Saethre-Chotzen Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease entity
Saethre-Chotzen syndrome. ICD-10: Q87.0.
Disease
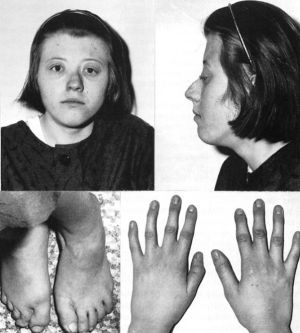
Also known as acrocephalosyndactyly type III, Saethre-Chotzen syndrome (SCS) is characterized by craniofacial abnormalities in conjunction with neurological, skeletal, and cardiac defects. Although the specific phenotypic categorization of SCS remains unclear, common ocular manifestations include eyelid ptosis (up to 90%) and strabismus (over 50%).[1] More variable ophthalmological considerations include hypertelorism, refractive error (usually myopia/astigmatism), optic atrophy, and lacrimal duct stenosis.[2] Other reported ophthalmic features include downward slanting palpebral fissures, epicanthal folds, and blepharophimosis.[3]
Non-ophthalmic characteristics that are typically observed in the condition include coronal craniosynostosis, brachycephaly (74%), soft tissue syndactyly, prominent ear crus (56%), broad depressed nasal bridge (65%), facial asymmetry, high forehead (56%), and maxillary hypoplasia.[3] [4] Other less common findings include low-set frontal hairline, digital webbing, diminished hearing, and highly arched or cleft palate with dental anomalies.[4][5] [6]
While intelligence in these patients is often normal, there are reports of mild-moderate mental retardation, seizures, and schizophrenia.[7] Mental retardation occurs less frequently in SCS patients with a TWIST1 mutation as compared to patients with a TWIST1 deletion. [3]
Etiopathogenesis
SCS is inherited in an autosomal dominant pattern. Based on murine and human genetic analyses, the phenotypic variation in SCS has been attributed to mutations in the TWIST1 gene. Over 100 TWIST1 mutations have been identified. The significant variability in mutation may reflect the variation in phenotype.[2] Although the specific mutation e.g., missense, point, etc., may differ, it is likely that ensuing haploinsufficiency is the underlying etiological mechanism.[5][8] TWIST1 is speculated to play an essential role in the formation of the head mesenchyme and as such, qualitative or quantitative impairment of the protein product leads to the observed craniosynostosis.[9]
Strabismus: Strabismus has been noted in over 50% of individuals with SCS.[1][7] See Craniosynostosis Syndromes for further discussion of the underlying mechanism for strabismus. Most patients specifically present with V-pattern strabismus with exotropia larger in upgaze.[10]
Vertical strabismus is another commonly noted abnormality with Jadico, et. al., observing it in approximately 60% of their patients with SCS.[10]
Ptosis is commonly reported in patients, although the exact prevalence has varied in studies, ranging from 59% to 82% of patients.[11][12] Ptosis often results from a defective functioning or agenesis of the levator palpebrae muscle. [3]
Other ocular manifestations seen in craniosynostosis, including the etiologies of increased intracranial pressure, exposure keratopathy, amblyopia, and astigmatism, are described in Craniosynostosis Syndromes.
Epidemiology
The prevalence of SCS is estimated to range from 1 in 25,000 to 1 in 50,000 live births, although this is probably underestimated due to extensive phenotypic variability.[4][11] Occurrence is equal in males and females.[13] Given that this is an autosomal dominant condition, many individuals have an affected parent. However, although rarer, de novo mutations have also been observed.[14]
Diagnosis
Diagnosis of SCS begins with history and physical examination. However, there is no single pathognomonic feature associated with the syndrome, and it is challenging to diagnose based on clinical features alone. Genetic testing is important to confirm the diagnosis.[2]
History
Classically, patients will present with typical clinical findings such as craniosynostosis. Given its autosomal dominant inheritance, patients may have a familial history of related, albeit perhaps less severe, clinical findings such as abnormal skull shape. However, the family history may appear unremarkable due to the wide phenotypic variability.[15] The diagnosis of several types of malignancies including Hodgkins disease, testicular carcinoma, and nasopharyngeal carcinoma were reported in several family members of patients diagnosed with SCS.[7]
Physical examination
Physical examination is an integral component of the diagnosis of SCS.
Eyes: As discussed above, commonly observed aberrations include strabismus, ptosis, amblyopia, and lacrimal duct stenosis.
Sensorimotor examination should be performed with strabismus measurements in the cardinal positions, specifically looking for V-pattern strabismus with exotropia larger in upgaze than in downgaze. The V-pattern is often accompanied by over-elevation in adduction (pseudo-inferior oblique overaction), with possible superior oblique underaction occurring ipsilateral to the fused coronal suture.[16]
In contrast to other craniofacial abnormalities, severe midfacial hypoplasia is less commonly noted in SCS, and so proptosis is less common. Instead, ptosis is more prevalent.[7] Ophthalmic examination should include measurements of eyelid position and function such MRD1 and levator function.
Other observed ocular characteristics include astigmatism, amblyopia, myopia/hyperopia, monocular elevation deficit, rotary nystagmus, extraocular muscle agenesis, and lower lid entropion.[10] Finally, one significant consideration in patients with SCS is an increased probability of optic nerve damage due to a high risk of intracranial hypertension.[5]
Ear: SCS characteristically presents with small, low-set ears that are posteriorly rotated and have prominent crura.[9] Conductive, mixed, and sensorineural hearing deficits may also be present.[17]
Face: Dysmorphic facial findings are a major feature of SCS with typical abnormalities that include facial asymmetry, hypertelorism, and maxillary hypoplasia. Less frequently observed characteristics are a high forehead, low-set frontal hairline, and deviated nasal septum.[4][9]
Musculoskeletal: Commonly noted defects in the skull include craniosynostosis and dilated parietal foramina. Potential limb abnormalities include brachydactyly, cutaneous syndactyly, particularly of the second interdigital space, hallux valgus with bifid distal phalanx, and triangular-shaped epiphyses of the hallux.[4] [5] [9] [18]
Other systems: Orthodontic, cardiac, and neurological abnormalities have also been documented in patients with SCS.[5][19] [20]
Diagnostic procedures
Diagnosis of SCS is primarily clinical, although diagnostic tests are useful for confirmation. Given that most patients have mutations in the TWIST1 gene, genetic evaluations are integral.[21] Even if the ophthalmologist, or clinician, does not already suspect SCS, patients with craniosynostosis and syndactyly or clinodactyly need to be provided a complete genetic workup. Where SCS is presumed despite normal molecular testing and clinically inconsistent findings, karyotyping should be a consideration.[22] [23]
Ultrasound may be utilized as early as the 19th week to assess for the presence of diagnostic markers such as an irregularly shaped fetal skull, which may be indicative of coronal synostosis. However, given the phenotypic variability of the condition, ultrasound cannot provide a definitive diagnosis. If SCS is suspected prenatally, either due to familial history or suspicious sonographic findings, genetic testing should be offered to families.[24]
The complex anatomy of patients with SCS can make strabismus difficult to treat.[1] Orbital imaging can be useful for guiding the surgical plan through visualization of the extraocular muscles. MRI using T2 fast spin echo sequences is the recommended imaging modality. Direct coronal images are preferred.[25] MRI of the brain can also be performed for additional evaluation when increased intracranial pressure is a concern.
Differential diagnosis
Muenke syndrome: Patients may present with features clinically similar to SCS. However, some work has been done to differentiate between the two, with patients with Muenke syndrome having a higher incidence of intellectual disabilities and patients with SCS having a higher incidence of intracranial hypertension and clinically significant ptosis.[5] Moreover, patients with SCS may have more ophthalmic abnormalities such as strabismus, ptosis, astigmatism, and amblyopia. [10]
Isolated unilateral coronal synostosis: Patients who have this abnormality may develop facial asymmetry if not treated.
Baller-Gerold syndrome: Individuals with this condition have bilateral craniosynostosis with brachycephaly. However, they typically additionally present with proptosis and poikiloderma, which are uncommon in SCS.[15]
Apert syndrome: A highly arched palate, cleft palate, and dental anomalies have been reported both in patients with Apert syndrome and SCS.[2] However, the severe midfacial hypoplasia that is common in Apert, Crouzon, and Pfeiffer syndromes is less common in SCS.[7]
Management and treatment
Given the variety of systems implicated in SCS, a multidisciplinary approach is required for the appropriate management and treatment of this condition. Pediatricians, ophthalmologists, otolaryngologists, and orthopedists should be consulted for comprehensive evaluation and development of a treatment plan for these patients.
Immediate post-natal care
Post-natal care is centered around immediate treatment of airway-related issues, if applicable. Nutrition may also be of concern, particularly if facial abnormalities prevent requisite oral intake.
Ophthalmological considerations
Because ophthalmological issues are common within this patient population, routine comprehensive evaluations with an ophthalmologist are indicated. Examination is recommended at the time of diagnosis, before and after craniofacial surgery, biannually until 7-9 years of age, and yearly through adolescence. These examinations should assess for strabismus, amblyopia, refractive error, keratopathy, optic neuropathy, nasolacrimal outflow abnormalities, papilledema, and ptosis. Dilated fundoscopic examinations are integral within this population due to the greater prevalence of elevated intracranial pressure.[2]
Children who have clinically significant ptosis or strabismus should be treated early to prevent amblyopia, either using patching or surgery. Recommendations for timing are not well established. Some studies have found that waiting until after craniofacial surgery reduces the likelihood of having to conduct further corrective operations.[10][2] However, another study found that improved binocularity with early strabismus surgery may outweigh the risk of future changes in craniofacial anatomy.[26]
Craniofacial considerations
Given the possibility of elevated intracranial pressure in patients with SCS, surgical treatment to address this may be necessary within the first year of life. Cranial vault expansion, which is needed to enable appropriate brain growth, traditionally occurs between 9 and 12 months of age. Further corrective procedures may be necessary to further increase cranial volume. By the age of 3 or 4, surgery can be utilized to close remaining full-thickness cranial defects. If required, patients may also receive corrective surgery for midface hypoplasia during late childhood or early adolescence.[27]
For prevention of exposure keratopathy and exophthalmos from postoperative orbital edema after craniofacial surgery, ophthalmology follow-up should be performed 2 months after the surgery to monitor for corneal risk factors. [1]
General considerations
Management and treatment of other concerns such as ocular, auditory, cardiac, developmental, etc., also necessitate consideration. Audiologic examination for hearing loss is indicated in these patients. If needed, they are treated in the standard manner using binaural amplification or possibly cochlear implantation.[17] Other routine assessments include a routine cardiac exam, examining for sleep apnea, and screening for musculoskeletal anomalies and developmental delays.[15]
References
- ↑ 1.0 1.1 1.2 1.3 Duan M, Skoch J, Pan BS, Shah V. Neuro-Ophthalmological Manifestations of Craniosynostosis: Current Perspectives. Eye Brain. 2021;13:29-40. Published 2021 Jan 29. doi:10.2147/EB.S234075
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Revere K.E., Forbes B.J., Katowitz W.R., Katowitz J.A. Congenital Craniofacial Deformities: Ophthalmologic Considerations. In Katowitz J., Katowitz W. ed. Pediatric Oculoplastic Surgery. New York: Springer, Cham; 2018: 801-830.
- ↑ 3.0 3.1 3.2 3.3 de Heer IM, de Klein A, van den Ouweland AM, et al. Clinical and genetic analysis of patients with Saethre-Chotzen syndrome. Plast Reconstr Surg. 2005;115(7):1894-1905. doi:10.1097/01.prs.0000165278.72168.51
- ↑ 4.0 4.1 4.2 4.3 4.4 Howard TD, Paznekas WA, Green ED, et al. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat Genet. 1997;15(1):36-41. doi:10.1038/ng0197-36
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Kress W, Schropp C, Lieb G, et al. Saethre-Chotzen syndrome caused by TWIST 1 gene mutations: functional differentiation from Muenke coronal synostosis syndrome. Eur J Hum Genet. 2006;14(1):39-48. doi:10.1038/sj.ejhg.5201507
- ↑ Pantke OA, Cohen MM Jr, Witkop CJ Jr, et al. The Saethre-Chotzen syndrome. Birth Defects Orig Artic Ser. 1975;11(2):190-225.
- ↑ 7.0 7.1 7.2 7.3 7.4 Fries PD, Katowitz JA. Congenital craniofacial anomalies of ophthalmic importance. Surv Ophthalmol. 1990 Sep-Oct;35(2):87-119. doi: 10.1016/0039-6257(90)90067-6. PMID: 2237761.
- ↑ El Ghouzzi V, Legeai-Mallet L, Aresta S, et al. Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum Mol Genet. 2000;9(5):813-819. doi:10.1093/hmg/9.5.813
- ↑ 9.0 9.1 9.2 9.3 El Ghouzzi V, Le Merrer M, Perrin-Schmitt F, et al. Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet. 1997;15(1):42-46. doi:10.1038/ng0197-42
- ↑ 10.0 10.1 10.2 10.3 10.4 Jadico SK, Huebner A, McDonald-McGinn DM, Zackai EH, Young TL. Ocular phenotype correlations in patients with TWIST versus FGFR3 genetic mutations. J AAPOS. 2006;10(5):435-444. doi:10.1016/j.jaapos.2006.06.008
- ↑ 11.0 11.1 Paznekas WA, Cunningham ML, Howard TD, et al. Genetic heterogeneity of Saethre-Chotzen syndrome, due to TWIST and FGFR mutations. Am J Hum Genet. 1998;62(6):1370-1380. doi:10.1086/301855
- ↑ Foo R, Guo Y, McDonald-McGinn DM, Zackai EH, Whitaker LA, Bartlett SP. The natural history of patients treated for TWIST1-confirmed Saethre-Chotzen syndrome. Plast Reconstr Surg. 2009;124(6):2085-2095. doi:10.1097/PRS.0b013e3181bf83ce
- ↑ Chun K, Teebi AS, Jung JH, et al. Genetic analysis of patients with the Saethre-Chotzen phenotype. Am J Med Genet. 2002;110(2):136-143. doi:10.1002/ajmg.10400
- ↑ Clauser L, Galiè M, Hassanipour A, Calabrese O. Saethre-Chotzen syndrome: review of the literature and report of a case. J Craniofac Surg. 2000;11(5):480-486. doi:10.1097/00001665-200011050-00007
- ↑ 15.0 15.1 15.2 Gallagher ER, Ratisoontorn C, Cunningham ML. Saethre-Chotzen Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle (WA): University of Washington, Seattle; May 16, 2003.
- ↑ Ganesh A, Edmond J, Forbes B, et al. An update of ophthalmic management in craniosynostosis. J AAPOS. 2019;23(2):66-76. doi:10.1016/j.jaapos.2018.10.016
- ↑ 17.0 17.1 Lee S, Seto M, Sie K, Cunningham M. A child with Saethre-Chotzen syndrome, sensorineural hearing loss, and a TWIST mutation. Cleft Palate Craniofac J. 2002;39(1):110-114. doi:10.1597/1545-1569_2002_039_0110_acwscs_2.0.co_2
- ↑ Trusen A, Beissert M, Collmann H, Darge K. The pattern of skeletal anomalies in the cervical spine, hands and feet in patients with Saethre-Chotzen syndrome and Muenke-type mutation. Pediatr Radiol. 2003;33(3):168-172. doi:10.1007/s00247-002-0823-3
- ↑ Molpeceres, R. G., Rodriguez, E. U., García, H. G., Vázquez, M. A., Sanz, J. L., & Guisasola, F. J. A rare case of acrocephaly: Saethre-Chotzen syndrome or Crouzon? Case Reports in Perinatal Medicine. 2016;5(2):151-155. doi:10.1515/crpm-2015-0057
- ↑ Pelc A, Mikulewicz M. Saethre-Chotzen syndrome: Case report and literature review. Dent Med Probl. 2018;55(2):217-225. doi:10.17219/dmp/91050
- ↑ Cai J, Goodman BK, Patel AS, et al. Increased risk for developmental delay in Saethre-Chotzen syndrome is associated with TWIST deletions: an improved strategy for TWIST mutation screening. Hum Genet. 2003;114(1):68-76. doi:10.1007/s00439-003-1012-7
- ↑ Shetty S, Boycott KM, Gillan TL, et al. Cytogenetic and molecular characterization of a de-novo cryptic deletion of 7p21 associated with an apparently balanced translocation and complex craniosynostosis. Clin Dysmorphol. 2007;16(4):253-256. doi:10.1097/MCD.0b013e3281e668eb
- ↑ Touliatou V, Mavrou A, Kolialexi A, Kanavakis E, Kitsiou-Tzeli S. Saethre-Chotzen syndrome with severe developmental delay associated with deletion of chromosomic region 7p15 --> pter. Genet Couns. 2007;18(3):295-301.
- ↑ Gebb J, Demasio K, Dar P. Prenatal sonographic diagnosis of familial Saethre-Chotzen syndrome. J Ultrasound Med. 2011;30(3):420-422. doi:10.7863/jum.2011.30.3.420
- ↑ Clark RA M.D. Orbital Imaging in Strabismus. J Binocul Vis Ocul Motil. 2018;68(3):87-98. doi:10.1080/2576117X.2018.1486678
- ↑ Diamond RG, Whitaker RL, Diamond GR. Ocular motility in craniofacial reconstruction. Plast Reconstr Surg. 1984;73(1):31–35. doi:10.1097/00006534-198401000-00007
- ↑ Buchanan EP, Xue AS, Hollier LH Jr. Craniofacial syndromes. Plast Reconstr Surg. 2014;134(1):128e-153e. doi:10.1097/PRS.0000000000000308