All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Corneal collagen crosslinking (CXL) is a minimally invasive procedure used to prevent progression of corneal ectasia such as keratoconus and post-LASIK ectasia.
Background
Cross-linking of collagen refers to the ability of collagen fibrils to form strong chemical bonds with adjacent fibrils. In the cornea, collagen cross-linking occurs naturally with aging due to an oxidative deamination reaction that takes place within the end chains of the collagen. It has been hypothesized that this natural cross-linkage of collagen explains why keratectasia (corneal ectasia) often progresses most rapidly in adolescence or early adulthood but tends to stabilize in patients after middle-age.
While crosslinking tends to occur naturally over time, there are other pathways that may lead to premature crosslinking. Glycation refers to a reaction seen predominantly in diabetics that can lead to the formation of additional bonds between collagen. In the pathway most relevant to this topic, oxidation has been shown to be able to trigger corneal crosslinkage through the release of oxygen free radicals.
The bases for the currently employed corneal collagen cross-linking techniques were developed in Europe by researchers at the University of Dresden in the late 1990s. UV light was used to induce collagen cross-linking in riboflavin soaked porcine and rabbit corneas via the oxidation pathway. The resultant corneas were shown to be stiffer and more resistant to enzymatic digestion. The investigation also proved that treated corneas contained higher molecular weight polymers of collagen due to fibril crosslinking. Safety studies showed that the endothelium was not damaged by the treatment if proper UV irradiance was maintained and if the corneal thickness exceeded 400 microns.[1]
Human studies of UV-induced corneal cross-linking began in 2003 in Dresden, and early results were promising. The initial pilot study enrolled 16 patients with rapidly progressing keratoconus and all of the patients stopped progressing after treatment. Additionally, 70% had flattening of their steep anterior corneal curvatures (decreases in average and maximum keratometric values), and 65% had an improvement in visual acuity. There were no reported complications. [2]
In late 2011, orphan drug status was awarded by the FDA to Avedro for its formulation of riboflavin ophthalmic solution to be used in conjunction with the company's particular UVA irradiation system. Corneal collagen cross-linking using riboflavin and UV received FDA approval on April 18, 2016.
In 2015, a Cochrane systemic review analyzing CXL for treating keratoconus revealed that the evidence for the use of CXL in the management of keratoconus is limited due the lack of properly conducted Randomized Controlled Trials.
Basic Concepts
The main components of CXL are a photosensitizer, a UV light source, and the resulting photochemical reaction.
Riboflavin
A photosensitizer is a molecule that absorbs light energy and produces a chemical change in another molecule.
In CXL, Riboflavin is used as the photosensitizer. It is safe systemically and can be adequately absorbed by the corneal stroma topically. It has an absorption peak at 370 nm.[3]
UV Light
As the absorption peak of riboflavin was noted to be 370 nm, UV-A light was found to be ideal for CXL, while at the same time protecting the other ocular structures. The total fluence required was found to be 5.4J/cm2.
The Bunsen Roscoe law states that the photochemical effect should be similar if the total fluence remains constant. Based on this, various protocols have been devised with different combinations of the intensity and duration of UV-A exposure.[4] However, it has been noted that CXL fails to be effective once the energy intensity exceeds 45mW/cm2.
Photochemical Reaction
Once exposed to UV-A light, the riboflavin generates Reactive oxygen species, which induce the formation of covalent bonds both between collagen molecules and between collagen molecules and proteoglycans.[5]
Oxygen
Studies indicate that the presence of oxygen is essential for effective CXL.[5]
Patient Selection
Indications
The primary purpose of cross-linking is to halt the progression of ectasia. Likewise, the best candidate for this therapy is an individual with a progressive ectatic disease of the cornea. The most common indication is keratoconus. Other diseases that may be candidates include Pellucid Marginal Degeneration, Terrien Marginal Degeneration, and post-refractive surgery (such as LASIK, PRK, or Radial Keratotomy) ectasia. There currently are no definitive criteria for progression, but parameters to consider are change in refraction (including astigmatism), uncorrected visual acuity, best corrected visual acuity, and corneal shape (topography and tomography).
Contraindications
- Corneal thickness of less than 400 microns is a contraindication to the standard treatment protocol
- Prior herpetic infection is a contraindication because it may result in viral reactivation
- Concurrent infection
- Severe corneal scarring or opacification
- History of poor epithelial wound healing
- Severe ocular surface disease (ex. dry eye)
- Autoimmune disorders
Surgical Technique
The standard treatment protocol, called the Dresden protocol[2], was formulated by Wollensak et al. for corneas with minimal thickness of 400µm, and is as follows:
- Instill topical anesthetic drops in the eye
- Debride the central 7-9mm of corneal epithelium
- Instill 0.1% riboflavin 5-phosphate drops and 20% dextran solution every 5 minutes for 30 minutes
- Exposure to UVA (370nm, 3mw/cm2) for 30 minutes while continuing instilling the above drops every 5minutes.
- At the end of the procedure, apply topical antibiotics and soft BCL with good oxygen permeability.
In the video shown, anesthetic drops are given, then the speculum is placed and the epithelium is removed. Next, drops of riboflavin are administered, followed by UV exposure.
Variations in Surgical Technique
Variations in Riboflavin
Delivery
Epithelium-off method
As the corneal epithelium offers a barrier to the diffusion of riboflavin to the stroma, the epithelium is manually debrided to enable better penetration. The epithelium-off method is the standard method and remains the most effective.[4]
Epithelium-on method / Trans-epithelial method
Various techniques have been tried to avoid epithelium debridement. These include the use of pharmacological agents to loosen the intraepithelial junctions, the creation of intrastromal pockets for direct introduction of riboflavin, and iontophoresis.
Even though debridement induced complications like postoperative pain and corneal haze are avoided, studies thus far have demonstrated lower effectiveness of CXL in this method.[5]
Osmolarity
Hypo-osmolar riboflavin is used in thin corneas with a thickness between 400 and 320 µm to thicken the cornea to a minimum of 400 microns.[6]
Variations in UV Exposure
Treatment Time - Accelerated CXL
Several protocols have been tried to reduce the treatment time by increasing the intensity of UV exposure. Studies have shown that a middle path with an irradiation dose of 10mW/cm2 for 9 minutes has a better therapeutic and safety profile than higher irradiation doses for shorter periods.[5]
Positioning
Traditionally, CXL is performed in the supine position. There are few reports on the nuances of crosslinking performed at the slit lamp by Hafezi et al. [7]
Effects and Safety of CXL
Crosslinking effect are seen more in the anterior cornea, as riboflavin concentration reduces with increasing depth. IOP measurements are not affected significantly post CXL.
The debrided epithelium is replaced in 3-4 days. Limbal stem cells are not damaged by CXL, as riboflavin is kept away by the remaining peripheral epithelium.
CXL causes apoptosis of keratocytes in the anterior stroma, and in the following weeks, new keratocytes are found migrating from the periphery to the center. As the stroma heals, collagen compaction, and a hyperdense extracellular matrix are seen.
No endothelial damage is caused by CXL when correctly performed. The subepithelial basal nerve plexus is obliterated by this procedure; however, it starts to regenerate after seven days.
Hence, CXL alters normal corneal structure and cellularity at least for 36 months.[6]
Applications and Results
Keratoconus
Controversies exist as to when to perform CXL. Given the natural history of the disease, it is prudent to perform CXL when progression is documented. It's also very important to advise keratoconus patients to stop eye rubbing and to avoid specific sleeping positions as those factors appear to play a major role in the progression of keratoconus.
For further visual rehabilitation along with stabilization of keratoconus, different approaches to combining CXL with refractive surgery, CXL Plus, have been described. The Athens protocol involves performing topography-guided PRK immediately followed by corneal collagen crosslinking. CXL with Intracorneal stromal rings (INTACS) or phakic IOL are other techniques employed to improve both best corrected and uncorrected visual acuity.[4][8]
Pellucid Marginal Degeneration
Being a rare ectatic disorder usually involving the inferior peripheral cornea, CXL has been attempted in these eyes by decentering the focus of irradiation to involve the pathological site. Reports suggest improvement in visual acuity, keratometry, and astigmatism parameters. Even though long-term stability is yet to be studied, in the absence of serious complications, CXL does buy time to postpone further tectonic surgical interventions.[3][9]
Ectasia following Refractive Surgery
CXL for post-Lasik ectasia has been found to stabilize or improve visual acuity and keratometric parameters. The ‘Athens protocol’ described by Kanellopoulos et al. combines CXL with PRK for managing post Lasik ectasia. Lasik Xtra is a new procedure for Lasik followed by modified CXL to prevent post-Lasik ectasia. However there is no proof regarding the benefit, safety and stability of such approach. [3][8][9]
Infective Keratitis – PACK CXL
PhotoActivated Chromophore for infectious Keratitis – corneal collagen crosslinking
Strengthening of the cornea by CXL and the microbicidal activity of UV irradiation has been utilized successfully in the management of keratitis with stromal melt. As the consistency of results is yet to be demonstrated, currently CXL is considered only in the cases resistant to standard antimicrobial therapy.[8]
Bullous Keratopathy
Studies have shown that CXL causes a reduction in corneal edema and thickness with improvement in visual acuity in patients with bullous keratopathy due to different causes. However, these changes last for only about six months and due to this transient effect, CXL may only have a palliative role, if at all, for now.[9]
Studies and Trials
C.G. Carus University Hospital, Dresden, Germany Study [10]
The strength of this study is its large sample size at 1 year. The weaknesses are its poor definition of the disease being treated and poor sample size after 1 year.
- Enrolled 480 eyes of 272 patients
Definition of progression: ≥1D change in keratometry value over 1 year,or, Need for a new contact lens fit ≥1 in 2 years,or,"Patient reports of decreasing visual acuity"
- 241 eyes with ≥6 months data post-CXL, 33 eyes with ≥3 years data post-CXL
- Significant improvement in BCVA at 1 year (-0.08 logMAR BCVA) and 3 yrs (-0.15 logMAR BCVA)
- Significant decrease in mean keratometry in 1st year (-2.68D)
- 53% of eyes with ≥1 lines improvement BCVA 1st year; Another 20% stable in 1st year
- 87% of eyes were stable or improved at 3 yrs (However, very low numbers were included in this analysis so conclusions must be drawn carefully)
Siena Eye Cross Study [11]
The strength of this study is also its sample size at 1 year. Similar to the Dresden study, its interpretation and application to a wider set of patients is limited by a poorly defined patient population. There is also a small sample size at 4 years.
- Enrolled 363 eyes with progressive keratoconus
Definition of progression: Only states that it was defined "clinically and instrumentally within 6 months"
- 44 eyes with ≥48 months of data post-CXL
- Significant improvement in manifest spherical equivalent at 1 year (+1.62D) and 4yrs (+1.87D)
- Significant reduction of mean keratometry values by 1 year (-1.96D) and 4 years (-2.26D)
- No significant change in pachymetry
- No significant change in UCVA/BCVA
- No significant change in cylinder
Australian Study [12]
This study had the best published study design and definition of progression. The researchers looked at patients with clearly defined progressive keratoconus and followed them for 5 years. The three-year data was published in April 2014 and is described below. [13] The five-year data were not published because the results were very similar (personal communication with one of the authors).
Definition of progression (all over 12 months): Increase in cylinder on manifest refraction by ≥1D; or Increase in steepest keratometry value (on Sim K or Manual) by ≥1D; or Decrease in back optic zone radius of best-fitting contact lens by >0.1mm
Methods:
- Eligible eyes randomized independently to either cross-linking or control group
- Primary outcome measure: Maximum simulated keratometry value (Kmax)
- Secondary outcome measures: Uncorrected visual acuity (UCVA), Best-spectacle corrected visual acuity (BSCVA); spherical and cylindrical error on manifest refraction, spherical equivalent, minimum simulated keratometry value (Kmin), corneal thickness at thinnest point; endothelial cell density; and intraocular pressure
- Assessments were performed at 3,6,12,24, and 36 months.
- Treatment: Dresden Protocol (Epi-off): Riboflavin 0.1% drops applied (after epithelial removal with a 57 Beaver blade) every 1-3 minutes x 15 minutes and continued every 1-3 minutes as needed over the 30 minute UV exposure period. UV-X device delivered UV-A 370nm at 3.0mW/cm2 through a 9mm aperture at a distance of 50mm from the corneal apex.
- Control eyes did not receive sham. At 6 months compassionate treatment with CXL was allowed in control eyes, but this then excluded further patient inclusion in the study. Therefore, final results are comparing only treated vs untreated eyes.
- Recruitment ended in 2009 with 50 control eyes and 50 treatment eyes.
Results:
- Three year study reports on 46 treated eyes and 49 control eyes. Out of the 49 control eyes 12 underwent CXL and 5 had corneal transplantation. Five treated and 4 control withdrew for personal reasons.The results are not described by an intention to treat analysis (ITT), so the data after drop-out or crossover on the study patients is not included in the reported results.
- Primary outcome results: Significant difference in Kmax at all time points.
- Treated: Average Kmax flattening was -1.03 +/-0.19D. 6/46 eyes (13%) flattened by ≥ 2.0 D. 1 eye steepened by ≥ 2.0 D.
- Control: Average Kmax steepening was +1.75 +/- 0.38 D. No eyes flattened by ≥ 2.0 D. 19/49 eyes (39%) steepened by ≥ 2.0 D.
- A negative correlation reported between baseline Kmax and change in Kmax at 36 months. Greatest improvement with eyes having a baseline Kmax ≥ 54.0 D in treatment group.
- A negative correlation reported between patient's age at enrollment and change in Kmax in control group.
- Secondary outcome results:
- UCVA: Improved in treatment group compared to baseline at 12, 24, 36 months (P < 0.001). Worsened in control group compared to baseline at 36 months (P<0.001). -
- BSCVA: Improved in treatment group compated to basline at 12, 24, and 36 months (P<0.007). No significant change in control group compared to baseline at 36 months. No significant difference between treated and control at any time point.
- Manifest spherical refraction: No significant difference at any time point.
- Manifest cylindrical error: No significant change from baseline in treatment group.
- Corneal thickness at thinnest point on ultrasound: No significant change in treatment group at any time point. Decreased in control group at 36 months (p=0.029).
- Corneal thickness at thinnest point on Orbscan: Treatment group showed significant decrease most marked at 3 months of -93.00 +/- 7.98 microns (p<0.001). This reversed over the follow-up period of 36 months to -19.52 +/- 5.06 microns.Control group showed progressive decrease at 12, 24, 36 months (p<0.001).
- Intraocular pressure: No significant change using Tonopen in either group. Using Goldmann, significant decrease at 36 months in both groups, but no significant difference between groups.
- Adverse Events:
- Keratitis and corneal edema: 1 case. Authors attributed to premature resumption of RGP wear. Did not adversely affect outcome but did cause scar.
- Keratitis and iritis: 1 case. Started two days after treatment and presumed to be microbial keratitis. Resolved on ofloxacin and fluorometholone acetate 0.1%. Culture negative.
- Peripheral corneal neovascularization: 1 case. Noted at 36 months and attributed to acne rosacea and not CXL.
- Haze: All patients had some degree of haze and this resolved with time.
Conclusions:
The authors of this study declared in their three year report the following pertinent statments :
- "The findings of this study suggest that CXL should continue to be considered as a treatment option for patients with progressive keratoconus"
- "Despite the growing body of literature and continuing efforts to optimize the treatment protocol, there remains a lack of randomized controlled studies with longer-term follow-up to support the widespread clinical use of CXL for keratoconus"
US Multicenter clinical trials
The unique strengths of the Avedro studies, NCT00674661 and NCT00647699, were an actual sham control group. However, the corneal epithelium was not removed in the sham control groups. Patients in the sham control groups were allowed to cross over to the CXL treatment after the 3 month evaluation. These left very few patients in the control groups at the end of the study. The definition of progressive disease was not as rigorous as in the Australian study but was more clearly defined than most other reported RCCTs.
11 U.S. sites
- US Multicenter clinical trial of corneal collagen cross linking for keratoconus treatment: 204 eyes enrolled
- US Multicenter clinical trial of corneal collagen cross linking for treatment of corneal ectasia after refractive surgery: 178 eyes enrolled
- Eyes of patients 14 years or older with progressive keratoconus or corneal ectasia after refractive surgery (defined as one or more of the following: increase in 1D in steepest keratometry or increase of 1D or more in manifest cylinder or increase of 0.5D or more in spherical equivalent in manifest refraction over a 24 month period) were randomized to either CXL treatment (Dresden protocol) or sham (application of riboflavin but no epithelial debridement or UV light treatment)
- The main outcome was the change in maximum keratometry value over a 1 year period
- In both studies, in the CXL treatment group, the maximum keratometry value decreased by 1.6D in the progressive keratoconus group and by 0.7D in the corneal ectasia after refractive surgery group from baseline to 1 year, whereas keratoconus or corneal ectasia after refractive surgery continued to progress in the control groups.
- In the CXL treatment groups, the maximum keratometry value decreased by 2D or more in about 30% of the progressive keratoconus patients and in about 20% of the corneal ectasia after refractive surgery patients and increased by 2 D or more in about 5% of both CXL treatment groups of progressive keratoconus and corneal ectasia after refractive surgery.
- About a third of the CXL treated eyes in both studies gained some corrected visual acuity and about 5% of CXL treated eyes lost some corrected visual acuity.
- The most frequent adverse event in both studies was corneal haze
- No significant changes were noted in endothelial cell count 1 year after treatment in both studies
- "These trials demonstrated the efficacy and safety of CXL for the treatment of keratoconus/corneal ectasia. In addition to decreasing disease progression, CXL also can have beneficial visual and optical effects such as decrease in corneal steepness and improvement in visual acuity in some patients"
Complications
- Temporary stromal edema (up to 70%), temporary haze (up to 100%), and permanent haze (up to 10%)
- Corneal scarring and sterile infiltrates[16][17]
- Infectious keratitis: Bacterial/protozoan/herpetic [18][19][20]
- Diffuse lamellar keratitis (DLK) in a post-LASIK patient[21]
Special Situations
Pediatric Keratoconus
Keratoconus diagnosed in children is usually associated with an unfavorable prognosis and an increased need for a corneal transplant. Hence, in this age group, CXL is advised immediately without the need for documenting progression, as the disease tends to be more aggressive.
The Siena Paediatrics CXL study was conducted on 152 keratoconus patients between 10 and 18 years of age. It demonstrated rapid functional improvement and better long-term stability irrespective of initial corneal thickness in 80% of patients. As expected, patients with thicker corneas did better than the patients with thinner corneas.[4][9]
Thin Cornea
According to the Dresden protocol, for safe crosslinking of the cornea, the minimal corneal thickness required is 400 µm. However, as many cases of keratoconus have thin corneas, different modifications have been tried to cross-link these eyes safely.
In corneas with a minimum thickness of 350 µm, hypo-osmolar riboflavin solution has been used to cause corneal swelling. The CXL effect is not compromised as the anterior stroma remains the same while it is the posterior corneal stroma that swells considerably, in turn also protecting the endothelium.
Prior to the advent of hypo-osmolar riboflavin, other methods like reducing the UV irradiation intensity and not debriding the central epithelium were tried; however, CXL effectiveness was compromised.
Using higher concentration riboflavin of 0.2% has been tried to increase the UV absorption in the anterior stroma and hence protect the endothelium.[6]
Corneal Ectasia in Pregnancy
Pregnancy is associated with hormonal changes that can negatively impact corneal biomechanics. Hence it is advisable to monitor pregnant patients with keratoconus or recent refractive surgery closely. CXL is avoided during pregnancy, keeping in mind the possibility of complications that may require systemic therapy or additional procedures. If needed, CXL may be done after delivery. Despite CXL being done, these patients need to be informed that the ectasia may progress during subsequent pregnancy due to the hormonal changes. For the same reason, it would be prudent for these patients to avoid hormonal contraception methods.[9]
Advances
Pulsed CXL
It has been postulated that pulsed delivery of UVA radiation, would permit better oxygen diffusion into the stroma. As oxygen has an essential role in the photochemical reaction, the presence of more oxygen would translate to greater effect. Further studies are required to determine the ideal pulsing approach.[8]
Adapted Fluence
UV-A fluence delivered in all cases irrespective of the protocol has remained constant at 5.4J/cm2. Hafezi and Kling have put forward the concept of adapted fluence where the energy delivered is customized for the cornea. While this may increase the safety profile of CXL especially in thin corneas, it remains to be validated.[8]
LASIK XTRA
Lasik Xtra is a procedure where CXL is combined with Lasik. After raising the flap and performing laser ablation, higher concentration riboflavin (0.25%) is applied to the stromal bed for about 90 seconds. After this, the interface is washed, and flap replaced. Then half fluence high-intensity UV irradiation is performed. Results so far have shown promise, with better refractive stability and reduced incidence of post Lasik regression and ectasia.[8]
Photorefractive Intrastromal CXL
High‑Fluence CXL is being tried in patients with low myopia to induce subsequent flattening and refractive correction.[8]
Scleral CXL in Axial Myopia
Progressive myopia is accompanied by scleral thinning and elongation. In vitro studies are being conducted to crosslink the sclera and hence stall the progression.[8]
Other CXL methods
Photochemical CXL with other agents like Rose Bengal dye and derivatives of photosynthetic agents like chlorophyll is being studied. Using a contact lens to increase the corneal thickness in thin corneas, during crosslinking has been suggested and termed CACXL – contact lens assisted cross linking. Purely chemical CXL, using molecules like Genipin and β-nitro alcohols, is being investigated.[3]
Summary
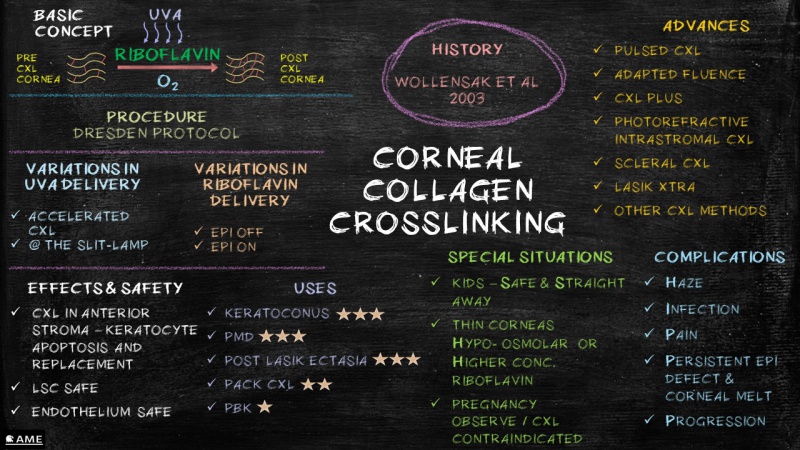
References
- ↑ P T Ashwin, P J McDonnell. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol 2010;94:965e970.
- ↑ Jump up to: 2.0 2.1 Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus.Am J Ophthalmol. 2003 May;135(5):620-7.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Sorkin N, Varssano D. Corneal Collagen Crosslinking: A Systematic Review. OPH. 2014;232(1):10-27. doi:10.1159/000357979
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Randleman JB, Khandelwal SS, Hafezi F. Corneal cross-linking. Surv Ophthalmol. 2015;60(6):509-523. doi:10.1016/j.survophthal.2015.04.002
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Subasinghe SK, Ogbuehi KC, Dias GJ. Current perspectives on corneal collagen crosslinking (CXL). Graefes Arch Clin Exp Ophthalmol. 2018;256(8):1363-1384. doi:10.1007/s00417-018-3966-0
- ↑ Jump up to: 6.0 6.1 6.2 Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul Surf. 2013;11(2):65-74. doi:10.1016/j.jtos.2013.01.002
- ↑ Salmon B, Richoz O, Tabibian D, Kling S, Wuarin R, Hafezi F. CXL at the Slit Lamp: No Clinically Relevant Changes in Corneal Riboflavin Distribution During Upright UV Irradiation. J Refract Surg. 2017;33(4):281.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Sachdev GS, Sachdev M. Recent advances in corneal collagen cross-linking. Indian Journal of Ophthalmology. 2017;65(9):787. doi:10.4103/ijo.IJO_648_17
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. Part II. Clinical indications and results. Ocul Surf. 2013;11(2):93-108. doi:10.1016/j.jtos.2013.01.003
- ↑ Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008 May;34(5):796-801.
- ↑ Caporossi A, Mazzotta C, Baiocchi S, Caporossi T.Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010 Apr;149(4):585-93. Epub 2010 Feb 6.
- ↑ Wittig-Silva, C; Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A Randomized Controlled Trial of Corneal Collagen Cross-linking in Progressive Keratoconus: Preliminary Results. Journal of Refractive Surgery. 2008 (24): S720 - S725.
- ↑ Wittig-Silva C et al. A Randomized, Controlled Trial of Corneal Collagen Cross-linking in Progressive Keratoconus: Three-Year Results. Ophthalmology. 2014. Volume 121 (4); 812-821.
- ↑ Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United States Crosslinking Study Group. United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment. Ophthalmology. 2017 Sep;124(9):1259-1270. doi: 10.1016/j.ophtha.2017.03.052. Epub 2017 May 7. Erratum in: Ophthalmology. 2017 Dec;124(12):1878. doi: 10.1016/j.ophtha.2017.09.014. PMID: 28495149.
- ↑ Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; U.S. Crosslinking Study Group. U.S. Multicenter Clinical Trial of Corneal Collagen Crosslinking for Treatment of Corneal Ectasia after Refractive Surgery. Ophthalmology. 2017 Oct;124(10):1475-1484. doi: 10.1016/j.ophtha.2017.05.036. Epub 2017 Jun 24. PMID: 28655538.
- ↑ Mazzotta C, Balestrazzi A, Baiocchi S, et al. Stromal haze after combined riboflavineUVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Experiment Ophthalmol 2007;35:580e2.
- ↑ Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009 Aug;35(8):1358-62.
- ↑ Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg 2009;35:588e9.
- ↑ Rama P, Di Matteo F, Matuska S, et al. Acanthamoeba keratitis with perforation after corneal crosslinking and bandage contact lens use. J Cataract Refract Surg 2009;35:788e91.
- ↑ Kymionis GD, Portaliou DM, Bouzoukis DI, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg 2007;33:1982e4.
- ↑ Kymionis GD, Bouzoukis DI, Diakonis VF, et al. Diffuse lamellar keratitis after corneal crosslinking in a patient with post-laser in situ keratomileusis corneal ectasia. J Cataract Refract Surg 2007;33:2135e7.

