Traumatic Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Definition
Traumatic glaucoma is a type of secondary glaucoma that develops following blunt or penetrating ocular trauma. This subtype of glaucoma usually entails several mechanisms that interplay to produce increased intraocular pressure (IOP) and it is important to consider all the pathophysiological mechanisms leading to increased IOP as they influence management of patients. Furthermore, it is important to recognize traumatic glaucoma given the significant number of patients affected every year as discussed in the incidence and prevalence sections below.
Incidence and prevalence
The risk of developing glaucoma after penetrating injury is estimated to be 2.67%, while it is 3.39% after blunt trauma according to latest studies in the U.S.[1]. This constitutes a significant number of patients who are at risk of developing vision threatening glaucoma after trauma to the eye since the incidence of ocular trauma is around 2.5 million patients every year in the U.S.[2]. Traumatic injuries are particularly prevalent among certain age groups like the elderly with falls leading to globe injury and younger individuals with injuries related to sports or assault.
Classification
Glaucoma after trauma can be classified into two broad categories; occurring after blunt or after penetrating trauma. Glaucoma occurring after blunt trauma can be further classified into early onset and late onset glaucoma[3]. Other entities of post traumatic glaucoma include post chemical, thermal, electric and radiation injury[3].
Etiology and pathophysiology
There are many etiologies for increased IOP in traumatic glaucoma due to the pathophysiology and healing responses that occur after injury to the eye. Therefore, the pathophysiology of increased IOP will be discussed separately for each entity of post traumatic glaucoma taking the above mentioned classification as a framework. It is important to keep in mind though that several of these mechanisms might be present at the same time thus contributing to the increased IOP.
Traumatic Glaucoma secondary to Blunt trauma
Early onset Glaucoma pathophysiology
1)Trabecular meshwork disruption:
The trabecular meshwork can get mechanically disrupted following globe trauma. On gonioscopy, superficial tears may be visualized and often manifest as trabecular flaps that are hinged at the level of the scleral spur[2]. Full thickness tears might be seen on gonioscopy as torn iris processes or blood in the angle or in Schlemm's canal[2]. These changes may not lead to an immediate and prolonged increase in IOP and may eventually resolve over time. However, the healing process leading to scarring from ciliary muscle disruption and angle injury along with trabecular meshwork tears might lead to late onset rise in IOP due to damage to the outflow apparatus and decreased draining of aqueous humor. Moreover, in certain injuries, a decrease in IOP might be observed due to the disruption of the ciliary body attachment to the scleral spur leading to a ciliary body cleft. Careful gonioscopy is essential for the diagnosis of angle recession and ciliary body clefts.
2) Hyphema:
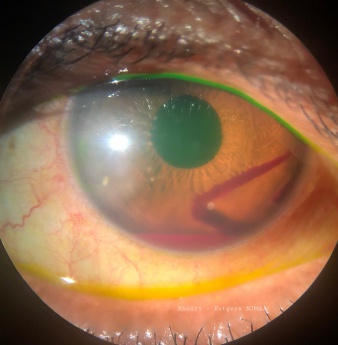
The source of bleeding is proposed to occur through two main mechanisms. First, the initial trauma to the globe leads to anteroposterior compression of the globe with simultaneous equatorial expansion leading to tearing of the blood vessels of the ciliary body and stromal iris[2]. Another mechanism is the sudden rise of IOP leading to damage of the fragile blood vessels of the iris/ciliary body[3]. The accumulation of blood in the anterior chamber (Figure 1) leads to obstruction of the trabecular meshwork with erythrocytes, fibrin, platelets and other inflammatory debris (by products of fibrinolysis) and this might later lead to prolonged increase in the IOP[3]. In cases where the patient is a carrier of sickle cell trait, even small amounts of RBCs in the anterior chamber might lead to significant increase in IOP since sickle cells tend to deform and block trabecular meshwork to a greater extent than normal healthy RBCs[1]. Obtaining a sickle cell test is paramount to diagnosis and proper treatment particularly in avoiding medications that may induce further sickle crisis like oral carbonic anhydrase inhibitors and in ensuring patients remain properly hydrated.
3)Traumatic Iritis:
Blunt trauma might lead to inflammation of the iris leading to compromise of the blood aqueous barrier and leakage of proteins and white blood cells that may then increase resistance at the trabecular meshwork leading to a rise in IOP. Trauma also might lead to direct inflammation of the trabecular meshwork (trabeculitis) leading to decrease in aqueous outflow[2].
4)Choroidal hemorrhage:
This is a cause of acute increase in IOP following severe eye trauma[1] or in patients who are on anticoagulation. The mechanism of increased IOP is related to the mass effect in the posterior segment leading to forward displacement of the lens-iris diaphragm by the choroidal hemorrhage leading to angle closure glaucoma[4].
Late onset Glaucoma pathophysiology
1)Angle-Recession:
Angle recession is defined as a tear that occurs between the longitudinal and circular layers of the ciliary muscles leading to a widened ciliary body band that can be seen on gonioscopy[5]. It is postulated that this damage in the ciliary muscle signifies a more prominent damage in the trabecular meshwork[1]. Such damage in the trabecular meshwork leads to scarring and decrease in the outflow of aqueous humor leading to increase IOP over time. Angle recession of greater than 180° leads to an increased risk of glaucoma [5].
2)Ghost cell Glaucoma:
This form of secondary glaucoma occurs following trauma causing bleeding in the vitreous cavity[1]. One to three weeks after the initial trauma, the red blood cells change their shape and consistency becoming spherical, rigid and containing Heinz bodies (degenerated hemoglobin)[1]. Those rigid cells are called ghost cells and being rigid and non flexible, these cells clog the trabecular meshwork leading to increased IOP[6]. The ghost cells reach the anterior chamber through a disrupted anterior hyaloid face after trauma[6]. Ghost cells do not form, however, in the case of anterior chamber bleeding due to the the high oxygen pressure in the anterior chamber and the rapid circulation[6].
3)Hemolytic Glaucoma (Rare):
This form of glaucoma is due to the hemolysis of red blood cells from bleeding in the vitreous body. The released debris, free hemoglobin and macrophages laden with hemoglobin obstruct the trabecular meshwork[1]. The difference from ghost cell glaucoma is in the absence of ghost cells in the trabecular meshwork (on careful slit lamp examination or on histopathologic evaluation of anterior chamber aspirate which is mostly of academic interest and not performed clinically).
4)Hemosiderotic Glaucoma (Very Rare):
This form of glaucoma is caused by the toxic effects of iron on the cell of the trabecular meshwork. The source of the iron is either longstanding bleeding in the vitreous body or a retained foreign body containing iron[1].
5)Lens dislocation/subluxation:
Globe trauma may lead to the anterior dislocation of the lens leading to pupillary block and acute angle closure glaucoma[2]. In some cases, the lens might dislocate posteriorly leading to vitreous prolapse into the anterior chamber and pupil block glaucoma[2]. According to Masaru et al., most cases of glaucoma secondary to lens subluxation are of the open angle type[7]. Postulated mechanisms for open angle glaucoma secondary to traumatic subluxation are; intermittent closure of the angle by the dislocated lens, intraocular inflammation associated with uveitis secondary to the dislocated lens, occult phacolytic glaucoma (described below) or obstruction of the trabecular meshwork by vitreous displaced in the anterior chamber[7].
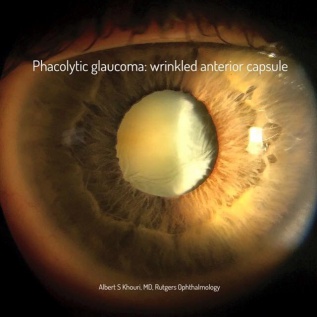
6)Phacolytic Glaucoma:
In the trauma setting, a traumatic mature cataract (but more commonly a mature cataract without history of trauma) may lead to leakage of proteins through an intact lens capsule causing glaucoma (Figure 2) due to the trabecular meshwork being overwhelmed with inflammatory cells and proteinaceous debris[8]. Those proteins lead to an intense inflammatory response in the trabecular meshwork in response to their high molecular weight nature leading to blockage of the meshwork with inflammatory cells and leaked proteins[8].
Traumatic Glaucoma secondary to Penetrating injury
Penetrating injuries will lead to glaucoma through many mechanisms including ones that are already discussed in the blunt trauma section. Those mechanisms include presence of a hyphema, lens dislocation, phacolytic or ghost cell glaucoma[2].
Other mechanisms include;
- Injury to the cornea leading to a shallow anterior chamber which in the setting of significant inflammation might lead to extensive anterior synechiae producing secondary angle closure glaucoma[2].
- Posterior synechiae can also occur with seclusion of the pupil and iris bombe leading to secondary angle closure glaucoma[2].
- Injury to the lens with disruption of the lens capsule can lead to severe inflammation due to liberation of lens material within the globe. Lens particles can also mechanically obstruct the trabecular meshwork.
- Hydrated lens with an enlarged anteroposterior dimension can lead to anterior iris bowing and angle closure.
- Penetrating trauma might create a conduit for the epithelial cells to get into the anterior chamber leading to epithelial down growth in the anterior chamber and obstruction of the meshwork by epithelial sheets of cells[1]. Anterior synechiae might also result from contracting epithelial cells leading to angle closure glaucoma[1].
- Retained foreign bodies containing iron might produce siderotic glaucoma due to the toxic effect of iron on cells of the trabecular meshwork[1]. As is evident from the above, depending on the nature and extent of the penetrating injury, the mechanisms leading to glaucoma can be multiple, complex and inter-related.
Diagnostic Workup
History
Obtaining an elaborate history is important to identify the setting and nature (blunt vs penetrating), the severity and possibility of an intraocular foreign body (e.g., high speed projectiles like shrapnel, glass, bullets or balls[9]), the timeline for developing increased IOP (e.g., delayed with angle recession and acute with hyphema or lens fragment), the setting of the injury (school, work, etc.[9]) and past ocular history (e.g., patients with history of cataract or pre-existing glaucoma)[9]. All of this will help guide the physical exam and identify the mechanisms of increased IOP and hence will help directing the management.
Physical Exam
- Visual Acuity assessment
- Visual inspection of the globe and adnexa for signs of retained foreign bodies.
- Slit lamp exam:
- Conjunctiva: Apparent or occult lacerations (self sealed) or hemorrhagic chemosis which may hide deeper penetrating lacerations or a foreign body.
- Cornea: Laceration or entry of a foreign body, self sealed laceration with high velocity projectiles or corneal blood staining signifying a possibility of long standing bleeding and increased IOP.
- Anterior chamber: Presence of hyphema, inflammatory cells and fibrin, debris (in iritis), shallow anterior chamber (in case of angle closure glaucoma), reddish - brown RBCs (in hemolytic glaucoma)[2]and anterior synechiae or peripheral synechiae. Careful examination for foreign material (lashes, glass, other foreign bodies or lens particles in case of lens damage).
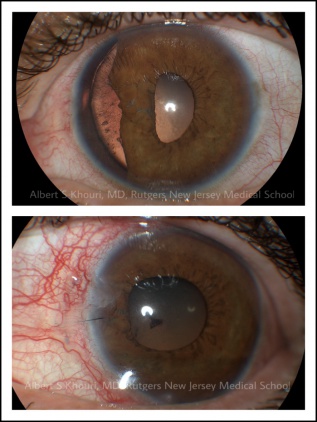
- Iris: Iridodialysis (in case of angle recession) (Figure 3), sphincter tears or iris transillumination defects (with foreign body suspicion).
- Lens: Lens subluxation, phacodonesis (in lens dislocation)[1], lens opacification (localized or diffuse), damage to lens capsule, pigment or blood within the lens or on the lens surface or vitreous prolapse around zonular damage among others.
- Vitreous: Prolapse, pigment (tobacco dust denoting posterior segment RD), foreign material, blood or reduced red reflex and retrolenticular mass (in choroidal hemorrhage). If view to the fundus is precluded, then a CT scan is indicated. Once globe penetration is excluded a B scan ultrasound is indicated. Magnetic Resonance Imaging is clearly contraindicated if a metallic foreign body is suspected.
- Gonioscopy: Careful Gonioscopy should be performed once globe penetration is ruled out in order to examine the angle for traumatic injury (blood, recession, cleft) and to rule out a foreign body (if suspected) that may be lodged in the angle and not visible on slit lamp examination.
- Gonioscopic signs of injury include:
- Iris root displacement posteriorly and a widened ciliary body face (in angle resection)[1].
- White- gray membrane covering the angle (also in angle resection)[1].
- Cyclodialysis cleft[2] (disinsertion of the ciliary body from the sclera leading to aqueous flow to the suprachoroidal space and often associated with hypotony).
- Trabecular meshwork flaps that are hinged at the level of the scleral spur (in superficial tears of trabecular meshwork disruption) [2].
- Torn iris processes with blood in the angle (in angle recession)[2].
- Gonioscopic signs of injury include:
Ocular imaging
- As mentioned earlier, Ultrasonography (B-scan is a 2 dimensional ultrasound scan. A-scan is a single beam of ultrasound that is meant to better identify the acoustic nature of the imaged ocular tissues. A-scan is useful if a suspected intraocular foreign body exists as hard matter like glass or metal will display a higher signal intensity versus blood clots or ocular tissues, for example) for posterior segment imaging.
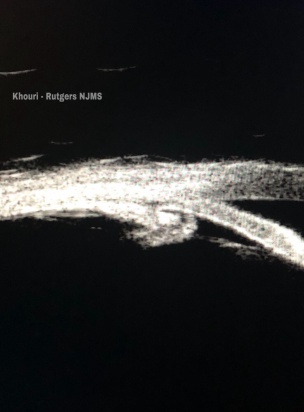
- Ultrasound Biomicroscopy (UBM: provides ultrasound examination of the anterior segment including the ciliary body and can be useful in the identification of a ciliary body cleft) (Figure 4).
- Anterior segment OCT, CT and MRI (MRI can only be performed once a magnetic FB is ruled out).
- All those modalities are used to provide useful information when physical exam and gonioscopy are non conclusive, when physical exam is not possible or when ocular structures are masked for example by hyphema or vitreous hemorrhage[2].
Diagnostic Taps
Might be used only when the clinical picture is not conclusive for the mechanism of glaucoma. This might be useful when a histopathologic diagnosis is imperative for the management[2].
Management
The main stay of glaucoma treatment is the reduction of IOP since elevated IOP remains the main risk factor for glaucomatous optic neuropathy. The treatment typically is through a stepwise approach where topical medications are instituted first. Depending on the severity of the IOP elevation and the degree of optic neuropathy, first line agents are instituted and then adjunctive agents are added until IOP is controlled (topical beta blockers, topical carbonic anhydrase inhibitors, and alpha agonists). Systemic glaucoma medications can be used to further control IOP like carbonic anhydrase inhibitors (orally or intravenously once sickle cell disease is ruled out) and hyperosmotic agents like mannitol (also caution needs to be exercise to avoid dehydration in elderly patients or patients with heart disease or sickle cell disease among others). Surgical interventions are needed when the disease is uncontrolled on medical therapy. Surgical management includes mainly tube shunting among other interventional methods. Tube shunting was found to have good outcomes in controlling IOP in post traumatic glaucoma that is not controlled with medical therapy[10]. Management of post traumatic glaucoma is aimed mainly at the main underlying mechanism leading to increased IOP. Therefore, management will be discussed within the framework of pathophysiological mechanism discussed above.
Traumatic Glaucoma secondary to Blunt trauma
Early onset Glaucoma management
1)Trabecular meshwork disruption:
This type of injury is a common cause of early increase in IOP in cases of blunt trauma[2].The management is typically limited to medical management to counteract the initial increase in IOP[2].However, it is important to discuss with patients that if healing leads to angle recession then incremental medical therapy is indicated and incisional surgery might be required to resolve the long term increase in IOP[2].
2)Hyphema:
The management of hyphema should be directed with the goal of preventing re-bleeding which will increase the chances of complications caused by hyphema like secondary glaucoma[3](refer to eye wiki article on hyphema for detailed information about the management of hyphema). In the case of the development of glaucoma secondary to hyphema, the initial management should be directed at lowering IOP using medical therapy. Topical therapies like carbonic anhydrase inhibitors, alpha agonists and Beta-blockers or combination of these drugs are the first line therapy[2]. Prostaglandin analogs and miotic agents should be avoided since they may enhance the acute inflammatory process causing possibly more damage to the ocular structures[1]. Oral therapies like acetazolamide and methazolamide are used if topical therapy is not enough to control the IOP[11] (and after sickle disease is ruled out). Moreover, I.V Mannitol might also be used in the acute setting in patients where IOP is not controlled on topical therapy in symptomatic patients or in patients with pre-existing optic nerve damage that are at higher risk for loss of vision[11].
However, as mentioned earlier in patient with sickle cell disease acetazolamide and mannitol are avoided due to the risk of increased sickling caused by decreased blood pH (metabolic acidosis) or volume depletion[11].
Surgical intervention generally should be considered in the following cases[1][2][11];
- Resistant IOP despite maximum medical therapy.
- Persistent 100 % hyphema (also known as eight ball hyphema).
- When hyphema is persistent for a period of days without signs of improvement: example more than 50 % for 10-14 days (According to some guidelines).
- Eyes manifesting early corneal blood staining.
- Eyes in which there is a detection of active rebleeding.
- Sickle cell anemia or trait.
Among the surgical options, the most intuitive is an anterior chamber washout to evacuate the blood[1]. Trabeculectomy may also be used to normalize pressure[1] but tube shunt surgery remains the main stay surgical treatment[10].
3)Traumatic Iritis:
The increased pressure is usually reversible and responds effectively to anti-inflammatory topical agents like steroids or NSAIDS[2]. Moreover, cycloplegics are used to decrease pain caused by the spasm of the ciliary body[2]. Topical glaucoma therapy is used to control IOP.
4)Choroidal hemorrhage:
The initial management should be directed at lowering the IOP using topical agents, oral carbonic anhydrase and systemic hyperosmotic agents as needed[1]. Cycloplegics will help in deepening the anterior chamber while steroids help reducing the inflammation and stabilize compromised choroidal vasculature[1]. Surgical drainage of the clot is indicated in case of persistent angle closure glaucoma with high IOP, large choroidals with retina apposition (“kissing choroidals”) or lens-cornea apposition[1].
Late onset Glaucoma management
1)Angle-Recession:
Angle recession glaucoma usually present with late onset increase in IOP caused by fibrosis, degeneration and atrophy of the trabecular meshwork and the canal of Schlemn[2]. Therefore, it presents as a secondary chronic open angle glaucoma[1]. Medical therapy might be beneficial in lowering IOP, particularly if the recession is limited and did not involve the majority of the angle. Incisional surgeries including trabeculectomy with anti-metabolite and tube shunt surgery can be performed to control the IOP. Cyclodestructive procedures like diode laser cyclophotocoagulation (continuous wave or micropulse cyclophotocoagulation) are beneficial in refractory cases[2].
2)Ghost cell Glaucoma:
This mechanism is usually self limited and thus initial management revolves around the use of standard medical therapy to lower the IOP[6]. If the medical therapy fails to lower the IOP and in the case of the presence of a dense vitreous hemorrhage a pars plana vitrectomy is performed to remove the reservoir of ghost cells[6].
3)Lens dislocation/subluxation/phacolytic glaucoma:
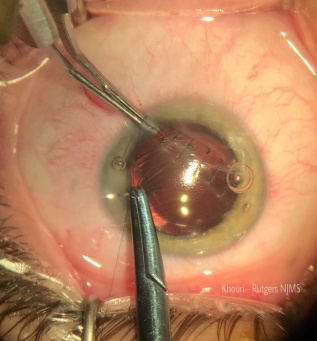
Initial management revolves around the relief of pupillary block caused by the lens through laser or surgical iridotomy[1]. In addition to medical therapy, lensectomy is indicated in cases of pupillary block or angle closure and when the lens is cataractous or hyper-mature[7]. (Figure5)
Traumatic Glaucoma secondary to Penetrating injury
It is important to consider that preventive management is of importance in the case of glaucoma secondary to penetrating injury[12]. Hence, the use of Viscoelastic material is recommended during the repair of open globe injury in order to preserve the structure of the anterior chamber and lower risks of anterior synechiae[12]. Furthermore, anti-inflammatory therapy with topical steroids is essential in order to reduce the iritis and lower the risks of anterior and posterior synechiae formation[12]. The management guidelines are similar to the ones discussed above in the blunt trauma section. Medical therapy is the mainstay in the acute setting unless the lens is the culprit for elevated IOP in which case a lensectomy may be indicated (pupil block, lens subluxation or lens particle among others).
Surgical management involves prompt closure of the globe to maintain a water sealed laceration and lower risks of infection (Videos 1 and 2). A high index of suspicion is essential to identify intraocular foreign bodies that need to be removed to prevent infection and degeneration due to oxidation (for example, hemosiderosis). The intraocular pressure is then monitored and managed often over the course of weeks to months to years after the injury with a paradigm that typically relies on the aforementioned medical then surgical interventions.
Video1.(Videos are courtesy of Albert S. Khouri, M.D, Rutgers NJMS Ophthalmology Department).
Video2.(Videos are courtesy of Albert S. Khouri, M.D, Rutgers NJMS Ophthalmology Department).
In the case of epithelial down growth, which is often identified weeks to months after the initial injury, treatment is challenging and the prognosis is usually guarded[1]. The surgical intervention aims to excise and remove the epithelial membrane and is often performed in conjunction with adjunctive cryotherapy[2]. In any case with a retained metallic foreign body within the globe, emergency surgery for removal of the foreign body should be done to prevent further complications[13](Figure 6).
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 Yanoff, Myron, MD, Duker, Jay S., MD. Ophthalmology, Fifth Edition.Philadelphia, Pennsylvania: Elsevier Inc; 2019:1089-1094.clinicalkey-com.proxy.libraries.rutgers.edu.web.25 July 2019.https://www-clinicalkeycom.proxy.libraries.rutgers.edu/#!/content/book/3-s2.0-B9780323528191003601.
- ↑ Jump up to: 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 Tarek M Shaarawy PD MD MSc,Mark B Sherwood FRCP FRCS FRCOphth, Roger A Hitchings FRCS FRCOphthand Jonathan G Crowston PhD FRCOphth FRANZCO. Glaucoma, second Edition. Philadelphia, Pennsylvania: Elsevier Inc; 2015:446-456. clinicalkey-com.proxy.libraries.rutgers.edu.web.25 July 2019.https://www-clinicalkey-com.proxy.libraries.rutgers.edu/#!/content/book/3-s2.0-B9780702051937001308.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Brandt MT, Haug RH. Traumatic hyphema: a comprehensive review. J Oral Maxillofac Surg 2001; 59:1462.
- ↑ YANG, SAM S. MD; FU, ARTHUR D. MD; McDONALD, H. RICHARD MD; JOHNSON, ROBERT N. MD; AI, EVERETT MD and JUMPER, J. MICHAEL MD. Massive Spontaneous Choroidal Hemorrhage.Retina.23(2):139-144, April 2003.
- ↑ Jump up to: 5.0 5.1 Ng, D.SC., Ching, R.HY. & Chan, C.WN. Int Ophthalmol (2015) 35: 107. https://doi.org/10.1007/s10792-014-0027-5
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Alamri A, Alkatan H, Aljadaan I. Traumatic Ghost Cell Glaucoma with Successful Resolution of Corneal Blood Staining Following Pars Plana Vitrectomy. Middle East Afr J Ophthalmol. 2016;23(3):271–273. doi:10.4103/0974-9233.180778
- ↑ Jump up to: 7.0 7.1 7.2 Masaru Inatani, MD, Hidenobu Tanihara, MD, Megumi Honjo, MD, Noriaki Kido, MD, Yoshihito Honda, MD. Secondary glaucoma associated with crystalline lens subluxation.Journal of Cataract & Refractive Surgery. Volume 26, Issue 10, October 2000, Pages 1533-1536.www.siencedirect-com.proxy.libraries.rutgers.edu.web.10 August 2019.https://www-sciencedirectcom.proxy.libraries.rutgers.edu/science/article/pii/S0886335000004715?via%3Dihub.
- ↑ Jump up to: 8.0 8.1 Dhingra, D., Grover, S., Kapatia, G., Pandav, S. S., & Kaushik, S. (2019). Phacolytic glaucoma: A nearly forgotten entity. European Journal of Ophthalmology. https://doi.org/10.1177/1120672119841972
- ↑ Jump up to: 9.0 9.1 9.2 Gardiner, Matthew F., Susan B. Torrey MD, and Jonathan Trobe MD. "Approach to eye injuries in the emergency department." UpToDate. 26 July 2019 <https://www.uptodate.com/contents/approach-to-eye-injuries-in-the-emergency department? source=history_widget>.
- ↑ Jump up to: 10.0 10.1 Yadgarov A, Liu D, Crane ES, Khouri AS. Surgical Outcomes of Ahmed or Baerveldt Tube Shunt Implantation for medically Uncontrolled Traumatic Glaucoma. J Curr Glaucoma Pract 2017;11(1):16-21.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 Christopher M Andreoli, MDMatthew F Gardiner, MD.Traumatic hyphema: Management.UpToDate. 10 August 2019. https://www.uptodate.com/contents/traumatic-hyphema-management#H714240958.
- ↑ Jump up to: 12.0 12.1 12.2 Osman, Essam A. “Glaucoma after open globe injury.” Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society vol. 29,3 (2015): 222-4. doi:10.1016/j.sjopt.2014.10.006.
- ↑ Bloom WR, Ramsey JK, Ohr MP. Ocular Siderosis Secondary to Retained Intraocular Foreign Body: A Case Report. Cureus. 2019;11(5): e4660. Published 2019 May 14. doi:10.7759/cureus.4660.