Xerophthalmia
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Xerophthalmia refers to the spectrum of ocular disease caused by severe Vitamin A deficiency (VAD). Vitamin A serves several essential functions in the eye, and deficiency can lead to a constellation of ocular signs and symptoms that affect the conjunctiva, cornea, and retina. Xerophthalmia continues to be a leading cause of preventable blindness in developing countries[1]. Unfortunately, xerophthalmia signifies a severity of VAD that causes significant morbidity and mortality from malnutrition and increased susceptibility to mucosal infections.
Epidemiology
VAD is among the leading causes of blindness worldwide, estimated to blind half a million children each year[2]. Although VAD is rarely seen in developed countries, it remains a public health concern in more than half of all countries, mostly affecting young children in impoverished regions. The World Health Organization (WHO) estimates that 228 million children have VAD, causing 1-3 million childhood deaths and 5-10 million cases of eye disease. VAD is especially prevalent in Africa and South-East Asia, where young children and pregnant women in low-income countries are disproportionally affected[3][4].
In the United States, VAD is rare. In 2013, it was estimated at 0.3%[5] [6]. VAD usually involves a malabsorptive process, such as inflammatory bowel disease or post-gastric bypass surgery, or a severely restrictive diet such as seen in some children with autism spectrum disorder[7][8].
Etiology
In resource-poor regions, the most common cause of VAD is insufficient nutrition complicated by chronic inflammation from regular gastrointestinal infections. In the developed world, the leading causes of VAD are pancreatic, liver, and intestinal pathology. Some of the more common causes of VAD are listed below:
| Reduced intake of Vitamin A | Impaired absorption of Vitamin A | Reduced Storage of Vitamin A |
|---|---|---|
|
|
|
Risk Factors
- Living in an endemic area
- Low socioeconomic status
- Malnutrition
- Maternal malnourishment (affects Vitamin A concentration in breastmilk)
- Zinc deficiency: inadequate zinc can depress the hepatic synthesis of retinol-binding protein (RBP), which is required for metabolism of retinol from the liver. Zinc may also play a role in the conversion of beta-carotene to retinol via the enzyme 15-15’-dioxygenase.
- Co-existing measles or other respiratory/diarrheal illness
- Autism spectrum disorder with severely restricted diet
Pathophysiology
Vitamin A is a fat-soluble vitamin that humans derive primarily from diet. It has several essential functions in the body, including cell development, metabolism, immune function, vision, and reproductive function.
Dietary sources of preformed vitamin A include dark leafy greens, orange-colored vegetables, fish liver oils, liver, egg yolks, butter, and vitamin A-fortified dairy products. A variety of other foods contain beta-carotene and other provitamin carotenoids, which get converted into vitamin A. These include green leafy and yellow vegetables, carrots, and deep- or bright-colored fruits. Once consumed, Vitamin A is hydrolyzed by pancreatic and intestinal enzymes, emulsified with dietary fats and bile acids, and absorbed in the duodenum. The liver stores 80-90% of the body’s vitamin A in hepatic stellate cells, and the remainder is stored in adipose tissue and the pancreas. Stored vitamin A is released into the circulation bound to prealbumin (transthyretin) and retinol-binding protein. Disruption in any of these processes can lead to VAD[5].
The recommended dietary allowance of vitamin A is 700ug/day in females and 900ug/day in the males. For children and pregnant or lactating women, the recommended amount is 300-900, 770, and 1300ug/day, respectively. Children aged 1-5 years old require a minimum of 200ug/day to prevent symptomatic VAD[5].
In the eye, Vitamin A is essential for maintenance of conjunctival and corneal epithelia as well as night vision. VAD causes metaplasia and keratinization of mucus-secreting epithelium, which can cause conjunctival and corneal xerosis, corneal ulcers, keratomalacia, and corneal scarring. Rods are the retinal photoreceptor that is responsible for night vision. Rods have a singular photopigment, rhodopsin. Retinol is a vitamin A-derived cofactor that is required for the formation of rhodopsin; thus, VAD leads to impairment of rod function and causes nyctalopia, or night blindness due to the eye’s inability to adapt from light to dark[9].
Diagnosis
Clinical Signs[10]
Night blindness (Grade XN): Defective vision in dim light or night blindness is one of the most common manifestations of VAD, especially in children age 2-6 or pregnant or lactating women. Although it is considered one of the earliest manifestations, children with VAD may develop one of the more severe signs, such as corneal ulcers, after infection or diarrhea without any of the classically early signs. Children may not be able to verbalize their symptoms, and parents need to be asked if they have noticed their children behaving differently in the dark, e.g. becoming less active or fearful. Night blindness is considered to be both a sensitive and specific indicator for serum retinol levels.
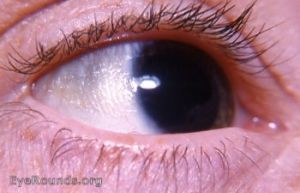
Conjunctival xerosis (Grade X1A): Conjunctival xerosis is characterized by a dull and dry appearance of the conjunctiva with slight wrinkling. It is caused by the loss of goblet cells and insufficient mucin secretion, and it can be subtle and difficult to detect clinically.
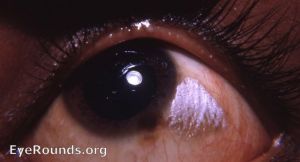
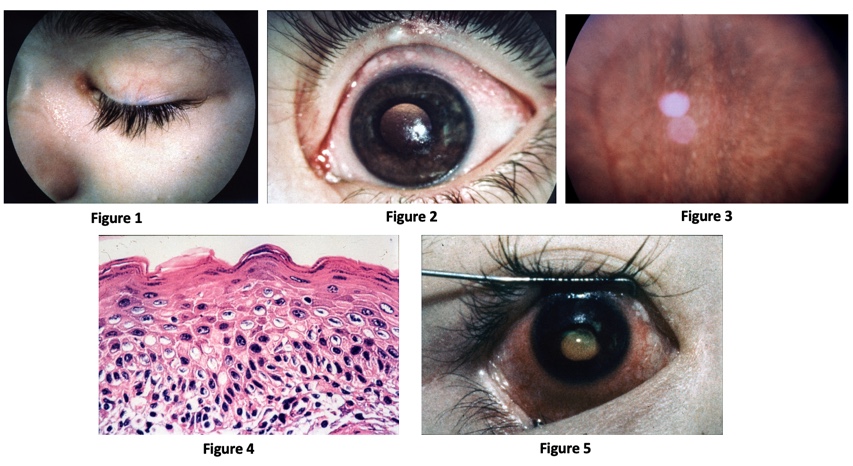
Bitot spots (Grade X1B): Bitot spots are collections of desquamated, keratinized epithelial cells mixed with the gas-forming bacteria Corynebacterium xerosis. They appear as triangular patches of foamy, whitish, opaque deposits, typically located on the bulbar conjunctiva near the limbus at the 3 and 9 o’clock positions. They are more common temporally.
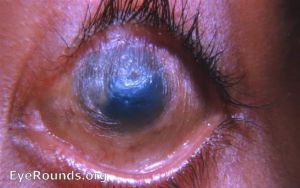
Corneal xerosis (Grade X2): Corneal xerosis is characterized by a dull and hazy appearance of the cornea that is caused by drying of the cornea secondary to conjunctival gland dysfunction. It may initially present with bilateral punctate corneal epithelial erosions, and it can quickly progress to the stage of corneal ulceration. Up to this stage, high-dose Vitamin A supplementation can result in the full preservation of vision.
Corneal ulceration (Grade X3A and B): Corneal xerosis can lead corneal ulceration and melting if not treated urgently. Keratomalacia, the melting away of the cornea by liquefactive necrosis, is the most severe form of xerophthalmia. It can perforate and destroy the cornea in just a matter of days. A child who appears relatively healthy but develops keratomalacia should be questioned about a recent history of measles or diarrhea, which could rapidly deplete already deficient vitamin A stores.
Corneal scar (Grade XS): Corneal scarring due to VAD is often symmetric and bilateral. Other causes of corneal scarring must be ruled out.
Xerophthalmic fundus (Grade XF): prolonged VAD can lead to structural changes in the retina. Fundus changes appear as small, white, deep retinal lesions scattered throughout the posterior pole.
Grading System
The World Health Organization (WHO) has a grading system for xerophthalmia as seen in the following table[11]. More detailed descriptions of the clinical signs of Xerophthalmia are below.
| Grade of xerophthalmia | Peak age group (years) | Type of deficiency |
|---|---|---|
| XN: Night blindness | 2-6; adult women | Longstanding. Not blinding |
| X1A: Conjunctival xerosis | 3-6 | Longstanding. Not blinding |
| X1B: Bitot's spots | 3-6 | Longstanding. Not blinding |
| X2: Corneal xerosis | 1-4 | Acute deficiency. Can be blinding |
| X3A: Corneal ulcer <1/3 cornea | 1-4 | Severe acute deficiency. Blinding |
| X3B: Corneal ulcer/keratomalacia
1/3 cornea or greater |
1-4 | Severe acute deficiency. Blinding |
| XS: Corneal scarring (from X3) | >2 | Consequence of corneal ulceration |
| XF: Xerophthalmos fundus | Adults | Longstanding. Not blinding. Rare |
Work-up
Patients presenting with clinical signs of xerophthalmia (or, in the case of a pediatric patient, their parents) must be questioned on dietary, medical, and social history, including alcohol intake. Be sure to ask about any malabsorptive process, as described above, or risk factors such as living in a resource-poor country or current pregnancy or lactation. Because xerophthalmia is a manifestation of moderate-to-severe VAD, it is important to ask about the systemic signs of milder VAD, including frequent GI and respiratory tract infections, anemia, iron deficiency, and development of xeroderma and phrynoderma (follicular hyperkeratosis often found on extensor surfaces, shoulders, and buttocks)[12] [9].
Physical exam
Aside from the ocular exam, the physical exam should include assessments for weight/body habitus, jaundice, and abdominal exam for hepatomegaly.
Blood tests[13]
- Serum vitamin A/retinol (reference range: 20-60 mcg/dL). These levels can be normal due to maintenance of circulating retinol levels by hepatic stores. VAD-related ocular symptoms have been shown to develop at concentrations <10mcg/dL.
- Serum retinol binding protein (reference range: 30-75 ug/ml).
- Serum zinc (reference range: 75-120 mcg/dL)
Other testing[5] [14]
- Dark adaptometry and night vision threshold tests
- Electroretinogram (ERG): Retinopathy from VAD is associated with decreased amplitude
- Impression cytology: conjunctival specimens can be viewed for the presence of goblet cells. A decrease in normal amount is considered an effective measurement of VAD.
- Liver biopsy: considered the gold standard for evaluating total body vitamin A, although it is not routinely used outside of the research setting due to procedural risks.
Differential Diagnosis
- Parasitic eye disease, e.g. Acanthamoeba keratitis or Onchocerciasis
- Trachoma
- Allergic conjunctivitis
- Viral conjunctivitis
- Dry eye syndrome
- Retinitis pigmentosa and other retinal dystrophies that can present with night blindness
- Pinguecula/pterygium (may resemble Bitot spots)
Management
Keratomalacia should be treated as a medical emergency, as it is an indicator of very severe VAD. High-dose vitamin A is the treatment for all patients, and treatment can either be oral or intramuscular. Recommended Vitamin A deficiency treatment regimens are described in the following table[15]. Treatment can be adjusted as needed based on regular serum retinol level monitoring
| Vitamin A dosage (IU) | |
|---|---|
| Young infants 0-5 mo1 | 50,000 |
| Older infants 6-11 mo1 | 100,000 |
| Children (males: 12 mo or more;
females 12 mo to 12 y and 50 y or more)1 |
200,000 |
| Women (13-49 y) with
night blindness and/or Bitot's spots |
10,000 every day or 25,000 every week for at least 3 mo |
| Women (13-49 y) with active
corneal lesions |
200,000 on days 1, 2, and 14 |
Patients with concomitant zinc deficiency should also undergo zinc supplementation. VAD associated with malabsorptive or other processes is treated based on the severity of deficiency and provider discretion, usually involving daily treatment.
Localized ocular treatment includes intense lubrication, topical retinoid acid, and management of perforation.
Patient education is a large component of management, and patients and their families should be educated on dietary sources of vitamin A and well-balanced, nutrient-rich diets. Alcohol abuse should be addressed if applicable. In resource-poor countries, large-scale vitamin A supplementation programs are in place.
Prognosis
Xerophthalmia signifies a severity of VAD that can cause mortality from malnutrition and increased susceptibility to mucosal infections. Nearly 2/3 of children with keratomalacia die within months. Mortality in children with night blindness is triple the mortality found in children with subclinical VAD, and mortality in children with both Bitot spots and night blindness is reported to be nine times that of children with subclinical VAD[11].
With prompt treatment of high-dose vitamin A, the early ophthalmologic signs, such as a rod function, conjunctival xerosis, and night blindness, can resolve completely within about 2 months of supplementation, without long-term sequelae[16] [17]. Corneal xerosis and ulceration may also improve; however, they can lead to scarring and permanent vision loss[5].
References
- ↑ Feroze KB, Kaufman EJ. Xerophthalmia. [Updated 2021 Apr 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431094/.
- ↑ Sommer A. Vitamin A deficiency, child health, and survival. Nutrition 1997; 13: 484–5.
- ↑ World Health Organization. Prevention of Childhood Blindness. Geneva: The Organization, 1994.
- ↑ World Health Organization. Vitamin A Deficiency. Retrieved May 30, 2021, from https://www.who.int/data/nutrition/nlis/info/vitamin-a-deficiency.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 Hodge C, Taylor C. Vitamin A Deficiency. [Updated 2021 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567744/
- ↑ Pfeiffer CM, Sternberg MR, Schleicher RL, Haynes BM, Rybak ME, Pirkle JL. The CDC's Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population is a valuable tool for researchers and policy makers. J Nutr. 2013 Jun;143(6):938S-47S.
- ↑ Song A, Mousa HM, Soifer M, Perez VL. Recognizing vitamin A deficiency: special considerations in low-prevalence areas. Curr Opin Pediatr. 2022 Apr 1;34(2):241-247. doi: 10.1097/MOP.0000000000001110. PMID: 35125379; PMCID: PMC8891082.
- ↑ Marek S, Forbes G, Avery RA, Zanganeh T, Davidson S, DeCarlo E, Kumar P, Hammersmith K. Potential blindness from nutritional xerophthalmia in autistic patients. J AAPOS. 2023 Aug;27(4):198.e1-198.e4. doi: 10.1016/j.jaapos.2023.05.009. Epub 2023 Jul 14. PMID: 37453663.
- ↑ Jump up to: 9.0 9.1 Mehra D, Le PH. Physiology, Night Vision. [Updated 2020 Oct 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545246/
- ↑ Gilbert C. The eye signs of vitamin A deficiency. Community Eye Health. 2013;26(84):66-67.
- ↑ Jump up to: 11.0 11.1 Gilbert C. The eye signs of vitamin A deficiency. Community Eye Health. 2013;26(84):66-67.
- ↑ Chiu M, Dillon A, Watson S. Vitamin A deficiency and xerophthalmia in children of a developed country. J Paediatr Child Health. 2016 Jul;52(7):699-703. doi: 10.1111/jpc.13243. PMID: 27439630.
- ↑ Krishna U, Kamath SJ, Nayak MK. Management of Bitot’s Spots. EyeNet Magazine. Accessed May 30, 2021. Available from: https://www.aao.org/eyenet/article/management-of-bitot-s-spots#chart.
- ↑ Lietman TM, Dhital SP, Dean D. Conjunctival impression cytology for vitamin A deficiency in the presence of infectious trachoma. Br J Ophthalmol. 1998 Oct;82(10):1139-42. doi: 10.1136/bjo.82.10.1139. PMID: 9924300; PMCID: PMC1722377.
- ↑ Ross DA. Recommendations for Vitamin A Supplementation. The Journal of Nutrition. Volume 132, Issue 9, September 2002, Pages 2902S–2906S, https://doi.org/10.1093/jn/132.9.2902S.
- ↑ Chow CC, Mieler WF. Vitamin A deficiency and xerophthalic fundus in autoimmune hepatitis and cirrhosis. Retinal Cases & Brief Reports. 2014;8(3):164–166. doi: 10.1097/ICB.0000000000000031.
- ↑ Genead MA, Fishman GA, Lindeman M. Fundus white spots and acquired night blindness due to vitamin A deficiency. Doc Ophthalmol 2009;119:229–233.
- ↑ Steinemann TL, Christiansen SP. Vitamin A deficiency and xerophthalmia in an autistic child. Arch Ophthalmol. 1998 Mar;116(3):392-3. doi: 10.1001/archopht.116.3.392. PMID: 9514500.