All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Multiple sclerosis (MS) is a neurodegenerative disease of the central nervous system (CNS) that results from the immune-mediated inflammation and demyelination of axons. It is characterized by focal demyelinating lesions in the white matter of the brain or spinal cord that vary temporally and spatially, thereby leading to the classic presentation of episodic neurologic symptoms. These symptoms vary depending on the location of lesions, and can include changes in autonomic, motor or sensory functions. Of interest, MS often presents with ocular manifestations secondary to inflammatory demyelination of the visual pathway, and a majority of patients experience at least one episode of ocular involvement in the course of their disease.
Epidemiology
MS affects an estimated 400,000 people in the United States and 2.1 million people worldwide, with a more prevalent geographic distribution among the northern and southern hemispheres.[2] The average age of onset ranges from 15 to 45 years while the average age at diagnosis is 30 years. Females are more commonly affected than males, at a ratio of about 2 to 1.[3]
Etiology
The underlying cause of MS remains enigmatic, though multiple factors have been proposed to contribute to the development and progression of the disease. The leading theories suggest a complex interplay between genetic predisposition and environmental factors.
A genetic component in MS is supported by twin studies showing a concordance rate of 25-30% in monozygotic twins, 5% in dizygotic twins, and 3% in non-twin siblings.[4] In addition, a number of genome-wide association studies have identified more than 100 risk loci in MS, and a majority of these genes have been found to encode for proteins involved in immune modulation. Although polymorphisms in the human leukocyte antigen (HLA) have been identified as the strongest susceptibility loci, other non-HLA genes have also been found.[5][6]
Despite data suggesting significant genetic influence in MS, genes alone do not fully contribute to the development of the disease. A number of environmental factors such as infection, location, climate, stress, occupation and diet have been postulated to be involved.[7] There is evidence suggesting the development or exacerbation of MS following post-viral syndromes, such as those from Epstein-Barr virus (EBV) and human herpes virus (HHV).[8][9] The geographic variation of MS further supports an environmental role. Although the underlying cause remains unclear, factors such as decreased sun exposure and low levels of vitamin D have been proposed to explain the latitudinal gradient.[2]
Pathophysiology
MS is thought to be an autoimmune disease and this theory is supported by its shared characteristics with other autoimmune disorders. These features include the predominance of women affected, the association with HLA polymorphisms, and the presence of autoantibodies to myelin antigens in CSF.[7]
The chronic inflammation, degeneration and demyelination of axons in the CNS lead to the clinical presentation of neurologic dysfunction. The inflammatory process is mediated by T lymphocytes that recognize myelin as a foreign entity and trigger other immune cells, macrophages, cytokines and antibodies that together lead to myelin and axonal breakdown. These T cells are thought to gain entry into the brain from disruptions in the blood-brain barrier, which can result from post-viral infections.[10] With the loss of myelin, the protective and insulating fatty sheath that surrounds axons, the conduction of electrical impulses is compromised.
This impaired transmission of neurologic signal can occur anywhere within the CNS, including the visual system. Ophthalmic involvement can be categorized into lesions of the afferent or efferent visual pathways. The afferent pathway is responsible for the transmission of sensory information from the retina to the brain. The optic nerve is most commonly involved and leads to a number of visual symptoms. Infrequently, the optic chiasm and optic tracts are involved which lead to visual field defects such as bitemporal hemianopsia and homonymous hemianopsia, respectively.
The efferent pathway is responsible for motor output to the pupillary muscles and extraocular muscles. Disorders of ocular movement affect more than 40% of patients with MS.[11] Lesions can involve the brainstem, cerebellum or cranial nerves and result in ocular misalignment resulting in diplopia, and nystagmus resulting in oscillopsia. A condition termed internuclear ophthalmoplegia (INO) occurs with lesions in the medial longitudinal fasciculus (MLF), a highly myelinated tract between the abducens nucleus to the contralateral medial rectus sub-nucleus of the oculomotor nucleus. MLF lesions result in impaired ipsilateral adduction with contralateral abducting nystagmus.
Diagnosis
History
Demographic information such as age, sex, race, country of origin and migration status is relevant. MS is more common in women ages 15-45. Family history of MS is also relevant as there is a genetic predisposition to the disease. Recent stressors, viral infections, and the presence of sick contacts should also be explored.
MS is classically described as a condition in which neurologic symptoms vary in time and space. Because demyelination can have diffuse involvement within the CNS, patients often complain of a constellation of seemingly unrelated symptoms. In 75% of patients, the initial presentation of MS involves an isolated complaint; 45% are motor or sensory and 20% are visual.[12] Therefore, a thorough review of systems is crucial. Symptoms of MS can be autonomic, visual, motor or sensory. Changes in sensation can present as pain, numbness, tingling, or pins and needles sensation. Motor involvement manifests as muscle weakness, muscle spasms, impaired coordination and balance or difficulty with speech and swallowing. Autonomic symptoms include bowel dysfunction (diarrhea or constipation) and bladder dysfunction (urinary incontinence). Cognitive complaints in MS include fatigue, decreased attention span, concentration, memory, and judgment. Psychological symptoms include depression and mood instability. MS is associated with other clinical findings such as: Charcot triad of dysarthria, ataxia, and tremor; Uhthoff’s phenomenon of worsening of symptoms in higher temperatures; Lhermitte’s sign of an electrical sensation running down the back when the neck is flexed; and trigeminal neuralgia presenting as facial pain or weakness.
Ocular manifestations are common in MS. Up to 20% of patients have optic neuritis as the initial clinical presentation and 75% of patients have at least one episode throughout the course of their lives.[13] Optic neuritis associated with MS typically presents as a monocular painful vision loss that occurs over hours to days and lasts a few weeks. Orbital pain occurs in 92% of patients and is usually worse with extraocular movement.
It is equally important to elicit the onset, duration and temporal relationship of symptoms. Exacerbations or “flare-ups” can be acute or subacute in onset and typically lasting days to months. These episodes are usually transient, with improvement or even complete resolution of symptoms in 85% of cases. However, symptoms can worsen or become permanent in the remaining 10-15% of those affected.[14]
Physical examination
A detailed neuro-ophthalmic examination should take place. This includes the afferent system: best corrected visual acuity, color vision, RAPD check, and visual field testing. Efferent exam includes checking for anisocoria, EOM range of motion, saccades, smooth pursuit, and presence of nystagmus along with an alignment testing. Orbits and cranial nerves should be assessed. Anterior segment and IOP. Fundus exam particularly assessing the optic nerves.
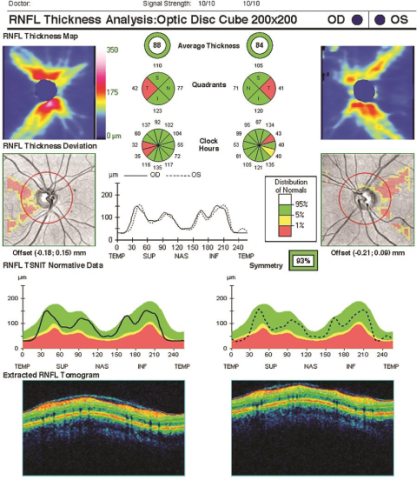
In addition, the use of optical coherence tomography (OCT) can provide information about the extent of involvement and allow for quantitative measures for disease progression over time.
In patients with optic neuritis, changes in visual acuity can range from mild to severe; 10% of patients are 20/20, 25% are 20/30-20/40, 29% are 20/50-20/190 and 36% are 20/200 or worse.[15] Patients will also present with diminished color vision (dyschromatopsia in 88%, best assessed by red desaturation), diminished contrast sensitivity, visual field loss (most commonly central scotoma) and a relative afferent pupillary defect (RAPD). Slit lamp examination will reveal optic disc swelling in one-third of patients.[15] Papillitis is more common in postviral and infectious neuritis than demyelinating neuritis, but considerable overlap exists. Retinal nerve fiber layer (RNFL) thinning reflecting axonal damage is observed in about 70% of patients with acute optic neuritis, while visual evoked potential (VEP) increases in latency and decreases in amplitude are found in 65%.[16][17]
Internuclear ophthalmoplegia occurs in about 30% of patients.[18] This condition presents with diplopia, nystagmus, and loss of depth perception (stereopsis) secondary to an adduction deficit or adduction lag on conjugate gaze. Other patterns of ocular misalignment include skew deviation, gaze paresis and impaired smooth pursuit.[19] Uveitis can occur in 1-2% of patients with MS, a rate that is 10 times more common than that of the general population.[20] Symptoms of uveitis include pain, photophobia, and conjunctival injection.
The neurologic exam can provide useful information regarding localization of the lesion(s), as well as severity and extent of involvement. The exam consists of the evaluation of cranial nerves, deep tendon reflexes, motor strength, sensation, gait, balance and coordination.
Clinical diagnosis
The 2010 revised McDonald Criteria is widely used for the clinical diagnosis of MS.[21] The criteria include the presence of two or more attacks involving two or more clinical lesions; two or more attacks of one clinical lesion with evidence for dissemination in space as demonstrated by MRI or CSF; one attack involving two or more lesions demonstrated by MRI or followed by a second attack; one attack of one lesion demonstrated by MRI, CSF or followed by a second attack. An attack is defined as any subjective or objective neurologic disturbance of 24-hour duration occurring 30 days apart, in the absence of fever or infection. MS is classified into four subtypes depending on its clinical course: relapsing-remitting (RRMS), secondary progressive (SPMS), primary progressive (PPMS), and progressive relapsing (PRMS).[22]
Diagnostic procedures
Repeated episodes of demyelination result in the formation of plaques that are seen as hyperintense T2 lesions or post-gadolinium enhancement on magnetic resonance imaging (MRI). MRI of the head and spine is performed to evaluate for these demyelinating plaques in the periventricular, juxtacortical, infratentorial or spinal cord white matter. Optic neuritis in MS is typically seen as a unilateral T2 lesion of the optic nerve. Optical coherence tomography can aid in diagnosis of optic neuritis and MS and monitor progression[23]. Thinning of the peripapillary retinal nerve fiber layer (RNFL) and macular ganglion cell layer and inner plexiform layer (GCIPL) are observed in MS patients both with and without optic neuritis[24]. Spectral domain optic coherence tomography (SD-OCT) is a recommended diagnostic tool for identifying and monitoring these retinal changes in MS patients[24]. Serial imaging is useful for comparing episodes and monitoring disease progression.
Laboratory test
Lumbar punctures can be performed for evaluation of the CSF for oliclonal bands and intrathecal immunoglobulin G (IgG). In an atypical presentation of optic neuritis, suspicion for a non-MS cause should prompt additional laboratory testing. Atypical features include bilaterality, painless vision loss, lack of characteristic MRI findings, lack of response to corticosteroids, and presence of severe optic nerve head swelling or peripapillary hemorrhages to name a few.[25]
The following laboratory tests should be considered given a patient’s history, risk factors and clinical findings:
Infectious:
- Venereal Disease Research Laboratories [VDRL], rapid plasma reagin [RPR] and fluorescent treponemal antibody (FTA-ABS) for syphilis
- Tuberculin skin test, chest x-ray or Quantiferon-TB for tuberculosis
- Lyme titers for Lyme disease (in an endemic area)
Inflammatory:
- Antinuclear antibody (ANA) for systemic lupus erythematosus
- Angiotensin-converting enzyme (ACE) and lysozyme for sarcoidosis
- Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) for inflammatory disorders
Other demyelinating:
- Anti-aquaporin 4 [AQP4] IgG antibody for neuromyelitis optica (NMO)
- Myelin oligodendrocyte glycoprotein antibody (MOG)
- Serum neurofilament light chain test
Lymphoproliferative
- CBC with differential
Leber's hereditary optic neuropathy
should be considered in cases that are painless, steroid resistant unilateral or bilateral simultaneous or sequential subacute optic neuropathy with mild or no optic nerve head swelling especially with cecocentral scotomas that might be overall be mistaken for optic neuritis
Differential diagnosis
The differential diagnosis for optic neuritis is broad, and accurate assessment is important for the proper treatment of the underlying etiology.[26][27] They include:
- Inflammatory conditions: NMO or Devic’s disease, acute disseminated encephalomyelitis (ADEM), MOGAD, sarcoidosis
- Infections: Tuberculosis, Syphilis, Lyme disease and Bartonella
- Compressive lesions: orbital tumors or aneurysms
- Connective tissue or vascular diseases: lupus, granulomatosis with polyangiitis, Sjogrens, Behcet
- Nonarteritic anterior ischemic optic neuropathy (NAION)
- Severe systemic hypertension
- Acute papilledema from increased intracranial pressure
- Leber’s hereditary optic neuropathy (LHON)
- Optic nerve glioma
- Toxic or metabolic optic neuropathy: alcohol, malnutrition, ethambutol, heavy metals
- Central serous retinopathy or other maculopathies
Management
There is no cure for MS. Management involves pharmacotherapy for immune modulation and symptomatic relief. The Optic Neuritis Treatment Trial (ONTT) has shown that high-dose intravenous methylprednisone improves recovery time for visual function, contrast sensitivity and color vision. However, corticosteroids have not been shown to improve final visual outcomes.[28] Intravenous immunoglobulin (IVIg) has not been shown to improve long-term visual acuity, contrast sensitivity or color vision.[29]Plasmapheresis may be used short term for severe attacks if steroids are contraindicated or ineffective.[30] There is a total of 13 medications approved by the FDA for MS.[31] Interfeon-beta and glatiramer acetate are generally used as first-line disease-modifying agents in MS, with relapse reduction rates of 18-34% in some trials.[32] Other FDA-approved disease-modifying drugs include fingolimod, teriflunomide, dimethyl fumarate, natalizumab, alemtuzumab, ocrelizumab and siponimod.
Prognosis
Multiple sclerosis can have severe debilitating effects within 20-25 years of onset. Up to 30% of patients eventually require assistance for ambulation, and 22% become wheelchair bound.[33] There is a 5 to 10 year decrease in life expectancy, and death secondary to cardiovascular disease, infection and suicide is higher in MS compared to the general population.[34]
Higher lesion load, more numerous active lesions, and higher cortical lesions at disease onset are associated with less favorable prognosis of overall MS disease[35][36]. Higher NfLc levels is also associated with less favorable prognosis and progression of MS[37].
Many of the clinical findings of optic neuritis come from the Optic Neuritis Treatment Trial (ONTT), a multi-institutional study of 454 patients with acute unilateral optic neuritis carried out between 1988 and 1991. The 15-year follow-up of the Optic Neuritis Treatment Trial (ONTT) showed that clinically definite MS developed in 50% of ONTT patients in 15 years. However, the probability of developing clinically definite MS based on MRI scan appearance ranged from 25% for patients with no lesions on the brain MRI to 72% with 1 or more lesions.
Visual prognosis following the first episode of optic neuritis is typically good with or without the use of corticosteroids. Visual symptoms such as decreased visual acuity, visual field defects, and color vision defects recover within weeks to months. A majority of patients achieve 20/20 vision one year after an acute episode while 8% of patients retain a visual acuity worse than 20/40.[38] However, recurrent episodes are associated with less favorable prognosis.
References
- ↑ American Academy of Ophthalmology. OCT of the nerve fiber layer. https://www.aao.org/image/oct-of-nerve-fiber-layer Accessed July 31, 2019.
- ↑ 2.0 2.1 Simpson S Jr., Blizzard L, Otahal P, et al. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J Neurol Neurosurg Psychiatry 2011; 82: 1132–1141.
- ↑ World Health Organization (2008). Atlas: Multiple Sclerosis Resources in the World 2008 (PDF). Geneva: World Health Organization. Pp. 15–16. ISBN 92-4-156375-3
- ↑ Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC; Twin concordance and sibling recurrence rates in multiple sclerosis. Canadian Collaborative Study Group. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):12877-82.
- ↑ Bashinskaya VV, Kulakova OG, Boyko AN, Favorov AV, Favorova OO. A review of genome-wide association studies for multiple sclerosis: classical and hypothesis-driven approaches. Hum Genet. 2015 Nov;134(11-12):1143-62.
- ↑ Bahreini SA, Jabalameli MR, Saadatnia M, Zahednasab H. The role of non-HLA single nucleotide polymorphisms in multiple sclerosis susceptibility. J Neuroimmunol. 2010 Dec 15;229(1-2):5-15.
- ↑ 7.0 7.1 Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004 Dec;3(12):709-18.
- ↑ Banwell B, Bar-Or A, Arnold DL, et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol 2011; 10: 436–445.
- ↑ Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, Calabresi PA, Brennan MB, Maloni HW, McFarland HF, Lin HC, Patnaik M, Jacobson S. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997 Dec;3(12):1394-7.
- ↑ Pender MP. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity: A unifying hypothesis. Autoimmun Dis 2012; 2012: 189-96.
- ↑ Prasad S, Galetta SL. Eye movement abnormalities in multiple sclerosis. Neurol Clin. 2010 Aug. 28(3): 641-55.
- ↑ Tsang BK, Macdonell R. "Multiple sclerosis- diagnosis, management and prognosis". Australian family physician Dec 2011. 40 (12): 948–55.
- ↑ Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005 May. 4(5):281-8.
- ↑ Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev Mar 2010. (5): A387–94.
- ↑ 15.0 15.1 The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch. Ophthalmol. 1991. 109(12),1673–1678.
- ↑ Costello F, Burton JM. An approach to optic neuritis: the initial presentation. Expert Rev Ophthalmol. 2013. 8(6):539–551.
- ↑ Costello F. The afferent visual pathway: designing a structural-functional paradigm of multiple sclerosis. ISRN Neurol. 2013. 2013:134858.
- ↑ Müri RM, Meienberg O. The clinical spectrum of internuclear ophthalmoplegia in multiple sclerosis. Arch Neurol 1985; 42:851.
- ↑ Amezcua M, Morrow MJ Jirawuthiworavong, G. Current Opinion in Ophthalmology Nov 2015. 2(6): 534-539.
- ↑ McClelland C, Galetta S. Eye Symptoms, Signs, and Therapy in Multiple Sclerosis. Rae-Grant A, Fox R, Bethoux F. Multiple Sclerosis and Related Disorders: Diagnosis, Medical Management, and Rehabilitation. Demos Medical Publishing; 2013. 179-86.
- ↑ Polman C et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Annals of Neurology. 2011;69:292-302.
- ↑ Rovira A, Auger C. Beyond McDonald: updated perspectives on MRI diagnosis of multiple sclerosis. Expert Rev Neurother. 2021;21:895–911
- ↑ American Academy of Ophthalmology. OCT of the nerve fiber layer. https://www.aao.org/image/oct-of-nerve-fiber-layer Accessed March 23, 2020, 2019.
- ↑ 24.0 24.1 Britze, J., Pihl-Jensen, G., Frederiksen, J. L, et al. Retinal ganglion cell analysis in multiple sclerosis and optic neuritis: a systematic review and meta-analysis. Lancelet Neurology 2017; 264(9): 1837–1853.
- ↑ Gaier ED, Boudreault K, Rizzo JF 3rd, Falardeau J, Cestari DM. Atypical Optic Neuritis. Curr Neurol Neurosci Rep. Dec 2015; 15(12):76.
- ↑ Kaiser PK. Friedman NJ. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 4th Edition. 2014.
- ↑ Costello F. Inflammatory optic neuropathies. Continuum (Minneap Minn). Neuro-ophthalmolog. Aug 2014; 20(4): 816-37.
- ↑ Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. Feb 1992. 326(9):581-8.
- ↑ Roed et al. A double-blind, randomized trial of IV immunoglobulin treatment in acute optic neuritis. Neurology Mar 2005; 64(5): 804-810.
- ↑ Ruprecht K, Klinker E, Dintelmann T, Rieckmann P, Gold R. Plasma exchange for severe optic neuritis: treatment of 10 patients. Neurology 2004. 63(6): 1081–1083.
- ↑ Maha Haki a, Haeder A AL-Biati a, Zahraa Salam Al-Tameemi a,b, Inas Sami Ali a, Hany A Al-hussaniy a,b,c,* Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine (Baltimore). 2024 Feb 23;103(8):e37297. doi: 10.1097/MD.0000000000037297. PMCID: PMC10883637 PMID: 38394496
- ↑ Scott LJ. Glatiramer acetate: a review of its use in patients with relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. CNS Drugs 2013; 27:971–988.
- ↑ Swingler RJ, Compston DA. The morbidity of multiple sclerosis. Q J Med. Apr 1992; 83(300):325-37.
- ↑ Manouchehrinia A, Tanasescu R, Tench CR, Constantinescu CS. Mortality in multiple sclerosis: meta-analysis of standardized mortality ratios. J Neurol Neurosurg Psychiatry. Mar 2016; 87(3):324-31.
- ↑ Luchetti, S., Fransen NL., van Eden CG., et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathology 2018; 135: 511–528.
- ↑ Rocca, M. A., Sormani MP., Rovaris M., et al. Long-term disability progression in primary progressive multiple sclerosis: a 15-year study. Brain 2017; 140: 2814–2819.
- ↑ Barro, C., Benkert P., Disanto G., et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141: 2382–2391.
- ↑ Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis. Experience of the optic neuritis treatment trial. Ophthalmology. 1994; 101(11):1771-8.