed, mechanical debridement of conjunctival cells should be promptly carried out.[16]
Similarly, patients should be followed regularly for signs of graft rejection and treated appropriately. Signs of rejection include sectoral limbal injection, edema and infiltration of the graft, punctate keratopathy, epithelial irregularities and defects, and surface keratinization.[53][16] Risk factors for failure of graft include blink-related microtrauma, conjunctival inflammation, increased intraocular pressure, aqueous tear–deficient dry eye, lagophthalmos, and pathogenic symblepharon, all of which should be addressed at follow-up visits should they arise.[54]
Complications
Untreated LSCD causes pain, decreased vision, and recurrent epithelial erosions that predispose to infection and loss of vision. Infectious keratitis is common with this disease, and patients who wear contact lenses for extended periods of time, have persistent epithelial defects and use topical immunosuppressive medications are at increased risk.[32] After surgical treatment, there is a risk of rejection from allogeneic transplants.[49] It is possible that the cornea will not remain clear and further surgery, such as repeat stem cell transplant or penetrating keratoplasty, may be necessary.[49]
Prognosis
Cultivated Oral Mucosal Epithelial Transplantation (COMET):
Patients with live-related stem cell transplantation, cultivated oral mucosal epithelial transplantation (COMET), and lamellar or penetrating keratoplasty have poor outcomes even with long-term immunosuppression.[54][55][56] The use of fibrin glue rather than amniotic membrane for COMET and optimizing the ocular surface prior to transplant improved outcomes in a recent study, and it is possible that future modifications to the technique may improve these outcomes further.[57]
Cultivated Limbal Epithelial Transplantation (CLET):
Studies have shown that CLET is as effective as direct limbal transplantation for LSCD while requiring less donor tissue and thus being safer for donor eyes.[45][58][59][60][61] Studies of CLET have shown a 68-80% success rate.[62][63] In a review of outcomes of cultured limbal epithelial cell therapy published from 1997 to 2011 with data from 583 patients, the overall success rate was 76%.[60] However, the success rate of a transplant is significantly higher with an increased number of transplanted stem cells and failures tend to happen within the first year.[63]
The largest study of xeno-free explant culture transplants showed a 71% success rate in 200 recipient eyes with a mean follow-up of approximately 5 years and up to 10 years.[46][49] Supplemental corneal transplant (PK) has a survival rate of 1 year, with a median survival of 3.3 years.[49]
In a recent meta-analysis of the outcomes of keratolimbal allografting for LSCD, postoperative corrected distance visual acuity (CDVA) was 2 or more lines better than the preoperative visual acuity in 31%to 67% of eyes .[55]
Simple Limbal Epithelial Transplant (SLET):
In a study of 6 patients with total unilateral LSCD, visual acuity improved from worse than 20/200 in all recipient eyes before SLET surgery to 20/60 or better in four eyes (66.6%), while none of the donor eyes developed any complications. The mean follow-up was 9.2 months.[48]
Boston Keratoprosthesis:
The Boston Keratoprosthesis (KPro) has been found to have good short-term visual and anatomical outcomes in patients with bilateral LSCD[64] with vision of 20/40 or better at 6 months. One large study found that the final postoperative CDVA was 2 or more lines better than the preoperative visual acuity in 86% (18 of 21) of eyes. CDVA was 20/50 or better in more than two-thirds of eyes up to 3 years after surgery, though these prostheses should be used with caution in eyes with SJS and other immune causes as there is an increased retention failure rate.[51]
References
- ↑ Ahmad S, Osei-Bempong C, Dana R, Jurkunas U. The culture and transplantation of human limbal stem cells. Journal of cellular physiology 2010;225(1):15-9 doi: 10.1002/jcp.22251[published Online First].
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 Sangwan VS. Limbal stem cells in health and disease. Bioscience reports 2001;21(4):385-405Â
- ↑ Jump up to: 3.0 3.1 Strungaru MH, Mah D, Chan CC. Focal limbal stem cell deficiency in Turner syndrome: report of two patients and review of the literature. Cornea 2014;33(2):207-9 doi: 10.1097/ICO.0000000000000040[published Online First].
- ↑ Skeens HM, Brooks BP, Holland EJ. Congenital aniridia variant: minimally abnormal irides with severe limbal stem cell deficiency. Ophthalmology 2011;118(7):1260-4 doi: 10.1016/j.ophtha.2010.11.021[published Online First].
- ↑ Hatch KM, Dana R. The structure and function of the limbal stem cell and the disease states associated with limbal stem cell deficiency. International ophthalmology clinics 2009;49(1):43-52 doi: 10.1097/IIO.0b013e3181924e54[published Online First].
- ↑ Di Iorio E, Kaye SB, Ponzin D, et al. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology 2012;119(1):74-83 doi: 10.1016/j.ophtha.2011.06.044[published Online First].
- ↑ Messmer EM, Kenyon KR, Rittinger O, Janecke AR, Kampik A. Ocular manifestations of keratitis-ichthyosis-deafness (KID) syndrome. Ophthalmology 2005;112(2):e1-6 doi: 10.1016/j.ophtha.2004.07.034[published Online First].
- ↑ Fernandes M, Sangwan VS, Vemuganti GK. Limbal stem cell deficiency and xeroderma pigmentosum: a case report. Eye 2004;18(7):741-3 doi: 10.1038/sj.eye.6700717[published Online First].
- ↑ Jump up to: 9.0 9.1 Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Seminars in ophthalmology 2009;24(3):139-48 doi: 10.1080/08820530902801478[published Online First].
- ↑ Aslan D, Ozdek S, Camurdan O, Bideci A, Cinaz P. Dyskeratosis congenita with corneal limbal insufficiency. Pediatric blood & cancer 2009;53(1):95-7 doi: 10.1002/pbc.21960[published Online First].
- ↑ Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology 1995;102(10):1476-85Â
- ↑ Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. The New England journal of medicine 2000;343(2):86-93 doi: 10.1056/NEJM200007133430202[published Online First].
- ↑ Meller D, Fuchsluger T, Pauklin M, Steuhl KP. Ocular surface reconstruction in graft-versus-host disease with HLA-identical living-related allogeneic cultivated limbal epithelium after hematopoietic stem cell transplantation from the same donor. Cornea 2009;28(2):233-6 doi: 10.1097/ICO.0b013e31818526a6[published Online First].
- ↑ Sangwan VS, Jain V, Vemuganti GK, Murthy SI. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea 2011;30(5):491-6Â
- ↑ Uchino Y, Goto E, Takano Y, et al. Long-standing bullous keratopathy is associated with peripheral conjunctivalization and limbal deficiency. Ophthalmology 2006;113(7):1098-101 doi: 10.1016/j.ophtha.2006.01.034[published Online First].
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian journal of ophthalmology 2000;48(2):83-92Â
- ↑ Dua HS, Azuara-Blanco A. Allo-limbal transplantation in patients with limbal stem cell deficiency. The British journal of ophthalmology 1999;83(4):414-9Â
- ↑ Sridhar MS, Vemuganti GK, Bansal AK, Rao GN. Impression cytology-proven corneal stem cell deficiency in patients after surgeries involving the limbus. Cornea 2001;20(2):145-8Â
- ↑ Ding X, Bishop RJ, Herzlich AA, Patel M, Chan CC. Limbal stem cell deficiency arising from systemic chemotherapy with hydroxycarbamide. Cornea 2009;28(2):221-3 doi: 10.1097/ICO.0b013e318183a3bd[published Online First].
- ↑ Lichtinger A, Pe'er J, Frucht-Pery J, Solomon A. Limbal stem cell deficiency after topical mitomycin C therapy for primary acquired melanosis with atypia. Ophthalmology 2010;117(3):431-7 doi: 10.1016/j.ophtha.2009.07.032[published Online First].
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 21.5 21.6 21.7 Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology 2014;121(10):2053-8 doi: 10.1016/j.ophtha.2014.04.025[published Online First].
- ↑ Jump up to: 22.0 22.1 22.2 Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea 2011;30(1):18-23 doi: 10.1097/ICO.0b013e3181e2d0f5[published Online First].
- ↑ Chan CC, Holland EJ. Severe limbal stem cell deficiency from contact lens wear: patient clinical features. American journal of ophthalmology 2013;155(3):544-49 e2 doi: 10.1016/j.ajo.2012.09.013[published Online First].
- ↑ Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology 1985;92(6):728-33Â
- ↑ Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. The Journal of cell biology 1986;103(1):49-62Â
- ↑ Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Investigative ophthalmology & visual science 1981;21(1 Pt 1):135-42Â
- ↑ Jump up to: 27.0 27.1 27.2 Tseng SC. Concept and application of limbal stem cells. Eye 1989;3 ( Pt 2):141-57 doi: 10.1038/eye.1989.22[published Online First].
- ↑ Gipson IK. The epithelial basement membrane zone of the limbus. Eye 1989;3 ( Pt 2):132-40 doi: 10.1038/eye.1989.21[published Online First].
- ↑ Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Survey of ophthalmology 2000;44(5):415-25Â
- ↑ Jump up to: 30.0 30.1 30.2 30.3 30.4 Osei-Bempong C, Figueiredo FC, Lako M. The limbal epithelium of the eye--a review of limbal stem cell biology, disease and treatment. BioEssays : news and reviews in molecular, cellular and developmental biology 2013;35(3):211-9 doi: 10.1002/bies.201200086[published Online First].
- ↑ Jump up to: 31.0 31.1 31.2 Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem cells translational medicine 2012;1(2):110-5 doi: 10.5966/sctm.2011-0037[published Online First].
- ↑ Jump up to: 32.0 32.1 32.2 Sandali O, Gaujoux T, Goldschmidt P, Ghoubay-Benallaoua D, Laroche L, Borderie VM. Infectious keratitis in severe limbal stem cell deficiency: characteristics and risk factors. Ocular immunology and inflammation 2012;20(3):182-9 doi: 10.3109/09273948.2012.672617[published Online First].
- ↑ Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Investigative ophthalmology & visual science 1991;32(1):96-105Â
- ↑ Dua HS, Joseph A, Shanmuganathan VA, Jones RE. Stem cell differentiation and the effects of deficiency. Eye 2003;17(8):877-85 doi: 10.1038/sj.eye.6700573[published Online First].
- ↑ Barbaro V, Ferrari S, Fasolo A, et al. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. The British journal of ophthalmology 2010;94(7):926-32 doi: 10.1136/bjo.2009.164152[published Online First].
- ↑ SCG. T. Application of Corneal Impression Cytology to Study Conjunctival Transdifferentiation Defect: Centro Grafico Editoriale, Univesita Degli Studi Parma, Parma, Italy,, 1988.
- ↑ Deng SX, Sejpal KD, Tang Q, Aldave AJ, Lee OL, Yu F. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Archives of ophthalmology 2012;130(4):440-5 doi: 10.1001/archophthalmol.2011.378[published Online First].
- ↑ Dua HS. The conjunctiva in corneal epithelial wound healing. The British journal of ophthalmology 1998;82(12):1407-11Â
- ↑ Poon AC, Geerling G, Dart JK, Fraenkel GE, Daniels JT. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. The British journal of ophthalmology 2001;85(10):1188-97Â
- ↑ Rathi VM, Sudharman Mandathara P, Vaddavalli PK, Dumpati S, Chakrabarti T, Sangwan VS. Fluid-filled scleral contact lenses in vernal keratoconjunctivitis. Eye & contact lens 2012;38(3):203-6 doi: 10.1097/ICL.0b013e3182482eb5[published Online First].
- ↑ Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Archives of ophthalmology 1998;116(4):431-41Â
- ↑ Sangwan VS, Matalia HP, Vemuganti GK, Rao GN. Amniotic membrane transplantation for reconstruction of corneal epithelial surface in cases of partial limbal stem cell deficiency. Indian journal of ophthalmology 2004;52(4):281-5Â
- ↑ Fernandes M, Sangwan VS, Rao SK, et al. Limbal stem cell transplantation. Indian journal of ophthalmology 2004;52(1):5-22Â
- ↑ Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. American journal of ophthalmology 2007;143(6):945-53 doi: 10.1016/j.ajo.2007.03.005[published Online First].
- ↑ Jump up to: 45.0 45.1 Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349(9057):990-3 doi: 10.1016/S0140-6736(96)11188-0[published Online First].
- ↑ Jump up to: 46.0 46.1 Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. The British journal of ophthalmology 2011;95(11):1525-9 doi: 10.1136/bjophthalmol-2011-300352[published Online First].
- ↑ Johnen S, Wickert L, Meier M, Salz AK, Walter P, Thumann G. Presence of xenogenic mouse RNA in RPE and IPE cells cultured on mitotically inhibited 3T3 fibroblasts. Investigative ophthalmology & visual science 2011;52(5):2817-24 doi: 10.1167/iovs.10-6429[published Online First].
- ↑ Jump up to: 48.0 48.1 Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. The British journal of ophthalmology 2012;96(7):931-4 doi: 10.1136/bjophthalmol-2011-301164[published Online First].
- ↑ Jump up to: 49.0 49.1 49.2 49.3 49.4 49.5 Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. The British journal of ophthalmology 2012;96(12):1504-9 doi: 10.1136/bjophthalmol-2012-301869[published Online First].
- ↑ Brown KD, Low S, Mariappan I, et al. Plasma polymer-coated contact lenses for the culture and transfer of corneal epithelial cells in the treatment of limbal stem cell deficiency. Tissue engineering. Part A 2014;20(3-4):646-55 doi: 10.1089/ten.TEA.2013.0089[published Online First].
- ↑ Jump up to: 51.0 51.1 Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea 2011;30(11):1187-94 doi: 10.1097/ICO.0b013e3182114467[published Online First].
- ↑ Espana EM, Di Pascuale M, Grueterich M, Solomon A, Tseng SC. Keratolimbal allograft in corneal reconstruction. Eye 2004;18(4):406-17 doi: 10.1038/sj.eye.6700670[published Online First].
- ↑ Daya SM, Bell RW, Habib NE, Powell-Richards A, Dua HS. Clinical and pathologic findings in human keratolimbal allograft rejection. Cornea 2000;19(4):443-50Â
- ↑ Jump up to: 54.0 54.1 Liang L, Sheha H, Tseng SC. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Archives of ophthalmology 2009;127(11):1428-34 doi: 10.1001/archophthalmol.2009.263[published Online First].
- ↑ Jump up to: 55.0 55.1 Cauchi PA, Ang GS, Azuara-Blanco A, Burr JM. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. American journal of ophthalmology 2008;146(2):251-59 doi: 10.1016/j.ajo.2008.03.018[published Online First].
- ↑ Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. The New England journal of medicine 2004;351(12):1187-96 doi: 10.1056/NEJMoa040455[published Online First].
- ↑ Satake Y, Yamaguchi T, Hirayama M, et al. Ocular surface reconstruction by cultivated epithelial sheet transplantation. Cornea 2014;33 Suppl 11:S42-6 doi: 10.1097/ICO.0000000000000242[published Online First].
- ↑ Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989;96(5):709-22; discussion 22-3Â
- ↑ Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology 1996;103(1):29-36Â
- ↑ Jump up to: 60.0 60.1 Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. Journal of cellular biochemistry 2011;112(4):993-1002 doi: 10.1002/jcb.23028[published Online First].
- ↑ Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Survey of ophthalmology 2007;52(5):483-502 doi: 10.1016/j.survophthal.2007.06.013[published Online First].
- ↑ Di Iorio E, Ferrari S, Fasolo A, Bohm E, Ponzin D, Barbaro V. Techniques for culture and assessment of limbal stem cell grafts. The ocular surface 2010;8(3):146-53Â
- ↑ Jump up to: 63.0 63.1 Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. The New England journal of medicine 2010;363(2):147-55 doi: 10.1056/NEJMoa0905955[published Online First].
- ↑ Basu S, Taneja M, Narayanan R, Senthil S, Sangwan VS. Short-term outcome of Boston Type 1 KPro for bilateral limbal stem cell deficiency. Indian journal of ophthalmology 2012;60(2):151-3 doi: 10.4103/0301-4738.94060[published Online First].