Craniosynostosis Syndromes
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Craniosynostosis is defined as a premature fusion of one or more cranial sutures during intrauterine or postnatal development. It may present either as an isolated entity, sporadically (70%) or may be associated with other abnormalities as part of a syndrome. Although the majority are sporadic, craniosynostosis syndromes may be associated with environmental and genetic factors. If untreated, it may not only result in abnormal skull shape but may also result in neurologic, visual and respiratory complications [1] [2] [3] [4]. There are close to 200 known syndromes related to craniosynostosis. These syndromes are primarily differentiated by the type of suture involved and gene mutation involved [5].
Crouzon, Apert and Pfeiffer syndromes are some of the most common craniosynostosis syndromes, the latter being more relatively uncommon of the two as it only appears in 1 out of 100,000 live births [1] [2] [3] [4]. Crouzon syndrome is one of the most common and occurs in about 1 out of 25,000 live births [1] [2] [3] [4]. The severity of the cranial bone deformations, associated systemic abnormalities and age of diagnosis may vary with therefore variable treatment outcomes and overall prognosis [6].
Signs and Symptoms
Generally, symptoms of craniosynostosis syndromes are specific to the suture involved and time of diagnosis. For instance, premature closure of the coronal suture would result in a short, broad skull, while premature closure of the sagittal suture would result in a long, narrow skull [5]. Infants with Crouzon or Apert syndromes face many similar potential clinical problems such as exorbitis (proptosis like presentation from shallow orbits), hypertelorism, strabismus, malocclusion, and hearing loss. Many of these features are directly associated with bilateral coronal synostosis, which gives the appearance of a flat skull shape and the head appears tall and wide [7]. Hydrocephalus and optic neuropathy may be present in craniosynostosis as well and should be monitored for especially in the first few decades of development.
Distinct deformations of the skull and extremities specific to Apert syndrome include a fused skull and an abnormally long or wide appearance to the skull. The hands and feet are comprised of soft tissue which, in many cases, leads to bone syndactyly [5], giving the extremities a webbed appearance [8]. Infants with Apert syndrome also face greater developmental impairments than those with Crouzon syndrome [9]. Crouzon syndrome manifests itself very similarly to Apert syndrome, but infants with this syndrome are more susceptible to exposure keratopathy, intranasal obstruction, and a v-shaped palate [3].
The most common ophthalmic manifestations of Crouzon syndrome are prominent eyes (exorbitism, secondary to shallow orbits) in almost all cases. Other features include strabismus, typically exotropia with loss of binocularity, exposure keratopathy, hypertelorism, and in rare cases, optic atrophy secondary to chronic papilledema[10]. Other ophthalmic manifestations may include globe luxation, ametropia, amblyopia and nystagmus[10]. Congenital glaucoma secondary to closed angles and FGFR2-related anterior segment dysgenesis has been reported[11].
Etiology of Ophthalmic Manifestations
Amblyopia has been reported as the primary cause of visual loss in varying rates in syndromic craniosynostosis, from 6.3% - 86%[12]. Any of the below processes which could affect afferent visual function are possible etiologies for amblyopia during the period of visual development in children.
Strabismus: Orbital development and resulting extraocular muscle displacement and dysfunction contribute to the development of strabismus. Most patients specifically present with V-pattern strabismus with exotropia larger in upgaze.[13] A variety of mechanisms have been proposed to explain this phenomenon including increased excyclorotation of ocular adductor muscles and sagittalization of the oblique muscles.[14][15] The excyclorotation is likely caused by abnormal bony development at the orbital apex, which displaces the superior rectus laterally and the lateral rectus inferiorly. Agenesis or anomalous insertions of extraocular muscles may also be a contributing factor. [16][17] [18] Orbital and secondary globe excyclorotation and retrusion of the superior orbital rim and trochlea can lead to over-elevation in adduction (pseudo inferior oblique overaction) and superior oblique underaction.[13]
Elevated Intracranial Pressure (ICP): Patients with Crouzon, Apert & Pfeiffer syndromes have a higher risk of elevated ICP. The elevated ICP can lead to neuronal death, optic nerve atrophy, and loss of vision. Previously, elevated ICP was thought to arise from cranio-cephalic disproportion. However, there is little to no correlation between intracranial volume and intracranial pressure. This hypothesis may only explain elevated ICP in patients under 1 year of age or in those with decelerating skull growth following decompressive surgery. Recently, the roles of hydrocephalus, respiratory pathologies, and venous hypertension have been shown to likely account for elevated ICP. Optic atrophy may also be caused by chronic compression of the optic nerve induced by anomalies of the orbit or optic canal from the craniosynostosis. [12]
Exposure Keratopathy: Dry eye may arise due to corneal exposure from shallow orbits, globe luxation, eyelid malformations, or eyelid malposition. Corneal exposure can progress to ulceration and lead to permanent vision loss from corneal scarring and deprivation amblyopia. [13]
Refractive Error: Hypermetropia and astigmatism are commonly associated with craniosynostosis. In patients with Crouzon syndrome, shallow orbital depth and resultant shortened axial length may predispose patients to hypermetropia. Patients with Crouzon, unicoronal synostosis, Apert, Pfeiffer, and craniofrontonasal dysplasia have the highest risk of astigmatism that is more than 1.25D.[12]
While typically afflicted children do not present with nasolacrimal duct obstruction, their abnormal anatomy (direction of bony nasolacrimal duct) may pose consequences when mid facial reconstructive surgery is considered.
Cranial Deformities
Under normal conditions in the absence of structural anomalies caused by craniosynostosis or other conditions, the infant's head is normocephalic and symmetrical along the sagittal suture. Craniosynostosis presents itself in different forms; bilateral coronal synostosis is the most common type of deformity associated with Apert and Crouzon syndromes, in which the head appears short from front to back [7], hence the more common name, brachycephaly [4]. Another form is scaphocephaly, in which the sagittal suture fuses prematurely, thus fusing the parietal bones [6][19].
Trigonocephaly is when the frontal or metopic suture fuses prematurely and the frontal bones become fused, as a result [6][19]. The head also takes a slightly triangular shape, as indicated by the name. In anterior plagiocephaly, the coronal suture between the frontal and parietal bones fuse, leaving the occipital bone unaffected [20] [21]. In contrast, the occipital and parietal bones may fuse as a result of premature fusion of the lambdoid suture leaving the frontal bones unaffected [6][19][21].
Another cranial deformity is known as deformational plagiocephaly, which is not a result of premature fusion of a particular suture, but occurs when tissues press against an infant’s developing head in utero and give the appearance of a flattened head [4][22]. This is typically of a lesser clinically significant in comparison, and the most common of the cranial deformities, occurring in about 5 to 45 percent of otherwise healthy infants [23]. This deformity can be treated non-surgically [19].
Inheritance Patterns
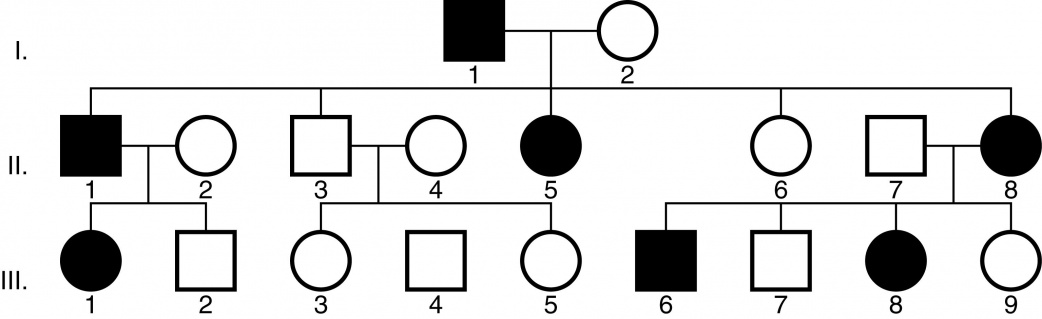
Craniosynostosis syndromes often occur spontaneously due to a de novo autosomal dominant mutation or they may be inherited by either an autosomal dominant (Figure 1) or autosomal recessive manner [5][24]. Apert and Crouzon syndromes are autosomal dominant conditions, meaning that only one copy of the altered gene is necessary to cause the disorder [24]. A minority of the craniosynostosis syndromes have an autosomal recessive transmission, in which mutations in both copies of the gene in each cell must be present [24].
Figure 1. Example of an autosomal dominant inheritance pattern. Image courtesy of the American Academy of Ophthalmology (http://www.aao.org/clinical-education).
Genetics and Testing
Many of the craniosynostosis syndromes are caused by mutations in the FGFR1, FGFR2, and FGFR3, TWIST1 and EFNB1 genes. FGFR2 mutations are present in Apert and Crouzon syndromes, as well as Pfeiffer syndrome (types 1-3), Jackson-Weiss syndrome, Beare-Stevenson syndrome and FGFR2-related isolated coronal synostosis [5]. Mutations in FGFR1 are associated with Pfeiffer syndrome (type 1) [5]. Mutations in FGFR3 are associated with Crouzon syndrome with acanthosis nigricans and also Muenke syndrome [5]. The FGFRs (fibroblast growth factor receptors) are normally responsible for suppressing excessive limb growth, therefore, a mutation in the FGFR gene is hypermorphic, as it excessively increases its gene’s product function [5][25]. The relatively similar phenotypic manifestations despite the variations in mutations is an example of allelic heterogeneity.
Craniosynostosis syndromes can be detected as early as in the prenatal period during ultrasound imaging, which may prompt the consideration of molecular genetic testing. Molecular genetic testing is only predictive if the disease causing mutation has already been identified in the family; furthermore the actual predictive value is generally considered to be poor [5]. It however aids genetic counselling and parental education. If prenatal images indicate signs such as ventriculomegaly and increased biparietal diameter caused by hydrocephalus, or a cloverleaf shaped skull, preimplantation genetic diagnosis (PGD) can be offered (prior identification of mutation must be known) [26] [27]. Otherwise, testing is generally rendered ineffectual in low-risk cases and a definitive diagnosis is made after birth.
Test methods include deletion and duplication analyses, such as quantitative PCR, long-range PCR, multiplex ligation dependent probe amplification (MLPA), and chromosomal microarray [5]. Sequence analyses are typically not used as the initial diagnostic method since the mutations are not readily found using this technique. Instead, sequence analysis is generally used to confirm diagnosis in the infant [28].
Management and Treatment
There is no uniform treatment that encompasses all the potential manifestations of the craniosynostosis syndromes; treatment methods are multifaceted and dependent on factors such as the age, time of diagnosis, suture(s) involved, severity of the condition, and whether or not other systemic clinical features are present [5][6]. A team approach of carniomaxillofacial surgeons comprising of facial plastic surgeons, neurosurgeons, pediatricians, otolaryngologists, orthodontists, geneticists, ophthalmologists, and other specialists are often needed to collaborate on a management and treatment plan [5]. Modern technology including 3-dimensional imaging, with printing, preoperative treatment planning, intraoperative navigation and simulation have greatly improved patient/family counselling, prepare surgery and deliver better outcomes
The primary goal of treatment is generally to increase cranial volume to make space for an infant’s developing brain, and to minimize intracranial pressure, which left untreated could lead to hydrocephalus or more developmental defects [6]. For this reason, when indicated, treatment should begin as soon as possible and preferably before the end of an infant’s craniofacial growth period; treating craniosynostosis syndromes as early as possible may also potentially decrease the need for additional surgeries and may yield better functional as well as cosmetic results in certain cases [5][29] [30]. Endoscopic strip craniectomy surgery can be performed in infants before 3 months of age; the surgeon creates small incisions in the scalp to view the anatomy of the skull, and then opens the affected suture to enable brain growth [31]. An early infant age may allow for an increased plasticity of the fontanelles and suture(s) involved [5]. A more traditional surgery known as calvarial vault remodeling is performed on infants older than 6 months of age, and involves moving affected areas and reshaping the skull [31]. A bilateral craniotomy with a fronto-orbital advancement can also be performed in older infants to create space for brain development [5]. For mid facial advancement it is imperative that the course of the bony nasolacrimal duct is taken into consideration and prophylactic stenting prior to mid facial osteotomy and advancement should be considered.
Generally, non-syndromic cases of craniosynostosis require fewer surgeries than syndromic cases [5][32]. For example infants with Apert syndrome, compared with non-syndromic cases, have a greater frequency of follow-up surgeries to correct their forehead shape, and may often experience minimal functional improvement after surgery to correct their bone syndactyly [33] [34].
Pediatric ophthalmologists should follow closely to monitor for papilledema or optic atrophy (following previous hydrocephalus), amblyopia and strabismus. Children with craniosynostosis often have a V-pattern exotropia. Traditionally, strabismus surgery has been recommended after the first 2 years of life, but Diamond, et. al., found that the benefit of achieving binocularity with earlier surgery may outweigh the risk of future shifts in ocular position.[35] [36] Furthermore, children with midface hypoplasia may have increased perspiration due to narrow airways and chronically increased respiratory effort. In these patients, adhesive occlusive patches may be ineffective.[13]
Individuals with craniosynostosis often require topical lubrication due to exposure keratopathy and eye protection. Lateral tarsorrhaphy may also be indicated.[12]
Refractive errors should be addressed with corrective lenses . However, glasses should be made to consider the midfacial hypoplasia that may be present. The eyelashes/globe may be easily touched by the lenses due to shallow orbits in these patients. Built-up nasal silicone nasal bridges can help in this situation.[12]
References
- ↑ Jump up to: 1.0 1.1 1.2 Corde Mason A, Bentz ML, Losken W. Craniofacial syndromes. In: Zitelli BJ, Davis HW, eds. Atlas of pediatric physical diagnosis. 4th ed. St. Louis: Mosby, 2002:803–17.
- ↑ Jump up to: 2.0 2.1 2.2 Haidar Kabbani, MD and Talkad S. Raghuveer, MD. Craniosynostosis. Am Fam Physician. 2004 Jun 15;69(12):2863-2870.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Craniosynostosis and Craniofacial Disorders. September, 2005. http://www.aans.org/Patient%20Information/Conditions%20and%20Treatments/Craniosynostosis%20and%20Craniofacial%20Disorders.aspx
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Craniofacial/Skull. Retrieved July 1, 2015 from http://www.kidsplastsurg.com/craniofacial-anomalies/#apert
- ↑ Jump up to: 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 Nathaniel H Robin, MD, Marni J Falk, MD, and Chad R Haldeman-Englert, MD. FGFR-Related Craniosynostosis Syndromes. GeneReviews® [Internet]. Initial Posting: October 20, 1998; Last Update: June 7, 2011.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 Craniosynostosis. Retrieved July 1, 2015 from http://www.chw.org/medical-care/neuroscience/conditions/craniosynostosis/
- ↑ Jump up to: 7.0 7.1 Bilateral Coronal Synostosis. Retrieved July 1, 2015 from https://www.childrens.com/specialties-services/specialty-centers-and-programs/plastic-craniofacial-surgery/programs-and-services/craniofacial-program/conditions-and-treatments/craniosynostosis/isolated-craniosynostosis/bilateral-coronal-synostosis
- ↑ Andrew O. M. Wilkie. Craniosynostosis: Genes and Mechanisms. Hum. Mol. Genet. (1997) 6 (10): 1647-1656. doi: 10.1093/hmg/6.10.1647
- ↑ Renier D, Arnaud E, Cinalli G, Sebag G, Zerah M, Marchac D. Prognosis for mental function in Apert's syndrome. J Neurosurg. 1996;85:66–72. [PubMed]
- ↑ Jump up to: 10.0 10.1 Kreiborg S, Cohen MM Jr. Ocular manifestations of Apert and Crouzon syndromes: qualitative and quantitative findings. J Craniofac Surg. 2010;21(5):1354-7.
- ↑ Alshamrani AA, Al-Shahwan S. Glaucoma With Crouzon Syndrome. J Glaucoma. 2018 Jun;27(6):e110-e112. doi: 10.1097/IJG.0000000000000946. PubMed PMID: 29557836.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 Duan M, Skoch J, Pan BS, Shah V. Neuro-Ophthalmological Manifestations of Craniosynostosis: Current Perspectives. Eye Brain. 2021;13:29-40. Published 2021 Jan 29. doi:10.2147/EB.S234075
- ↑ Jump up to: 13.0 13.1 13.2 13.3 Ganesh A, Edmond J, Forbes B, et al. An update of ophthalmic management in craniosynostosis. J AAPOS. 2019;23(2):66-76. doi:10.1016/j.jaapos.2018.10.016
- ↑ Tan KP, Sargent MA, Poskitt KJ, Lyons CJ. Ocular overelevation in adduction in craniosynostosis: is it the result of excyclorotation of the extraocular muscles?. J AAPOS. 2005;9(6):550-557. doi:10.1016/j.jaapos.2005.07.004
- ↑ Gobin MH. Sagittalization of the oblique muscles as a possible cause for the "A", "V", and "X" phenomena. Br J Ophthalmol. 1968;52(1):13-18. doi:10.1136/bjo.52.1.13
- ↑ Dagi LR, MacKinnon S, Zurakowski D, Prabhu SP. Rectus muscle excyclorotation and V-pattern strabismus: a quantitative appraisal of clinical relevance in syndromic craniosynostosis. Br J Ophthalmol. 2017 Nov;101(11):1560-1565. doi: 10.1136/bjophthalmol-2016-309996. Epub 2017 Mar 20. PMID: 28320694.
- ↑ Greenberg MF, Pollard ZF. Absence of multiple extraocular muscles in craniosynostosis. J AAPOS 1998;2:307–9
- ↑ Cuttone JM, Brazis PT, Miller MT, et al. Absence of the superior rectus muscle in Apert’s syndrome. J Pediatr Ophthalmol Strabismus 1979;16:349–54
- ↑ Jump up to: 19.0 19.1 19.2 19.3 Common variation in craniosynostosis presentations. Retrieved July 1, 2015 from http://www.uptodate.com/contents/image?imageKey=ALLRG%2F52793&topicKey=ALLRG%2F2910&source=see_link&utdPopup=true
- ↑ Lambdoidal Synostosis. Retrieved July 1, 2015 from http://www.ohsu.edu/xd/health/services/doernbecher/programs-services/lambdoidal.cfm
- ↑ Jump up to: 21.0 21.1 What are the different types of craniosynostosis? Retrieved July 1, 2015. http://www.hopkinsmedicine.org/neurology_neurosurgery/centers_clinics/pediatric_neurosurgery/conditions/craniosynostosis/types.html
- ↑ Plagiocephaly. Retrieved July 1, 2015 from http://smilesforkids.missouri.edu/common-conditions/plagiocephaly/
- ↑ Deformational Plagiocephaly. Retrieved July 1, 2015 from http://www.facesofchildren.org/Deformational%20Plagiocephaly
- ↑ Jump up to: 24.0 24.1 24.2 What is Craniosynostosis? Retrieved July 1, 2015 from http://www.hopkinsmedicine.org/neurology_neurosurgery/centers_clinics/pediatric_neurosurgery/conditions/craniosynostosis/
- ↑ Hypermorphic Mutation. June 23, 2015. http://www.informatics.jax.org/glossary/hypermorphic
- ↑ Chen CP, Su YN, Hsu CY, Ling PY, Tsai FJ, Chern SR, Wu PC, Chen HE, Wang W. Second-trimester molecular prenatal diagnosis of sporadic Apert syndrome following sonographic findings of mild ventriculomegaly and clenched hands mimicking trisomy 18. Taiwan J Obstet Gynecol. 2010;49:129–32.[PubMed]
- ↑ Weber B, Schwabegger AH, Vodopiutz J, Janecke AR, Forstner R, Steiner H. Prenatal diagnosis of apert syndrome with cloverleaf skull deformity using ultrasound, fetal magnetic resonance imaging and genetic analysis. Fetal Diagn Ther. 2010;27:51–6. [PubMed]
- ↑ Bochukova EG, Roscioli T, Hedges DJ, Taylor IB, Johnson D, David DJ, Deininger PL, Wilkie AO. Rare mutations of FGFR2 causing apert syndrome: identification of the first partial gene deletion, and an Alu element insertion from a new subfamily. Hum Mutat. 2009;30:204–11. [PubMed]
- ↑ Lajeunie E, Bonaventure J, El Ghouzzi V, Catala M, Renier D. Monozygotic twins with Crouzon syndrome: concordance for craniosynostosis and discordance for thumb duplication. Am J Med Genet. 2000;91:159–60.[PubMed]
- ↑ Renier D, Lajeunie E, Arnaud E, Marchac D. Management of craniosynostoses. Childs Nerv Syst.2000b;16:645–58. [PubMed]
- ↑ Jump up to: 31.0 31.1 Surgical Options for Craniosynostosis. Retrieved July 1, 2015 from http://www.hopkinsmedicine.org/neurology_neurosurgery/centers_clinics/pediatric_neurosurgery/conditions/craniosynostosis/surgery.html
- ↑ Definition of Nonsyndromic. August 28, 2013. http://www.medicinenet.com/script/main/art.asp?articlekey=21328
- ↑ American Cleft Palate-Craniofacial Association; Parameters for evaluation and treatment of patients with cleft lip/palate or other craniofacial anomalies. Cleft Palate Craniofac J. 1993;30:S1–16. [PubMed]
- ↑ Thomas GP, Wilkie AO, Richards PG, Wall SA. FGFR3 P250R mutation increases the risk of reoperation in apparent 'nonsyndromic' coronal craniosynostosis. J Craniofac Surg. 2005;16:347–52. [PubMed]
- ↑ Diamond RG, Whitaker RL, Diamond GR. Ocular motility in craniofacial reconstruction. Plast Reconstr Surg. 1984;73(1):31–35. doi:10.1097/00006534-198401000-00007
- ↑ Cohen MM Jr. Craniosynostosis diagnosis, evaluation, and management second edition. Oxford Chap. 2000;2000(24):316–353.