Systemic Lupus Erythematosus (SLE)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by chronic overactivation of the immune system, leading to inflammation.[1][2] SLE has the potential to present with a wide variety of clinical manifestations, with the most common features including fatigue, arthralgia/myalgia, sun sensitivity, pleurisy, and fever. A facial malar rash, while classic, is a less common finding.[3] Ocular manifestations are common in SLE, affecting 33-50% of patients.[4][5][6]
Etiology
Like many autoimmune diseases, the development of SLE is believed to have a strong genetic component. As of now, there are over 80 loci variations identified as SLE-related, suggesting a complex balance between susceptible and protective genes.[7] Several environmental factors, including viral antigen exposure, certain medication use, and UV light exposure, have been implicated in the development of SLE. As there is a significant female to male overrepresentation, it is believed that estrogen and other hormones may contribute.[1][8]
Epidemiology
SLE is most commonly diagnosed in women, with onset typically occurring between 20 and 30 years of age. The ratio of women to men with lupus is believed to be about 9:1, with higher prevalence in African American women.[9]
Pathophysiology
The pathogenesis of SLE is not completely understood however, it is believed it involves an unregulated, overproduction of autoantibodies and the deposition of immune complexes. A common theory is that in SLE, with genetic or environmental predisposition, there is a loss of self-tolerance and a subsequent increase in autoantibodies produced. This state is perpetuated by overactive T helper cells and a loss of regulatory immune cells. At the same time, B cells mature more quickly and are less likely to undergo apoptosis. This dysregulation in apoptosis is believed to contribute to longer-living plasma cells, releasing excess autoantibodies. The autoantibodies attach to various autoantigens (nucleic, nuclear or cytoplasmic), releasing inflammatory cytokines and leading to the formation of immune complexes. Chronic inflammation, immune complex deposition, along with poor apoptotic and cellular debris clearing, leads to tissue and organ damage.[1][8][9]
Systemic Findings
Clinical Presentation
SLE may present with a wide range of systemic signs and symptoms. Although considerable variation exists, the more common presentations are summarized in Table 1.[3]
| System | Signs/Symptoms |
| Constitutional | Fatigue (90%), fever (57%) |
| Musculoskeletal | Arthralgia/myalgia (90%), arthritis (50%) |
| Cutaneous | Sun sensitivity (70%), malar rash (35%), oral ulcerations (20%), discoid lesions (20%) |
| Renal | Proteinuria/nephritis (40%) |
| Cardiovascular | Pericarditis (25%), Raynaud’s (25%), hypertension (25%), CNS vasculitis (15%) |
| Pulmonary | Pleurisy (60%), pleural effusion (25%) |
| Hematologic | Leukopenia (50%), anemia (40%) |
| Neurologic | Intermittent cognitive impairment (30%) |
| Ophthalmologic | Keratoconjunctivitis sicca (35%), eye movement abnormalities (30%), retinopathy (15%) |
Diagnostic Criteria
The criteria for the diagnosis of SLE continues to evolve with a better understanding of immunologic markers and clinical manifestations. The first criteria used was developed by the American College of Rheumatology (ACR) criteria, last revised in 1997. In this system, SLE was diagnosed by the presence of 4 out of 11 qualifying clinical or immunologic manifestations. In 2012, this criterion was revisited by the Systemic Lupus Erythematosus International Collaborating Clinics group (SLICC). This expanded the criteria for a diagnosis of lupus by requiring 4 out of 17 separate elements.[1][2] More recently, in 2019, the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) established new criteria, where a positive ANA titer was required for diagnosis. This system includes various clinical or immunologic manifestations, weighted from 2-10. An ANA positive patient may be diagnosed with a score of 10 or more.[10]
Ocular Manifestations
Ocular complaints affect somewhere between 33-50% of patients with SLE. The ophthalmologic manifestations of SLE are varied, ranging from mild irritation to vision threatening retinopathy. The most common ophthalmologic manifestation of SLE is keratoconjunctivitis sicca (dry eye syndrome), affecting about a third of patients. The etiology of keratoconjunctivitis sicca in SLE patients is thought to be multifactorial, involving the development of secondary Sjogren’s syndrome.[4][5][6] While dry eye may be the most common complaint, the most common vision threatening ocular manifestation is that of lupus retinopathy, ranging from 3-29% of patients affected, depending on disease control. Such retinopathy is considerably less prevalent with available steroids and immunosuppressants; however, there is the possible sequela of blindness.[4][11] Of note, uveitis is not commonly associated with SLE; if uveitis is present, alternative etiologies should be explored.[4][5][11]
An expanded discussion of the most common ocular manifestations in patients with SLE is included below, and a more extensive list of ophthalmologic presentations is outlined in Table 2.
| Orbit | Myositis, proptosis, ptosis, panniculitis |
| Eyelids | Discoid rash |
| Anterior segment:
· Cornea · Episclera/sclera · Iris/ciliary body |
Keratoconjunctivitis sicca, punctate epithelial loss, peripheral ulcerative keratitis, chemosis, scleritis, episcleritis, iritis, uveitis (uncommon) |
| Posterior segment:
· Retina · Choroid |
Cotton wool spots, hard exudates, retinal hemorrhages, vascular tortuosity, large vessel occlusion, pigmentary changes, retinal detachment, choroidal ischemia |
| Neuro-ophthalmological | Optic neuritis, optic neuropathy, diplopia, internuclear ophthalmoplegia, eye movement abnormalities |
Cutaneous and Eyelid Involvement
Background
There are several subtypes of chronic cutaneous lupus erythematosus, with discoid lupus erythematosus being the most common. Up to 20% of SLE patients will manifest discoid lesions at some point during their disease process. Discoid lesions are most commonly found on the scalp, face, anterior neck and extensor surfaces of the arms.[12][13][14] Additionally, these lesions can affect the eyelids, resulting in cicatricial entropion or ectropion.
Signs and Symptoms
Discoid lesions start as a scaly, purple macule or papule that expands into a discoid plaque. Scaling and hyperpigmentation may be seen in the periphery while the center is atrophic. These lesions may coalesce and a significant percentage of patients, over 50%, will develop scarring. When discoid lesions affect the eyelids, cicatricial entropion or ectropion can develop as a consequence of this scarring.
Treatment
Treatment of discoid lesions first starts with sun protection, as these lesions are photosensitive. Often these lesions are treated with topical steroid creams, however due to the proximity to the globe the potential for steroid-induced pressure elevations must be with special attention to glaucoma patients. Topical calcineurin inhibitors (tacrolimus, pimecrolimus) and topical retinoids (tretinoin, tazarotene) have also shown to be effective. Antimalarials should be prescribed in patients with acute cutaneous flares, with discussion of ocular considerations below.[12][13]
Cicatricial entropion or ectropion may be managed surgically.[15]
Prognosis
The chronic inflammatory nature of SLE leads to possibility of recurrence.[15]
Keratoconjunctivitis Sicca
Background
Keratoconjunctivitis sicca in the context of SLE is believed to be due to chronic inflammation, immune complex deposition and, primarily, the development of secondary Sjogren’s syndrome. Primary Sjogren’s is manifested by the triad of dry eye, dry mouth, and parotid gland enlargement along with positive anti-SS-A or anti-SS-B antibodies. The presentation is similar in secondary Sjogren’s, though the etiology is related to the pathophysiology of SLE (described above).
Signs and Symptoms
Keratoconjunctivitis sicca, due to secondary Sjogren’s, is an aqueous tear deficiency. Symptoms include a burning or dry sensation, blurry vision, photophobia, worsening symptoms at the end of the day, and worsening symptoms with exposure to toxins (e.g., cigarette smoke). Signs may include conjunctival hyperemia, irregular corneal surface, and debris/mucus in the tear film.[4][5][16][17]
Diagnostic Testing
• Tear evaluation: A tear meniscus less than 1 mm or tear break up time under 10 seconds.
• Staining: Staining of conjunctiva can be done with fluorescein, rose bengal, or lissamine green to evaluate for corneal irregularities. Rose bengal and lissamine green may be preferred over fluorescein with subtle or fine conjunctival irregularities.
• Schirmer test: This test is used to evaluate tear production in a given time frame. The patient’s eye is first dried and then a Schirmer filter paper is placed in the lower fornix at the lateral one-third of the lower eyelid, adjacent to the lower palpebral conjunctiva. The filter paper is left in place for 5 minutes and the tear production is measured by saturation of the filter paper in millimeters. An un-anesthetized eye can be expected to produce sufficient tears to equal 15 mm of migration, while an anesthetized eye can be expected to produce at least 5 mm migration.
Treatment
Mild to moderate disease can be treated with artificial tears four times daily or preservative-free artificial tears hourly. A lubricating ointment may be added. More advanced disease may require punctal plugs and/or cyclosporine twice daily. Patients beginning treatment with cyclosporine should be advised that symptom improvement may take months to manifest. Less common treatments include the addition of autologous serum drops, short term topical corticosteroids, and/or tarsorrhaphy. These are typically reserved for severe cases, resistant to traditional therapies.[4][11][17]
Prognosis
Severe disease may lead to corneal thinning, with perforation possible. Band keratopathy may also develop.[17]
Lupus Retinopathy
Background
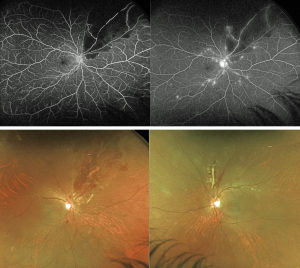
Lupus retinopathy is the most common posterior segment pathology in patients with SLE. It affects anywhere from 3-29% of patients, more likely to develop in patients with severe or chronically uncontrolled disease.[18][19] The pathophysiology of lupus retinopathy is twofold. First, patients with SLE are at risk of developing antiphospholipid syndrome leading to thrombosis of retinal vessels. Second, immune complex deposition in retinal vessels causes release of inflammatory mediators, leading to vasculitis.[18][19][20][21][22][23] Immune complex deposition at the vascular endothelium leads to complement activation and subsequent inflammatory mediators. Similarly, this inflammatory response can lead to nonperfusion and ischemia.
Anticardiolipin antibodies are relatively common in SLE, with around 40% of patients testing positive. However, in patients with known retinopathy, the percent with positive anticardiolipin can be as high as 86%, suggesting occlusive components play a significant role in its pathogenesis.[19][20][23]
Signs and Symptoms
Symptoms of lupus retinopathy range from asymptomatic fundoscopic changes to peripheral vision changes and/or decreased visual acuity. Retinopathy is thought to reflect the severity of systemic disease, with symptoms progressing with more severe, uncontrolled SLE.[19][23] CRAO/CRVO are less common than diffuse arteriolar and capillary nonperfusion.[19] Common presentations include cotton wool spots, microaneurysms, and hard exudates. This may be followed by ischemia, neovascularization and the potential for hemorrhage or retinal detachment.[6][18][20][21] As compared diabetic retinopathy and hypertensive retinopathy, the more occlusive nature of SLE retinopathy typically leads to more profound ischemia.[18]
Vasculitis may be identified in fundoscopic exam as sheathing of arterioles or venules or vascular tortuosity.[19][21]
Conditions that may mimic lupus retinopathy can be found in Table 3, along with a list of their distinguishing features.[24][25][26][27][28][29][30]
| Condition | Differentiating characteristics |
| Behçet’s disease | · History of oral or genital ulcers |
| Sarcoidosis | · Uveitis common |
| Lyme disease | · Annular skin lesions
· Endemic area |
| Hypertensive retinopathy | · AV nicking, copper wire vessels |
| Diabetic retinopathy | · History of elevated A1c |
| Polyarteritis nodosa | · More common in males
· ANCA negative |
| Syphilis | · Uveitis common
· Uniform distribution of inflammation in posterior pole |
Diagnostic Testing
In addition to fundoscopic examinations, patients with suspicion for retinal involvement or with active disease can undergo imaging to assist with diagnosis. The mainstay imaging has been fundus fluorescein angiography (FFA), which can be used to detect leakage, capillary dilation, blockages or microaneurysms. Indocyanine green may be used as an alternative, which can identify active choroidopathy with choroidal hyper-fluorescence when not visualized during exam or on FFA. Finally, the newer OCT-A could be advantageous in monitoring the structural changes in the retina, especially with subclinical retinopathy. However, as a newer imaging modality, its utility in SLE retinopathy has not been studied.[18][20][21]
Treatment
As development of retinopathy has been associated with poorly controlled disease, proper systemic disease treatment and management is paramount. Depending on the mechanism of the retinopathy, immunosuppressants and/or anticoagulant therapy may be advised.[6][11][19][21] Immunosuppression has shown to improve the appearance of retinopathy, but visual recovery is still not common. High-dose steroids should be used in patients with severe ocular manifestations as this is typically associated with high disease activity. Other treatments, such as plasmapheresis and plasma exchange have been utilized in severe disease.[6] Like other retinal occlusive diseases, PRP and/or vitreoretinal surgery may be indicated.[19][21]
Prognosis
Visual outcomes are often poor. Nearly 50% of patients end up with vision worse than 20/200, with vaso-occlusive disease leading to worse outcomes.[21]
Optic Neuritis
Background
Being a widespread, inflammatory disease, optic neuritis may occur in the context of SLE, affecting about 1% of patients.[31] Compared to other causes, SLE optic neuritis has a far worse prognosis with over half of all patients having a permanent central scotoma and eventual optic atrophy.[31][32]
Signs and Symptoms
Optic neuritis is characterized by monocular vision loss, occurring over days, and periorbital pain, exacerbated by eye movement. Almost all patients will experience dyschromatopsia, typically for red-green. Signs include RAPD in the affected eye, optic disc pallor or edema and possible central depression noted on perimetry.[33][34] As compared to other etiologies, namely multiple sclerosis, visual impairment is more profound with nearly 90% of patients below 20/200.[31]
Diagnostic Testing
Patients with suspected SLE optic neuritis can undergo fundus fluorescein angiography to note vascular changes, such as dilation of peripapillary capillaries, incomplete capillary and choroidal filling, or hyperfluorescent leakage. When FFA is either not diagnostic or normal, MRI may be warranted. Gadolinium-enhanced MRI imaging may show enlargement and enhancement of the optic nerves and/or chiasm. Also common are enhancing lesions noted in the anterior visual pathways.[32][35][36]
Treatment
Standard treatment currently is intravenous methylprednisolone boluses (1 g/day) for three days with transition to oral prednisone (1 mg/kg/day) with careful taper. Steroid-refractory disease may be present in up to a third of patients, necessitating use of IV pulse-dosed cyclophosphamide for six months.[31][35][37][38] Recrudescence is fairly common during steroid taper (37%) and re-treatment may be necessary.[31]
Prognosis
Prognosis is generally guarded in cases of SLE optic neuritis, with about 50% patients measuring a final VA ≥ 20/25 at resolution. Early detection and treatment (within 10 days) leads to a greater chance of visual recovery.[31]
Hydroxychloroquine/Chloroquine Considerations
Hydroxychloroquine and chloroquine are frequently prescribed in patients with SLE as they have great success in reducing lupus flares. Both their utility in SLE treatment and potential ocular complications have been well described.[39][40] These medications bind to the melanin in the RPE, prolonging their effect in the retina specifically and, as such, have the potential for maculopathy. Patients may notice paracentral scotomas, central visual decline or may appreciate difficulty reading. However, these symptoms are often preceded by loss of the foveal reflex and therefore are not reliable indicators of early toxicity.[33][39][40][41][42] Signs of toxicity include bilateral paracentral visual field defects, inner segment ellipsoid loss (“flying saucer” sign on SD-OCT), and progressive pigmentary changes +/- classic “bulls eye” maculopathy. Risk factors for maculopathy include: lifetime total dosage above 1,000 mg with hydroxychloroquine (or 450 mg of chloroquine), daily dose above 6.5 mg/kg, reduced liver or kidney function, obesity, age above 65, and previous maculopathy.[33][41] Hydroxychloroquine toxicity is not treatable, therefore early detection is paramount to preserve vision. The AAO recommends a baseline fundoscopic exam then annual exams after 5 years of use. A Humphrey 10-2 visual field exam along with an SD-OCT are advised at these exams with discontinuation of the medication if maculopathy is noted.[41][42]
Diagnosis
Diagnostic Testing
In addition to the diagnostic criteria outlined above, it is recommended those suspected of a SLE diagnosis should get a CBC, BMP, ANA testing with possible reflexive testing of anti-dsDNA, antiphospholipid antibodies, C3/C4 levels, ESR and/or CRP. Diagnostic testing in addition to a basic, dilated exam should be determined on a patient-by-patient basis.[10][3]
Differential Diagnosis
Because of the extremely variable presentation, SLE often takes several months before a definitive diagnosis is reached. However, differential diagnosis should include other autoimmune conditions (i.e., rheumatoid arthritis, mixed connective tissue disease, Sjogren’s syndrome), vasculitides, dermatomyositis/polymyositis, seronegative spondyloarthropathies, Behçet syndrome, infection, fibromyalgia, malignancies, and psychiatric disease.[10]
Management
Treatment
The first step in management of lupus involves lifestyle modifications including reducing sun exposure, healthy diet, and smoking cessation. In general, all patients with active disease should be treated with antimalarials, such as chloroquine or hydroxychloroquine (Plaquenil). Hydroxychloroquine is typically favored as it is less retinotoxic[3][8][10]. See the above discussion on ocular considerations related to antimalarial treatment. Depending on the severity of disease, patients may be prescribed oral steroid treatment, most commonly prednisone. Patients on extended steroid courses should have their eye pressure checked as IOP can increase on as few as 2 weeks of oral steroid use. Patients with known glaucoma may need more careful, frequent monitoring.[8][10][43] On top of these more generalizable recommendations, an individualized approach must be taken for each patient to address specific presenting symptoms. Along these lines, an expanded discussion of the treatment for the most common ophthalmologic conditions associated with lupus appears above.
Prognosis
The prognosis for patients with lupus is relatively good, with a five-year survival rate of over 90%. The worst prognosticator is renal involvement.[3]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Marquez, T. D., & Neto, D. U. (2012). Lupus: Symptoms, treatment and potential complications. Nova Science Publishers Incorporated.
- ↑ Jump up to: 2.0 2.1 Gordon, C., & Isenberg, D. (2016). Systemic lupus erythematosus. Oxford University Press.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Wallace, D. J. (2014). Lupus: The essential clinician's guide. Oxford University Press.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 Silpa-archa, S., Lee, J. J., & Foster, C. S. (2015). Ocular manifestations in systemic lupus erythematosus. British Journal of Ophthalmology, 100(1), 135–141.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Sivaraj, R. R., Durrani, O. M., Denniston, A. K., Murray, P. I., & Gordon, C. (2007). Ocular manifestations of systemic lupus erythematosus. Rheumatology, 46(12), 1757–1762.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Palejwala, N. V., Walia, H. S., & Yeh, S. (2012). Ocular manifestations of systemic lupus erythematosus: A review of the literature. Autoimmune Diseases, 2012, 1–9.
- ↑ Zucchi, D., Elefante, E., Calabresi, E., Signorini, Bortoluzzi, A., & Tani, C. (2019). One year in review 2019: systemic lupus erythematous. Clinical & Experimental Rheumatology, 37(5), 715–722.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Fortuna, G., & Brennan, M. T. (2013). Systemic lupus erythematosus. Dental Clinics of North America, 57(4), 631–655.
- ↑ Jump up to: 9.0 9.1 McPhee, S. J., Hammer, G. D., Gelber, A. C., Levine, S. M., & Darrah, E. (2019). Inflammatory Rheumatic Diseases. In Pathophysiology of disease: An introduction to clinical medicine. essay, McGraw-Hill Education Medical.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 Wallace, D. J., & Gladman, D. D. (2019, December 10). Clinical manifestations and diagnosis of systemic lupus erythematosus in adults. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-systemic-lupus-erythematosus-in-adults?_escaped_fragment_=.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 Dammacco, R. (2017). Systemic lupus erythematosus and ocular involvement: An overview. Clinical and Experimental Medicine, 18(2), 135–149.
- ↑ Jump up to: 12.0 12.1 Bolognia, J., Cerroni, L., Schaffer, J. V., Lee, L. A., & Werth, V. P. (2018). Lupus Erythematous. In Dermatology. essay, Elsevier.
- ↑ Jump up to: 13.0 13.1 Walling, H. W., & Sontheimer, R. D. (2009). Cutaneous lupus erythematosus. American Journal of Clinical Dermatology, 10(6), 365–381.
- ↑ Powers, D. B. (2008). Systemic lupus erythematosus and discoid lupus erythematosus. Oral and Maxillofacial Surgery Clinics of North America, 20(4), 651–662.
- ↑ Jump up to: 15.0 15.1 Foster, J. A. (2018). Orbit, eyelids, and Lacrimal system. American Academy of Ophthalmology.
- ↑ Jensen, J. L., Bergem, H. O., Gilboe, I.-M., Husby, G., & Axéll, T. (2007). Oral and ocular sicca symptoms and findings are prevalent in systemic lupus erythematosus. Journal of Oral Pathology & Medicine, 28(7), 317–322.
- ↑ Jump up to: 17.0 17.1 17.2 17.3 Weisenthal, R. W. (2018). External disease and cornea. American Academy of Ophthalmology.
- ↑ Jump up to: 18.0 18.1 18.2 18.3 18.4 Azevedo, L. G., Biancardi, A. L., Silva, R. A., Tavares, N. da, Abreu, M. M., Bica, B. E., & Moraes Júnior, H. V. (2021). Lupus retinopathy: Epidemiology and risk factors. Arquivos Brasileiros De Oftalmologia, 84(4).
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 Seth, G., Chengappa, K. G., Misra, D. P., Babu, R., Belani, P., Shanoj, K. C., Kumar, G., & Negi, V. S. (2018). Lupus retinopathy: A marker of active systemic lupus erythematosus. Rheumatology International, 38(8), 1495–1501.
- ↑ Jump up to: 20.0 20.1 20.2 20.3 Ushiyama, O. (2000). Retinal disease in patients with systemic lupus erythematosus. Annals of the Rheumatic Diseases, 59(9), 705–708.
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 21.5 21.6 Jabs, D. A., Fine, S. L., Hochberg, M. C., Newman, S. A., Heiner, G. G., & Stevens, M. B. (1986). Severe retinal VASO-OCCLUSIVE disease in systemic lupus erythematosus. Archives of Ophthalmology, 104(4), 558–563.
- ↑ Papagiannuli, E., Rhodes, B., Wallace, G. R., Gordon, C., Murray, P. I., & Denniston, A. K. (2016). Systemic lupus erythematosus: An update for ophthalmologists. Survey of Ophthalmology, 61(1), 65–82.
- ↑ Jump up to: 23.0 23.1 23.2 Stafford-Brady, F. J., Urowitz, M. B., Gladman, D. D., & Easterbrook, M. (1988). Lupus retinopathy. Arthritis & Rheumatism, 31(9), 1105–1110.
- ↑ Moarefi, M., Abdelaziz, M., Rostamizadeh, M., Ahmad, B., Palestine, A., Shukla, E. J., & Read, R. W. (2020, November 13). Behcet disease. EyeWiki. https://eyewiki.aao.org/Behcet_Disease.
- ↑ Hall, M. G., Bhat, N., Harish Bindiganavile, & Lee, A. G. (2021, June 28). Ocular manifestations of Sarcoidosis. EyeWiki. https://eyewiki.aao.org/Ocular_Manifestations_of_Sarcoidosis.
- ↑ Feldman, B. H., Rodriguez-Garcia, A., Lozano, A. F. I., Palestine, A., Altufeili, K. A., & Koretz, Z. (2021, February 3). Lyme disease. EyeWiki. https://eyewiki.aao.org/Lyme_Disease.
- ↑ Mehta, J., Feldman, B. H., Shah, V. A., Tripathy, K., Kiernan, D. F., Valikodath, N., Bhagat, N., Kim, J., & Lim, J. I. (2021, May 9). Hypertensive retinopathy. EyeWiki. https://eyewiki.aao.org/Hypertensive_Retinopathy.
- ↑ Feldman, B. H., Shah, V. A., Tripathy, K., Rana, H. S., Hsu, J., Tsui, J. C., & Lim, J. I. (2021, April 17). Diabetic retinopathy. EyeWiki. https://eyewiki.aao.org/Diabetic_Retinopathy.
- ↑ Vazquez-Romo, K. A., Rodriguez-Hernandez, A., Paczka, J. A., Nuño-Suarez, M. A., Rocha-Muñoz, A. D., & Zavala-Cerna, M. G. (2017). Optic neuropathy secondary TO Polyarteritis nodosa, Case report, and Diagnostic Challenges. Frontiers in Neurology, 8.
- ↑ Wiley, Z., Bhat, N., Bindiganavile, S. H., & Lee, A. G. (2019, September 20). Ophthalmologic manifestations of syphilis. EyeWiki. https://eyewiki.aao.org/Ophthalmologic_Manifestations_of_Syphilis.
- ↑ Jump up to: 31.0 31.1 31.2 31.3 31.4 31.5 Lin, Y.-C., Wang, A.-G., & Yen, M.-Y. (2009). Systemic lupus erythematosus-associated optic neuritis: Clinical experience and literature review. Acta Ophthalmologica, 87(2), 204–210.
- ↑ Jump up to: 32.0 32.1 Sagduyu, A., Çelebisoy, N., & Yünten, N. (2004). Optic neuropathy and chiasmopathy as presenting manifestations of systemic lupus erythematosus. Neuro-Ophthalmology, 28(4), 183–186.
- ↑ Jump up to: 33.0 33.1 33.2 Wajda, B. (2016). The wills eye manual. Lippincott Williams & Wilkins.
- ↑ American Academy of Ophthalmology. (2016). Neuro-ophthalmology.
- ↑ Jump up to: 35.0 35.1 Bertsias, G. K., Ioannidis, J. P., Aringer, M., Bollen, E., Bombardieri, S., Bruce, I. N., Cervera, R., Dalakas, M., Doria, A., Hanly, J. G., Huizinga, T. W., Isenberg, D., Kallenberg, C., Piette, J. C., Schneider, M., Scolding, N., Smolen, J., Stara, A., Tassiulas, I., … Boumpas, D. T. (2010). EULAR recommendations for the management of systemic lupus erythematosus with NEUROPSYCHIATRIC manifestations: Report of a task force of the EULAR standing Committee for clinical affairs. Annals of the Rheumatic Diseases, 69(12), 2074–2082.
- ↑ Heckman, A. J., Alsaad, A. A., Stewart, M. W., & Maniaci, M. J. (2019). Acute unilateral vision loss due to optic neuropathy in a patient with systemic lupus erythematosus. American Journal of Case Reports, 20, 97–100.
- ↑ Galindo-Rodrı́guez Griselda, Aviña-Zubieta, J. A., Pizarro, S., Dı́az de León Verónica, Saucedo, N., Fuentes, M., & Lavalle, C. (1999). Cyclophosphamide pulse therapy in optic neuritis due to systemic lupus erythematosus: An open trial. The American Journal of Medicine, 106(1), 65–69.
- ↑ Suri, D., Abujam, B., Gupta, A., Rawat, A., Saikia, B., Walker Minz, R., Gupta, V., Bansal, R., Kaushik, S., & Singh, S. (2015). Optic nerve involvement in childhood onset systemic lupus erythematosus: Three cases and a review of the literature. Lupus, 25(1), 93–96.
- ↑ Jump up to: 39.0 39.1 Watts, R. A., & Gordon, C. (2013). Systemic lupus erythematous- clinical features and aetiopathologies. In Oxford textbook of rheumatology. essay, Oxford University Press.
- ↑ Jump up to: 40.0 40.1 Wallace, D. J. (n.d.). Overview of the management and prognosis of systemic lupus erythematous in adults. Uptodate. https://www.uptodate.com/contents/overview-of-the-management-and-prognosis-of-systemic-lupus-erythematosus-in-adults#!
- ↑ Jump up to: 41.0 41.1 41.2 Ingraham, H. J. (2018). Update on general medicine. American Academy of Ophthalmology.
- ↑ Jump up to: 42.0 42.1 Lexicomp. (n.d.). Hydroxychloroquine: Drug information. Uptodate. https://www.uptodate.com/contents/hydroxychloroquine-drug-information/print#!
- ↑ Tsokos, G. C. (2011). Systemic lupus erythematosus. New England Journal of Medicine, 365(22), 2110–2121.