Toxic Optic Neuropathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Toxic optic neuropathy (TON) refers to visual impairment due to optic nerve damage caused by a toxin.[1] Toxic optic neuropathy is characterized by bilateral, usually symmetric vision loss, papillomacular bundle damage, central or cecocentral scotoma, and reduced color vision. This disease is often underdiagnosed or often diagnosed at a stage when recovery of vision is not possible.[2]
Epidemiology
The prevalence of toxic optic neuropathy varies among drug type and duration of usage. The effects are also usually dose-dependent.
Etiology
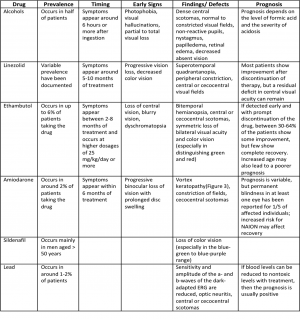
Any toxins, including drugs, metals, organic solvents, methanol, carbon dioxide, and tobacco. Below are the most common causes of toxic optic neuropathy[2]:
- Alcohols: Commercial alcohol, Methanol*, Ethylene glycol*
- Antibiotics: Chloramphenicol, Sulfonamides, Linezolid*
- Antimalarials: Hydroxychloroquine, Chloroquine, Quinine
- Antitubercular: Isoniazid, Ethambutol*, Streptomycin
- Antiarrhythmic: Digitalis, Amiodarone*
- Anticancer: Vincristine, Methotrexate, Tamoxifen
- PDE inhibitors: Sildenafil*
- Heavy metals: Lead*, Mercury, Thallium
- Other: Carbon Monoxide, Tobacco
* Please see Pathophysiology for selected drug mechanism and Management for selected drug treatment.
Risk Factors
Risk factors may include:
- Nutritional deficits, including the vitamins thiamine (B1), riboflavin (B2), niacin (B3), pyridoxine (B6), cobalamin (B12), folic acid, and proteins with sulfur-containing amino acids that can trigger or enhance toxic optic neuropathy
- The use of systemic medications in high doses or for a prolonged duration
- Exposure to a toxic substance in the environment.[1][2] Patients with decreased renal function or liver disease are at risk with certain substances.[1]
General pathology
The exact pathology is unknown. However, it is generally accepted that in most cases, the cause of the toxic neuropathy impairs the tissue’s vascular supply or metabolism. In addition, a common feature shared by some of these toxins is mitochondrial injury and an imbalance of intracellular and extracellular-free radical homeostasis.[1][2]
Pathophysiology
There are many causes of toxic optic neuropathy, but below are the selected drugs for review due to their prevalence in clinical practice:
- Alcohols (commercial alcohol, methanol, and ethylene glycol): Chronic alcoholism leads to vitamin B12 or folate deficiency. Over time, these deficiencies can cause accumulations of formic acid, which inhibits the electron transport chain and mitochondrial function. As a result, ATP production decreases and impairs the ATP-dependent axonal transport system. In methanol specifically, the drug can cause focal retrolaminar optic nerve delamination.[2] Poisoning with methyl alcohol can cause brain edema, hemorrhages, and an increase in cerebrospinal fluid. Reduced size of the ganglion cells and cortical atrophy has been observed in experimental animals, with optic nerve and cerebral involvement more than that of the kidney, liver, or muscles. Methyl alcohol is excreted very slowly and up to 1/3 may remain in the tissues after 48 hours of ingestion.[3]
- Linezolid: Prolonged linezolid usage can interfere with mammalian ribosomes and lead to the disruption of mitochondrial oxidative phosphorylation and protein synthesis.[4]
- Ethambutol: The chelating property of ethambutol has adverse effects on human cells. The exact mechanism is unknown, but it has been hypothesized that the induced optic neuropathy may possibly be due to a disruption of oxidative phosphorylation secondary to decreased free copper in the mitochondria or the inhibition of lysosomal activation due to the chelation of zinc.[5] This damage is irreversible.
- Amiodarone: Amiodarone can interact with polar lipids and accumulate as inclusion bodies in the optic nerve, leading to optic disc swelling and induced optic neuropathy.[6] Histological evaluation may reveal inclusion elements distributed in a lamellar fashion in the large axons of the optic nerve. In contrast, toxicity of amiodarone in peripheral nerves has been shown to induce loss of axons and demyelination. One hypothesis of these element deposits is the inhibition of lysosomal sphingomyelinases . Amiodarone deposits can be visible in the cornea in 90% of patients taking amiodarone.[7]
- Sildenafil: Sildenafil acts as an inhibitor of phosphodiesterase and when used at high therapeutic doses, it can influence individual steps of the phototransduction process.[8] It has been shown to increase the risk for non-arteritic anterior ischemic optic neuropathy (NAION) more than a direct toxicity to the optic nerve.[9]
- Lead: Lead can bind to specific target proteins, which results in a change in protein structure and function. It can also bind to melanin in the retinal pigment epithelium, choroid, iris, and ciliary body with high affinity and disrupt cellular biochemistry. Recent evidence also indicates that it can induce oxidative stress that result in lipid peroxidation, DNA damage, and depletion of cell antioxidant defense systems. These events make the optic nerve susceptible to optic neuropathy. In addition, low-level lead exposure produces scotopic vision deterioration and rod and bipolar apoptopic cell death.[10]
Diagnosis
Toxic optic neuropathy is a diagnosis of exclusion, with patients typically presenting with[1][2]:
- A history of drug, medications, or alcohol use
- Painless, bilateral, and symmetric vision loss
- Progressive loss of visual acuity that usually starts with a blur at the point of fixation
- Dyschromatopsia
- Central or cecocentral scotoma with preservation of the peripheral fields
- Papillomacular bundle loss
History
A detailed medical history is significant in elucidating toxic neuropathy and establishing the diagnosis. The following are important factors to consider when taking patient history[1][8]:
- Drug/toxin exposure in the workplace or environment
- Use of systemic medication – dosage and duration
- Use of tobacco and alcohol
- Metabolic disease, including but not limited to diabetes mellitus, kidney failure, liver disease, and thyroid disease
- Family history to rule out hereditary optic nerve disorders
- Any sensory disturbances in the peripheral nervous system
- Headache, vertigo, or loss of hearing
Physical examination[1][2]
- Humphrey or Goldman visual field evaluation
- Snellen visual acuity
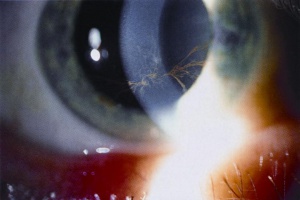
- Slit lam examination may show deposits of the drug, sus as Amiodarone. Figure 1. Vortex Keratopathy from Amiodarone crystals deposition. Obtained from AAO.org.
- Swinging flashlight test for relative afferent pupillary defect (RAPD)
- Direct ophthalmoscopy of the optic nerve and retina
Signs[2]
- No RAPD, when bilateral disease
- Normal, swollen, or hyperemic optic disc in early detection
- Temporal optic disc pallor in late detection
- Centrocecal scotoma in visual fields with preservation of the peripheral field. In rare cases, however, the peripheral field may be involved in case of Ethambutol causing bitemporal hemianopsia[11]
- Decreased visual acuity
- Greater impairment of blue/yellow hue discrimination than red/green if the drug targets a loss of perifoveal visual field
- Greater impairment of red/green hue discrimination than blue/yellow if the drug affects pathways dense in foveal cones
- Photophobia
- Abnormal Visual Evoked Potentials (VEP) or Electroretinogram (ERG)
- Loss of retinal nerve fibers on Optical Coherence Tomography (OCT) . Figure 2 shows an example of retinal nerve fiber thinning due to ethambutol and linezolid toxic optic neuropathy. Permission obtained from Dr Jane Libershteyn for publication March 18th,2018.
Symptoms[1][8]
- Loss of central or paracentral visual acuity
- Reduced contrast perception
- General loss of color perception, particularly red
- Photophobia
- Poor dark adaptation
Diagnostic procedures[1][2]
- Magnetic Resonance Imaging (MRI) of the optic nerves and chiasm with and without gadolinium contrast – An MRI study is usually required to rule out other causes, such as a compressive lesion, and to confirm the diagnosis of toxic optic neuropathy, especially when the etiology is unclear.
- Electrophysiological tests (VEP or PERG) – These tests can be useful in patients with early or subclinical optic neuropathy. In addition, it can be used to differentiate optic neuropathy from demyelinating disease and macular lesions.
- Optical coherence tomography – OCT is a helpful diagnostic tool that can detect signs of early toxicity, even before the fundus changes become apparent. It can quantify the loss of retinal nerve fibers and be used to monitor patients who are at risk.
- Ishihara plates or Panel D 15 test – The Panel D 15 test can detect a preferential loss of red/green hue discrimination and be used to diagnose early toxicity and prevent further optic neuropathy.
Laboratory test[1][2]
- Complete blood culture and urinalysis to screen for specific toxins
- Heavy metal screening if heavy metal toxicity is suspected
- Serum B-12 and red cell folate levels if the patient presents with bilateral central scotomas
Differential diagnosis
- NAION – The clinical presentation of NAION may be similar to amiodarone induced optic neuropathy. However, in amiodarone induced optic neuropathy, patients are typically male[7], have a slower and more progressive onset, a milder degree of visual loss, a longer duration of disc edema, and is more commonly bilateral than NAION. But due to vascular comorbidities, patients on amiodarone may also be at greater risk for NAION.[6]
- Nutritional optic neuropathy – anemia, polyneuropathy, and vitamin deficiencies[1]
- Leber hereditary optic neuropathy (LHON) and dominant optic atrophy – mitochondrial inheritance, degeneration of retinal ganglion cells, acute or subacute loss of central vision, usually bilateral sequential, predominantly occurs in young adult males[2]
- Lesion of the optic chiasm – blurred vision, unilateral optic disk pallor, RAPD [12]
- Bilateral inflammatory or demyelinating optic neuropathy – optic neuritis, RAPD, periorbital pain
- Maculopathies/macular dystrophies – loss of central vision
- Retinal degenerations – retinal detachment, photophobia, loss of peripheral vision
Management
Screening
- Alcohols – Screen for blood levels of formic acid and cyanide.[2]
- Linezolid – Patients who are on a multi-drug-resistant tuberculosis regimen should undergo a full ophthalmic evaluation, including visual acuity screening, slit lamp examination, and a dilated fundus examination.[13]
- Ethambutol – Patients who are taking ethambutol and asymptomatic should receive monthly screenings, including visual acuity and Amsler grid testing. For patients who are taking ethambutol and symptomatic, formal visual field testing and a full ophthalmic evaluation should also be included.[5]
- Amiodarone – Patients should be screened before given amiodarone. They should subsequently be evaluated at least every 6 months for changes in vision and disc swelling.[2]
- Lead – Screen for blood levels if exposure and toxicity is suspected.[2]
Treatment
The immediate step in treating toxic optic neuropathy is to remove the offending agent. In many cases, removal of the offending agent can reverse the process of optic neuropathy. Because toxic optic neuropathy can be multifactorial, the treatment is mainly dictated by the specific toxin. Treatment of the selected drugs for review are below:
- Alcohols (methanol and ethylene glycol): Use buffers like sodium bicarbonate to correct metabolic acidosis and an antidote such as ethanol; hemodialysis may be necessary in some cases if metabolic acidosis is severe. IV pulse steroids have shown promising results in salvaging vision in some patients.[2]
- Linezolid: Discontinuation of treatment, especially if the patient has been on the drug for at least 5-10 months.[14]
- Ethambutol: No specific treatment is available other than stopping the drug.[2]
- Amiodarone: Must weigh the visual complications with the cardiac benefits before discontinuing the drug, since the only way to stop optic nerve toxicity is stopping the medication.[2]
- Sildenafil: Limit the use of sildenafil if there are predisposing cardiovascular risk factors.
- Lead: Remove the source of exposure. Treat with a chelating agent for heavy metals, such as penicillamine, intramuscular dimercaprol, or disodium calcium EDTA. For patients with encephalopathy, consider treatment with oral succimer.[15][16]
Patients should be monitored every 4-6 weeks and depending on their recovery, every 6-12 months thereafter. Check-up examinations should include the patient’s visual acuity, pupils, optic nerves, color vision, and visual fields.[2]
Medical therapy
Depending on the offending agent, medical therapy can include vitamin supplementation, which is most often prescribed in patients with tobacco alcohol amblyopia.[2]
Prognosis
The prognosis varies by patient, depending on the type of drug, drug dose, duration of drug usage, and time of diagnosis. However, if detected early enough, patients can regain their vision over several weeks or sometimes months for full recovery, except for ethambutol toxicity. Visual acuity usually recovers before color vision (2).
Additional Resources
- Fox DA, Boyes W (2001) Toxic responses of the ocular and visual system. In: Klaassen CD (ed) Casarett & Doull’s toxicology: the basic science of poisons. McGraw-Hill, New York.
- Fraunfelder FT (2000) Drug-induced ocular side effects. Butterworth- Heinemann, Boston.
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Grzybowski A, Zülsdorff M, Wilhelm H, Tonagel F (2015): Toxic optic neuropathies: an updated review. Acta Ophthalmol 93: 402-410.
- ↑ Jump up to: 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 Sharma P, Sharma R (2011): Toxic optic neuropathy. Indian J Ophthalmol 59: 137-141.
- ↑ Walsh F, Hoyt W (2005). Neurotoxic substances: Methyl Alcohol. In: Rizo M & Barton J (eds), Walsh and hoyt's clinical neuro-ophthalmology (6th ed. Vol 3. pp: 2582-2586). Philadelphia, PA: Lippincott Williams & Wilkins.
- ↑ Karuppannasamy D, Raghuram A, Sundar D (2014): Linezolid-induced optic neuropathy. Indian J Ophthalmol 62: 497-500.
- ↑ Jump up to: 5.0 5.1 Chamberlain PD, Sadaka A, Berry S, Lee AG (2017): Ethambutol optic neuropathy. Current Opinion in Ophthalmology 28: 545-551.
- ↑ Jump up to: 6.0 6.1 Nagra PK, Foroozan R, Savino PJ, Castillo I, Sergott RC (2003): Amiodarone induced optic neuropathy. Br J Ophthalmol 87: 420-422.
- ↑ Jump up to: 7.0 7.1 Passman RS, Bennett CL, Purpura JM, Kapur R, Johnson LN, Raisch DW,…McKoy JM (2012): Amiodarone-Associated Optic Neuropathy: A Critical Review. Am J Med 125: 447–453.
- ↑ Jump up to: 8.0 8.1 8.2 Zrenner E, Hart W. Drug-Induced and Toxic Disorders in Neuro-Ophthalmology, Schiefer, Wilheim and Hart, Clinical Neuro-Ophthalmology - A Practical Guide. Berlin: Springer; 2007.
- ↑ Gorkin L, Hvidsten K, Sobel R, Siegel R (2006): Sildenafil citrate use and the incidence of nonarteritic anterior ischemic optic neuropathy. International Journal of Clinical Practice 60: 500-503.
- ↑ Erie JC, Butz JA, Good JA, Erie EA, Burritt MF, Cameron JD (2005): Heavy Metal Concentrations in Human eyes. American Journal of Ophthalmology 139: 888-893.
- ↑ Kho RC, Al-Obailan M, Arnold AC. Bitemporal visual field defects in ethambutol-induced optic neuropathy. J Neuroophthalmol. 2011 Jun;31(2):121-6. doi: 10.1097/WNO.0b013e318205a148.
- ↑ Meijico LJ, Miller NR, Dong LM (2004): Clinical features associated with lesions other than pituitary adenoma in patients with optic chiasmal syndrome. American Journal of Ophthalmology 137: 908-913.
- ↑ Mehta S, Das M, Laxmeshwar C, Jonckheere S, Thi SS, Isaakidis P (2016): Linezolid- Associated Optic Neuropathy in Drug-Resistant Tuberculosis Patients in Mumbai, India. PLoS ONE 11: e0162138.
- ↑ Libershteyn Y (2015): Ethambutol/Linezolid Toxic Optic Neuropathy. Optometry and Vision Science 93: 211-217.
- ↑ Baghdassarian SA (1968): Optic Neuropathy Due to Lead Poisoning. Arch Ophthal 80: 721-723.
- ↑ Fintak DR (2007, January 30). Wills Eye Resident Case Series. Review of Ophthalmology. https://www.reviewofophthalmology.com/article/wills-eye-resident-case-series-24966