Ghost Cell Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Summary
Ghost cell glaucoma is a secondary open-angle glaucoma caused by degenerated red blood cells (ghost cells) obstructing the trabecular meshwork.
Disease Entity
Other specified glaucoma
- ICD10 2015 H40.89
- ICD9 2015 365.89
Glaucoma associated with vascular disorders
- ICD9 2015 365.63
Disease
Following vitreous hemorrhage, blood breakdown products may accumulate in the anterior chamber (AC). Hemolyzed erythrocytes obstruct aqueous outflow through the trabecular meshwork leading to a secondary open-angle glaucoma.
Etiology
Ghost cell glaucoma is closely associated with vitreous hemorrhage. Causes of ghost cell glaucoma include causes of vitreous hemorrhage such as ocular trauma, systemic diseases such as diabetes or sickle cell disease/trait, iritis (Fuchs heterochromic iridocyclitis, herpes simplex, herpes zoster, etc.), intraocular tumors (retinoblastoma, malignant melanoma, etc.), uveitis glaucoma hyphema syndrome, rubeosis iridis, iris varices, papillary microhemangiomas, and ocular surgery including but not limited to cataract extraction, laser trabeculoplasty and iridotomy. One case report of ghost cell glaucoma after a snake bite has been reported. Ghost cell glaucoma can also be associated spontaneous ocular hemorrhages.
Risk Factors
Risk factors for posttraumatic glaucoma include advancing age, visual acuity on presentation worse than 20/200, iris injury, lens injury, hyphema, and angle recession. Risk factors for ghost cell glaucoma include vitreous hemorrhage.
Pathophysiology
Three to 10 days after vitreous hemorrhage, thrombi undergo fibrinolysis and red blood cells (RBCs) diffuse throughout the vitreous cavity. During this time, RBC breakdown also occurs. Loss of hemoglobin from the RBCs produces ghost cells and hemoglobin spherules. During the conversion of RGCs to their ghost cell form, intracellular hemoglobin is lost into the extracellular vitreous spaces and adhere to vitreous strands. Ghost cells appear as small, spherical, khaki-colored cells and do not adhere to each other or to the vitreous strands and are free to move into the AC. They gain access to the AC through a disrupted anterior hyaloid face, which can occur from previous surgery (e.g., pars plana vitrectomy, cataract extraction, or capsulotomy), trauma, or spontaneous disruption. Ghost cells are generally 4 to 7 micrometers in size and less pliable/more rigid than normal RBCs. As a result of their rigidity, ghost cells are not able to easily exist the AC through the trabecular meshwork. Their accumulation within the trabecular meshwork obstruct outflow resulting in secondary glaucoma. Ghost cells are visible within 1-3 months following vitreous hemorrhage. It is important to note that the presence of ghost cells do not necessarily lead to development of ghost cell glaucoma.
Diagnosis
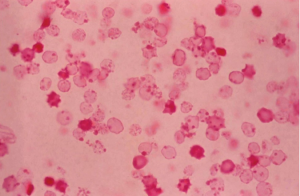
Ghost cell glaucoma is a clinical diagnosis. Diagnostic findings include presence of heme in the vitreous, ghost cells in the AC, delayed onset of increased intraocular pressure following vitreous hemorrhage, an open angle on gonioscopy, possible presence of ghost cells layering in the inferior angle due to gravity, and often a disrupted anterior hyaloid face. Histologically, the diagnosis of ghost cell glaucoma can be made by phase-contrast microscopy of AC aspirate, paraffin embedding of the pellet after centrifugation of the aspirate, or staining of the sample with 1 % methyl violet. Heinz bodies, and spherical erythrocytes with denatured hemoglobin granules bound to the internal surface of the cell membrane are observed with H&E staining.
History
Ghost cell glaucoma was originally described by Campbell et al in 1976. It was once also termed “hemophthalmitis” which was a misnomer as there is often no evidence of active inflammation. This term has been since abandoned.
Physical Exam
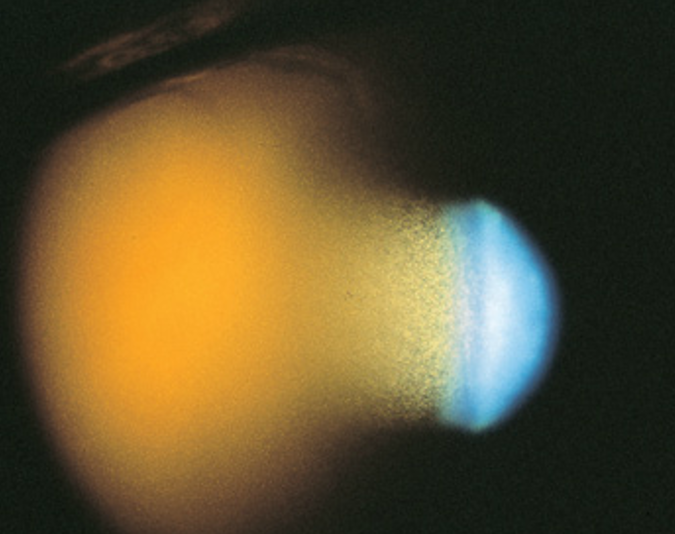
Clinically, patients present with intraocular pressure (IOP) spikes and a history of vitreous hemorrhage resulting from trauma, surgery, or preexisting retinal disease 1-3 months prior to presentation. The IOP may be markedly elevated (as high as 60 to 70 mmHg), causing corneal edema. The AC is filled with small, circulating, tan colored cells. If fresh RBCs exist, two or more different layers of cells can be seen, with the lighter khaki-colored layer of ghost cells appearing on top of a heavier, fresh RBC layer, imparting a candy-striped appearance. The cellular reaction may appear out of proportion to the aqueous flare. Conjunctival reaction tends to be minimal unless the IOP is markedly elevated or there is a history of previous surgery. Gonioscopy reveals either 1) a normal appearing open angle, 2) an open angle covered by a fine layer of khaki-colored cells, which may have discolored the trabecular meshwork, or 3) a pseudohypopyon layer of cells filling the angle, most often inferiorly. The vitreous has the appearance of an old hemorrhage, with characteristic khaki coloration of RBCs and clumps of extracellular pigmentation from degenerated hemoglobin.
Symptoms
The symptoms relate to the etiology. Pain secondary to trauma or surgery may be experienced by the patient. However, less pain is often reported than expected from a severely elevated IOP. Patients with high IOP may also present with blurry vision, headache, brow ache, nausea and/or vomiting.
Diagnostic Procedures
The diagnosis of ghost cell glaucoma is usually clinical. Patient history and paracentesis with phase-contrast microscopy of the aspirate would confirm this diagnosis.
Laboratory Tests
For spontaneous hemorrhages, complete blood count with coagulation profile, and sickle cell prep in African American patients are warranted.
Differential Diagnosis
Differential Diagnosis of Ghost cell glaucoma includes Angle Recession glaucoma, Hemolytic glaucoma, Hemosiderotic glaucoma, Uveitic glaucoma, Neovascular glaucoma and Acute angle closure (Mechanical secondary to trauma).
Hemosiderotic Glaucoma is a late onset glaucoma following intraocular hemorrhage secondary to iron deposition resulting in damage to the trabecular meshwork. This extremely rare glaucoma is more chronic, does not have ghost cells in the AC, and is characteristically associated with a slight discoloration of the trabecular meshwork. It most often occurs many years after the original injury, in contrast to ghost cell glaucoma, which occurs within weeks to months after the original injury.
Hemolytic Glaucoma is a type of secondary glaucoma where the trabecular meshwork is obstructed by macrophages containing RBC debris and hemosiderin after vitreous hemorrhage. In ghost cell glaucoma, little to no RBC debris and few to no macrophages are found in the trabecular meshwork.
Neovascular glaucoma is differentiated from ghost cell glaucoma by the absence of ghost cells within the AC and the presence of neovascularization at the papillary margin and in the angle. The angle may also be closed if the neovascularization is long-standing.
A history of vitreous hemorrhage, disruption of the hyaloid face, a multitude of tiny khaki-colored cells in the AC, a quiet conjunctiva, and an absence of keratic precipitates differentiates ghost cell glaucoma from uveitis and endophthalmitis.
Management
Ghost cell glaucoma usually resolves once the vitreous hemorrhage has cleared. Medical therapy with aqueous suppressants is the preferred initial approach. Surgical intervention is often necessary because of persistently elevated intraocular pressure despite maximum medical therapy.
Medical Therapy
Aqueous suppressants are the first line approach. Monotherapy or a combination of topical alpha adrenergic agonists, beta adrenergic blockers, parasympathomimetics, prostaglandin analogues, and carbonic anhydrase inhibitors may be used. An oral carbonic anhydrase inhibitor may be added. Intravenous Mannitol or Diamox may be used for extremely high IOPs in acute settings. This may occur once cells re-accumulate in the trabecular meshwork after initial clearing.
Surgery
Surgery might be required to remove the ghost cells from the AC and decrease the stress on the trabecular meshwork. This can be accomplished by AC paracentesis and irrigation, pars plana vitrectomy (PPV), and/or a trabeculectomy.
If AC washout successfully lowers the intraocular pressure but the pressure rises again secondary to reaccumulation of ghost cells originating in the vitreous, a washout of the AC can be repeated. If this relatively simple and safe procedure is unsuccessful, vitrectomy to remove the contents of the vitreous cavity may be required.
For refractory glaucoma caused by chronic obstruction of trabecular meshwork by ghost cells, trabeculectomy or usage of glaucoma drainage device is warranted. Certain MIGS can also be considered.
Complications
Uncontrolled IOP may lead to optic nerve damage. Ghost cell glaucoma usually resolves once the hemorrhage clears.
Prognosis
Prognosis of ghost cell glaucoma is excellent as the condition is typically transient, although it may last many months before fully resolving. Once the supply of ghost cells in the vitreous cavity is exhausted ghost cells cease to re-accumulate in the AC. If hemorrhage is large and ghost cells remain, surgical treatment options are available as discussed above.
Additional Resources
- Boyd K, McKinney JK. Glaucoma. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/glaucoma-list. Accessed March 13, 2019.
References
- Albert DM & Miller JW. Principles and Practice of Ophthalmology. Third Edition. Philadelphia, PA. W.B Saunders Company © 2000 and Elsevier, Inc © 2008.
- Campbell DG, Simmons RJ, & Grant WM. Ghost cells as a cause of glaucoma. Am J Ophthalmol. 1976; 81:441-440.
- Cioffi GA, Durcan FJ, Girkin CA, Gross RL, Netland PA, Samples JR, Samuelson TW, O’Connell SS & Barton K. Glaucoma. Last major revision 2008-2009. San Francisco, CA. American Academy of Ophthalmology. Copyright 2010.
- Girkin CA, McGwin G, Cherie L, Robert M & Ferenc K. Glaucoma after ocular contusion: A cohort study of the United States eye injury registry. Journal of Glaucoma. 2005; 14(6): 470-473.
- Ritch R, Shields MB & Krupin T. The Glaucomas. Volume 2. St. Louis, MI. C.V. Mosby Company. © 1989.
- Rojas L, Ortiz G, Gutierrez M & Corredor, S. Ghost Cell Glaucoma Related to Snake Poisoning. Arch Ophthalmol. 2001; 119 (8): 1212-1213.
- Shetlar DJ, Chevez-Barrios P, Dubovy S, Rosa RH, Syed N, Wilson MW, Pelton RW & Pe’er J. Ophthalmic Pathology and Intraocular Tumors. Last major revision 2007-2008. San Francisco, CA. American Academy of Ophthalmology. Copyright 2010.
- Spraul CW & Grossniklaus HE. Vitreous Hemorrhage. Surv Ophthalmol. 1997; 42(1): 3-39.
- Mansour AM, Chess J, Starita R. Nontraumatic ghost cell glaucoma- a case report. Ophthalmic Surg. 1986;17:34-36.