Internuclear Ophthalmoplegia
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Introduction
Internuclear ophthalmoplegia or ophthalmoparesis (INO) is an ocular movement disorder that presents as an inability to perform conjugate lateral gaze and ophthalmoplegia due to damage to the interneuron between 2 nuclei of cranial nerves (CN) VI and CN III (internuclear).[1] This interneuron is called the medial longitudinal fasciculus (MLF) and it may be affected by ischemia, demyelination, and ischemia, among others.
Anatomy
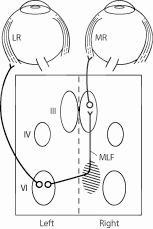
The MLF is a heavily myelinated nerve tract connecting the oculomotor nucleus (CN III) of the ipsilateral side with the paramedian pontine reticular formation (PPRF) and CN VI in the contralateral pons. Thus, demyelinating lesions in the midbrain or pons often produce a unilateral or bilateral INO, usually in young patients. The MLF is located at the dorsomedial brainstem tegmentum (midbrain and pons) ventral to the aqueduct or the fourth ventricle. MLF lies very near to the midline. As both MLFs are close to each other and near to midline, bilateral INO is not rare.
Foveation and binocular single vision of the mobile and immobile objects require coordination between the cranial nerves (II, III, IV, and VI), their interneurons, and various supranuclear influences. CN VI is the final common pathway for lateral horizontal gaze. The PPRF or paraabducens nucleus is the key structure in conjugate horizontal gaze and horizontal saccades. Damage to the PPRF results in impaired horizontal saccade in the same direction. Typically, the PPRF receives information from the higher cortical centers such as the frontal eye fields, occipital and parietal lobes, and the superior colliculus. The frontal eye field controls contralateral saccades and the parietal lobe controls ipsilateral pursuits.[2]
PPRF sends a signal to the CN VI. Some axons from the CN VI innervate the ipsilateral lateral rectus, resulting in the abduction of the ipsilateral eye. The signal for contraction of the contralateral medial rectus (for adduction of the contralateral eye) is sent from the CN VI to the contralateral medial rectus subnucleus of CN III via the internuclear pathway called MLF. The activation of the contralateral medial rectus and ipsilateral lateral rectus muscle produces horizontal conjugate eye movement. The side of the INO is named by the side of the adduction deficit, which is ipsilateral to the MLF lesion. The MLF is also involved in multiple other functions including oculovestibular reflex, vertical pursuit, and optokinetic (OKN) nystagmus, and it coordinates the conjugate ocular movements in response to movement of the head and neck.[3]
Clinical features
Symptoms
Symptoms of INO may vary in severity. They may include horizontal diplopia, difficulty in tracking high-speed objects, or dizziness on lateral gaze. Horizontal diplopia is caused by the limitation of adduction in the ipsilateral eye. The diplopia becomes more prominent when the patient looks at objects on the opposite side of the lesion, but it is not usually present in the primary gaze. The patient may also complain of headaches or other deficits due to the involvement of the brainstem. Vertical oblique diplopia related to associated skew deviation may also be another symptom. Interruption of binocular vision resulting from defective conjugate horizontal movements of eyes may cause reading fatigue, visual confusion, loss of stereopsis, oscillopsia, and diplopia. The difficulty in looking at the sides may lead to difficulties during driving or walking and may increase the risk of road traffic accidents or falls. Patients may complain of headaches and vertigo.
Signs
A good ocular examination is often all that is required to diagnose INO. The INO is characterized clinically by an ipsilesional adduction deficit (partial or complete) with a contralateral, dissociated, horizontal abducting saccade or nystagmus on attempted gaze to the contralesional side. There is a slow adducting saccadic velocity in the affected side. A skew deviation with the ipsilateral hypertropic eye may be noted. Vertical gaze nystagmus may be noted on upgaze. The INO can be unilateral or bilateral and may present with or without (neurologically isolated) other brainstem findings.
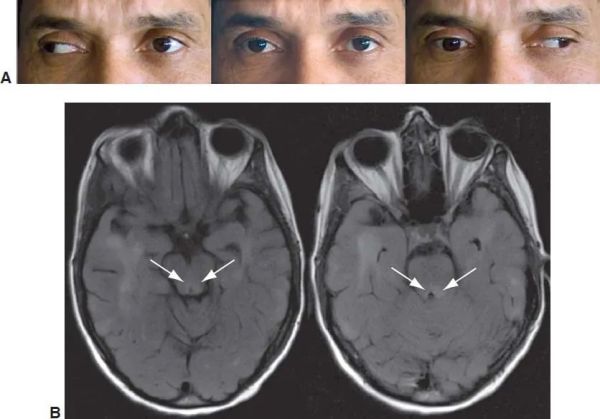
Ipsilesional adduction deficit (partial or complete)
The partial adduction defect may be made clinically more apparent by performing horizontal saccades. There is adduction lag or reduced adducting saccadic velocity on the ipsilateral eye. The OKN drum also can reveal such a subtle reduction in adducting saccadic velocity. The defective input to the medial rectus subnucleus of CN III from the CN VI via MLF causes this ipsilesional adduction deficit or lag.
Contralateral, dissociated, horizontal abducting saccade/nystagmus on attempted gaze to the contralesional side
Hering’s law of equal innervation has been hypothesized as a possible explanation for the dissociated contralateral horizontal gaze-evoked nystagmus in the abducting eye. Increased innervation to the underacting adducting muscle would result in an enhanced stimulus to the contralateral abducting muscle.[5] Damage to the adjacent structures including the vestibular nucleus may also contribute to gaze-evoked nystagmus. Dissociation of both eyes (abducting nystagmus in the opposite eye and the ipsilateral eye does not usually show clinically visible nystagmus on adduction) has been hypothesized to be caused by adductor weakness in the ipsilateral eye. Electro-ocular techniques may detect subclinical nystagmus in the eye with adduction deficit (ipsilateral eye).
Abnormal vertical eye movements
These may be present in INO and are not necessary for the diagnosis of INO. These are usually seen with bilateral INO and may represent INO plus syndromes. Such abnormal vertical ocular movements include:[3]
- hypertropic skew deviation in the ipsilateral eye due to pontine lesions (with incyclotorsion in the ipsilateral eye)[6]
- abnormal OKN nystagmus and pursuits
- vertical gaze-evoked nystagmus on upgaze
- abnormal vestibulo-ocular reflex
- contraversive ocular tilt reaction[7]
- reduced vertical gaze holding
- oblique saccades in adduction[8]
Normal convergence
Usually, convergence is not affected, even in the eye with adduction deficit in horizontal gaze. This is called ''dissociation of convergence.'' This is due to the sparing of the convergence pathway, including the fibers from the medial rectus subnucleus of the CN III in INO. However, the absence of convergence does not rule out the diagnosis of INO. Previously, the anterior INO of Cogan was thought to arise from a lesion at the level of the CN III nucleus, and it was characterized by the absence of convergence.[9] Posterior INO of Cogan was thought to involve the MLF below the CN III nucleus, and the convergence was preserved. However, recent studies have found this rule to be debatable.[10]
[Video Courtesy: Moran CORE, University of Utah, https://morancore.utah.edu/]
Associated Syndromes:
WEBINO
Location of lesion: Bilateral INO
Wall-eyed bilateral internuclear ophthalmoplegia exists when there is bilateral damage to the MLF.[11] This damage causes a primary position exotropia (eyes are looking at the opposite “wall,” thus the possibly outdated term “wall-eyed”). The most common etiology is infarction of the midbrain in older patients and demyelinating disease in young patients.[12] The exotropia (XT) is likely due to decompensation of fusional mechanisms, and the XT is not present in every case of bilateral INO. Bilateral INO may also show vertical gaze-evoked nystagmus on superior gaze. Such nystagmus occurs because of the lesion in the ascending tracks (involved in gaze holding and vertical vestibular pursuit) from the vestibular nuclei. Bilateral INO is characterized by bilateral adduction lag and bilateral abduction nystagmus.
See video: [1] https://www.aao.org/master-class-video/bilateral-internuclear-ophthalmoplegia-ino
WEMINO
Location of lesion: Monocular INO
This is a less common variant of INO, similar to the WEBINO above.[7][13] As in WEBINO, patients with a unilateral MLF lesion (monocular INO) have a primary position XT.[7]
Location of lesion: The MLF and the PPRF and/or CN VI nucleus on the same side
This syndrome[14] is characterized by no horizontal movement in the ipsilateral eye. The contralateral eye's abduction is preserved, while adduction is null and there is an abduction nystagmus.[15] However, the convergence, elevation, and depression on both eyes are usually intact. In primary gaze, the contralateral eye is exotropic (paralytic pontine exotropia).[3] When the CN VI nucleus is spared, on volitional horizontal gaze, a gaze palsy is present. However, the vestibulo-ocular reflex[16] and ocular response to caloric stimulus in the ear are normal.
[Video Courtesy: Instant Neuro, lesion on the right side]
Location of lesion: The MLF, the PPRF, and/or CN VI nucleus, and facial fascicular nerve (CN VII) on the same side
This syndrome[17] is characterized by having one-and-a-half syndrome and a facial fascicular nerve (CN VII) palsy. The fascicle of CN VII wraps around the nucleus of CN VI in the dorsal pons. The ipsilateral eye does not have any horizontal gaze movement and there is ipsilateral lower motor neuron CN VII palsy. The contralateral eye does not have adduction as a part of horizontal gaze palsy, but this eye abducts with abduction nystagmus. The proximity of the PPRF, facial nerve nucleus, and MLF located in the dorsal pons makes this syndrome much more likely. The lesion is most often vascular or demyelinating in the dorsal tegmentum of the caudal pons.[18]
[Video Courtesy: JAMA Network, Lesion is on the left side. Skaat A, Huna-Baron R. Eight-and-a-half syndrome: a rare pontine neuro-ophthalmologic syndrome. Arch Neurol. 2012 Jul;69(7):934-5. doi: 10.1001/archneurol.2011.2185. PMID: 22393168.]
Half-and-Half Syndrome:
Location of lesion: MLF and fascicle of CN VI
This syndrome[19] consists of an INO in 1 eye, combined with an ipsilateral CN VI fascicular involvement with sparing of the sixth nerve nucleus. Thus, there is “half” of a horizontal gaze palsy (INO) plus an additional “half” (abduction deficit from CN VI fascicular palsy).
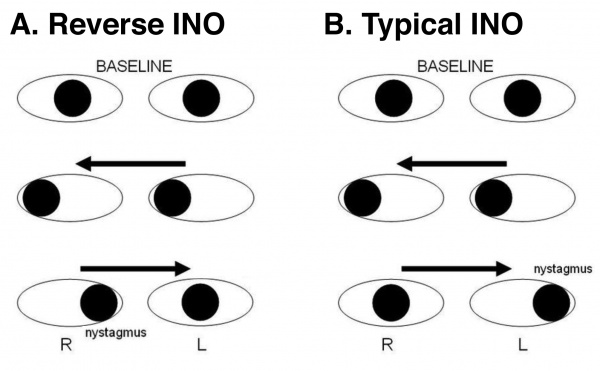
Internuclear Ophthalmoplegia of Abduction (Lutz Posterior INO)
Location of lesion: Variable (including paramedian pontine rostral formation (PPRF),[20] pontine MLF,[21] pre-nuclear rostral pons,[22] abducens fasiculus, and midbrain.[21] )
Other names for this entity include reverse INO and pseudo abducens palsy. This syndrome is a rare ophthalmoplegia, either bilateral or unilateral, that exhibits contralateral adducting eye (rather than abducting eye) nystagmus with abduction restriction on physical examination. It is the reverse of the typical INO, and although the lesion localization is not consistent, it likely is due to CN VI pre-nuclear input asymmetry.[23]
Etiology
A lesion in the MLF interrupts the neural communication to the CN III subnuclei that allows for conjugate horizontal gaze, arising from the final common pathway for horizontal gaze (CN VI nuclei). The MLF can be damaged by any lesion (eg, demyelinating, ischemic, neoplastic, inflammatory) in the pons or midbrain. The MLF is supplied by branches of the basilar artery, and ischemia in the vertebrobasilar system can produce an ischemic INO.[24]
An INO is a common presentation of multiple sclerosis (MS) in younger patients. Demyelination of the MLF in MS can involve any segment and is frequently bilateral. [25] Internuclear ophthalmoplegia can serve as an important sign for diagnosing MS, because it can occur as its first symptom. [26] The relationship between INO and MS has also been extended, showing that INO can be used as a biomarker of axonal and myelin integrity in MS. [27]
Additionally, other demyelinating etiologies such as myelin oligodendrocyte glycoprotein-IgG-associated disorder (MOGAD) [28] and neuromyelitis optica (NMO) previously known as “Devic’s disease” with unilateral or bilateral involvement,[29] even though their most typical neuro-ophthalmologic finding is optic neuritis.
Less common etiologies for an INO can be traumatic, neoplastic, inflammatory (eg, sarcoid, Behçet disease, lupus), infectious [eg, cryptococcosis, Borrelia burgdorferi [Lyme disease]), nutritional, or metabolic.[30] [31]
Wernekink commissure syndrome is a rare mesencephalic syndrome caused by lesions to the Wernekink commissure, which is the decussation of the superior cerebral peduncles. INO is the most frequent ophthalmoplegia associated with Wernekink commissure syndrome, due to its adjacency to the MLF. This is a rare syndrome caused by the rich blood supply to the midbrain preventing a pure midbrain infarct. Both Wernekink commissure and the MLF are supplied by the inferior paramedian mesencephalic arteries (IPMAs). [32]
INO is rare in children, but it may result from neoplasms (eg, medulloblastomas or pontine gliomas), hydrocephalus, or similar etiologies to those in adults. [33] In 1 review of INO, some of the most common causes included: 1) Infarction - 38%- Unilateral INO in 87% of cases; 2) Multiple sclerosis in 34% - Unilateral in 27%; and 3) Unusual causes included trauma, tentorial herniation, infection, tumor, iatrogenic injury, hemorrhage, and vasculitis. [34] The vascular lesions causing dorsal brainstem infarcts may involve the basilar artery, superior cerebellar artery, and posterior cerebellar artery.[24]
In the elderly population, medial longitudinal fasciculus lesions are most commonly due to CVA with a typical age of onset of 62 to 66 years. The MLF is found in the dorsal brainstem and projects to the nuclei controlling the extraocular muscles for CN VI and CN III. The CN VI nucleus is in the inferior aspect of the dorsal pons. This region is supplied by the small perforating paramedian arteries arising from the basilar artery. The CN III nucleus is in the midbrain and is supplied by the small perforating branches of the P2 segment of the posterior cerebral artery. Small embolic events, hypertensive stroke, vascular dissection, and vasculitides in these arteries are all potential causes of CVA that implicate the MLF. [35], [36]
The integrity of convergence can be used to differentiate between anterior (midbrain) and posterior (pons) INO. If the lesion to the MLF occurs at the level of the CN III nucleus (anterior INO) convergence is impaired. A lesion occurring below the CN III nucleus (posterior INO) will present with intact convergence. [37]
| Most common causes (around 70%) |
|
| Infections |
|
| Inflammation |
|
| Trauma |
|
| Congenital malformations |
|
| Neoplasia/ tumors |
|
| Nutritional disorders |
|
| Toxicity of drugs |
|
| Degenerative conditions | |
| Metabolic conditions |
|
| Other conditions |
|
Diagnosis
Evaluation
The diagnosis is made clinically with testing of the eye’s ability to perform conjugate movements.
Saccadic velocity and OKN drums or tapes are important tests to detect subtle disconjugate saccades in the affected eye.
Quantitative infrared oculography may increase the precision for the diagnosis of subtle INO cases. In a study, INO was not identified in cases with mild slowing of adduction velocity by 71% of physician observers. [38] Twenty-five percent of the physician observers could not detect intermediate disconjugacy.[38]
Imaging tests, such as a CT (computed tomography) scan or MRI (magnetic resonance imaging) brain (stroke protocol and with contrast for demyelination), are ordered after a diagnosis is made to discover where the damage is located so that the physician can then assess which approach to take. In general, MRI is superior to CT scan for evaluation of INO. MRI should involve fine overlapping cuts to ensure detection of lesions that may be very small and may otherwise be missed. Proton density imaging was noted to be the preferred modality to identify MLF lesion in multiple sclerosis with INO when compared to T2-weighted imaging, and fluid-attenuated inversion recovery (FLAIR) imaging.[39] Diffusion-weighted imaging with MRI may detect brainstem infarcts causing INO that may not be detected with T2-weighted imaging alone.[24]
Differential Diagnosis
The differential diagnoses include
- Pseudo-INO: This is usually seen in established cases of myasthenia gravis[40][41] and in Guillain-Barré syndrome.[42] Pseudo-INO may also be the presenting feature of these disorders. However, the vertical nystagmus on the upgaze is not seen. Myasthenia gravis is characterized by variable ptosis, fatigue of the upper lid, reduced response on repetitive nerve stimulation test, and presence of anti-acetylcholine receptor antibody. The adduction limitation of myasthenia typically improves with intravenous edrophonium or with appropriate therapy of myasthenia. The Miller-Fisher variant of Guillain-Barré syndrome is characterized by a triad of areflexia, ataxia, and ophthalmoplegia.
- Third nerve palsy: INO may simulate partial third nerve palsy. CN III palsy is also associated with pupillary dilation, loss of accommodation, ptosis, absence of abduction nystagmus in the fellow eye, impaired convergence, and limitation of elevation.
- Progressive supranuclear palsy (PSP): Abnormal ocular movements similar to binocular INO may be seen in PSP. Features of Parkinsonism may be noted. The ocular movement abnormalities may be overcome by oculocephalic maneuvers in PSP (and not in INO) as the lesion is supranuclear.
- Chiari malformation
- Other INO-mimics: There are reports on patients with Idiopathic intracranial hypertension (IIH) in association with INO, [43] as well as INO occurrence after placement of a fourth ventricle shunt for a dilated and ballooned fourth ventricle where the hypothesized mechanism was acute decompression affecting the MLF located in the floor of the fourth ventricle.[44] [45] Rarely, patients with orbital metastasis may present mimicking INO, as in 2 reports of rectal adenocarcinoma and urothelial carcinoma of the bladder. [46] [47] This confirms the importance of obtaining brain imaging.
More recently, medical treatment of cancers with the immune checkpoint inhibitor nivolumab has been associated with side effects of INO with an unremarkable MRI. [48],[49],[50]
Management
Dalfampridine, a potassium channel blocker prescribed for gait impairment, was used in a case series, and the authors reported improvement in saccades. [51] binocular conjugacy, and ocular motility in patients with INO secondary to demyelination in multiple sclerosis.[52] Improved neuronal conduction along MLF has been discussed as a possible explanation for this effect.[53] Some patients may also report "visual improvement" after using this drug.[53] If the etiology of INO is inflammation or infection, high-dose corticosteroids can help. If the etiology is MS and is not relapsing-remitting, then treatment becomes harder. Patients with WEBINO or WEMINO may benefit from patching, prism, or strabismus surgery to correct any residual primary position symptomatic deviation (XT) that does not recover.[54] Injection of botulinum toxin in 1 or 2 extraocular muscles may temporarily reduce the diplopia and improve binocular function in INO.[54] Prisms may not be helpful for most of the patients with INO.[3] Bothersome diplopia can be avoided by patching 1 eye. The patients can be taught to move the head to compensate for the defective adduction.
Prognosis
The prognosis of most patients with an INO is good, and most cases improve with time, but the final outcome depends in part on the treatment of the underlying etiology. Ischemic and demyelinating INO typically recover. All patients achieved resolution of INO within 1 day to 1 year in a study of 30 patients with INO.[24] Rapid recovery is usually seen in the absence of other neurological signs including facial palsy, ataxia, hemiparesis, vertigo, pyramidal tract dysfunction, sensory symptoms, dysarthria, and absent lesion in T2-weighted imaging .[55] [56] In a series of 65 patients with INO, persistence of INO was noted in around half of the patients, even after 1 year.[56] Another study noted that around 80% of patients with ischemic INO had resolution of diplopia in the primary position. The average time for resolution was 2.25 months.[55]
Suggested Reading
- https://www.uptodate.com/contents/internuclear-ophthalmoparesis
- https://www.ncbi.nlm.nih.gov/books/NBK441970/
References
- ↑ Feroze KB, Wang J. Internuclear Ophthalmoplegia. 2021 Jun 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 28722999.
- ↑ Sarkar S, Tripathy K. Cortical Blindness. [Updated 2022 Feb 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560626/
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Frohman TC, Petzold A, Frohman EM. Internuclear ophthalmoparesis [Internet]. Available from: https://www.uptodate.com/contents/internuclear-ophthalmoparesis
- ↑ Bilateral internuclear ophthalmoplegia. American Academy of Ophthalmology. https://www.aao.org/education/image/bilateral-internuclear-ophthalmoplegia Accessed October 1, 2024.
- ↑ Zee DS, Hain TC, Carl JR. "Abduction nystagmus in internuclear ophthalmoplegia." Ann Neurol. 1987;21(4):383-8.
- ↑ Virgo JD, Plant GT. Internuclear ophthalmoplegia. Pract Neurol. 2017 Apr;17(2):149-153. doi: 10.1136/practneurol-2016-001428. Epub 2016 Dec 7. PMID: 27927777.
- ↑ Jump up to: 7.0 7.1 7.2 Jeon, Sang Beom, et al. "Wall-eyed monocular internuclear ophthalmoplegia (WEMINO) with contraversive ocular tilt reaction." Journal of Clinical Neurology 1.1 (2005): 101-103.
- ↑ Rock, O., Albonico, A., Javadian, F., Ashkanani, M., Taylor, A. J. G., Dreyer, M., & Barton, J. J. S. (2022). Oblique saccades in internuclear ophthalmoplegia. Experimental brain research, 240(3), 861–869. https://doi.org/10.1007/s00221-021-06283-6
- ↑ Cogan DG. Neurology of the ocular muscles. Springfield, Ilinois: Charles C Thomas, 1956.
- ↑ Leigh RJ, Zee DS. The neurology of eye movements. 4th edn. New York: Oxford University Press, 2006.
- ↑ Petrik S, Lambeck J, Bardutzky J. WEBINO Syndrome Caused by Bilateral Pontine Microhemorrhages. Dtsch Arztebl Int. 2021;118(43):723-729. doi:10.3238/arztebl.m2021.0080
- ↑ Shinoda, Koji, et al. "Wall-eyed bilateral internuclear ophthalmoplegia (WEBINO) syndrome in a patient with neuromyelitis optica spectrum disorder and anti-aquaporin-4 antibody." Multiple Sclerosis Journal 17.7 (2011): 885-887
- ↑ Ullah R, Yasir M, Sarfaraz W, Hussain R, Rashid N. Wall-Eyed Monocular Inter-Nuclear Ophthalmoplegia (WEMINO) Syndrome, A Rare Decisive Manifestation Of An Isolated Unilateral Pontine Stroke. J Ayub Med Coll Abbottabad. 2021;33(4):698-701.
- ↑ de Seze J, Lucas C, Leclerc X, Sahli A, Vermersch P, Leys D. One-and-a-half syndrome in pontine infarcts: MRI correlates. Neuroradiology. 1999;41(9):666-669. doi:10.1007/s002340050821
- ↑ Menon, Vimla, et al. "Isolated “one and a half syndrome” with brainstem tuberculoma." The Indian Journal of Pediatrics. 71.5 (2004): 469-471.
- ↑ Simakurthy S, Tripathy K. Oculovestibular Reflex. 2021 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 31194421.
- ↑ Ingle V, Panda S, Penuboina T, Kashyap M. Eight-and-a-half syndrome: a rare presentation. BMJ Case Rep. 2021;14(9):e244338. Published 2021 Sep 3. doi:10.1136/bcr-2021-244338
- ↑ Bocos-Portillo, Jone, et al. "Eight-and-a-half syndrome." JAMA Neurol 72.7 (2015): 830
- ↑ Gómez Iglesias P, Sanesteban Beceiro E, Gómez Ruíz MN, Matías Guiu JA. Half and half syndrome as a presentation of multiple sclerosis. Síndrome del medio y medio como presentación de esclerosis múltiple. Neurologia (Engl Ed). 2021;36(3):246-248. doi:10.1016/j.nrl.2020.04.021
- ↑ Bogousslavsky J, Regli F, Ostinelli B, Rabinowicz T. Paresis of lateral gaze alternating with so-called posterior internuclear ophthalmoplegia. A partial paramedian pontine reticular formation-abducens nucleus syndrome. J Neurol. 1985;232(1):38-42.
- ↑ Jump up to: 21.0 21.1 Thömke F, Hopf HC. Abduction paresis with rostral pontine and/or mesencephalic lesions: Pseudoabducens palsy and its relation to the so-called posterior internuclear ophthalmoplegia of Lutz. BMC Neurol. 2001 Dec 18;1:4.
- ↑ Rothstein TL, Alvord EC, Jr. Posterior internuclear ophthalmoplegia. A clinicopathological study. Arch Neurol 1971;24:191-202.
- ↑ Bijvank, JA Nij, et al. "A rare cause for visual symptoms in multiple sclerosis: posterior internuclear ophthalmoplegia of Lutz, a historical misnomer." Journal of Neurology 264.3 (2017): 600-602.
- ↑ Jump up to: 24.0 24.1 24.2 24.3 Kim, Jong S. "Internuclear ophthalmoplegia as an isolated or predominant symptom of brainstem infarction." Neurology 62.9 (2004): 1491-1496.
- ↑ Fiester P, Baig SA, Patel J, Rao D. An Anatomic, Imaging, and Clinical Review of the Medial Longitudinal Fasciculus. J Clin Imaging Sci. 2020;10:83.
- ↑ Gil-Casas A, Piñero DP, Molina-Martin A. Binocular, Accommodative and Oculomotor Alterations In Multiple Sclerosis: A Review. Semin Ophthalmol. 2020;35(2):103-115.
- ↑ Serra A, Chisari CG, Matta M. Eye Movement Abnormalities in Multiple Sclerosis: Pathogenesis, Modeling, and Treatment. Front Neurol. 2018;9:31.
- ↑ Ophthalmic manifestations of myelin oligodendrocyte glycoprotein-IgG-associated disorder other than optic neuritis: a systematic review. Br J Ophthalmol. 2021;105(11):1591-1598.
- ↑ Garcia-Martin E, Pinilla I, Pueyo V, Gil L, Martinez-Morales J, Fernandez J. Bilateral Internuclear Ophthalmoplegia in a Patient with Devic’s Neuromyelitis Optica. Case Rep Neurol. 2010;2(3):139-144.
- ↑ Cogan, David G. "Internuclear ophthalmoplegia, typical and atypical." Archives of Ophthalmology 84.5 (1970): 583-589.
- ↑ Takeshige, Haruka, et al. "Pathways linked to internuclear ophthalmoplegia on diffusion-tensor imaging in a case with midbrain infarction." Journal of Stroke and Cerebrovascular Diseases 25.11 (2016): 2575-2579
- ↑ Dong M, Wang L, Teng W, Tian L. Wernekink commissure syndrome secondary to a rare ’V’-shaped pure midbrain infarction: a case report and review of the literature. Int J Neurosci. 2020;130(8):826-833.
- ↑ Chen, Ko-Ting, Tzu-Kang Lin, and Tsung-Che Hsieh. "Isolated Internuclear Ophthalmoplegia After Massive Supratentorial Epidural Hematoma: A Case Report and Review of the Literature." World Neurosurgery 100 (2017): 712-e5.
- ↑ Keane JR. Internuclear ophthalmoplegia: unusual causes in 114 of 410 patients. Arch Neurol. 2005 May;62(5):714-7
- ↑ Fiester P, Baig SA, Patel J, Rao D. An Anatomic, Imaging, and Clinical Review of the Medial Longitudinal Fasciculus. J Clin Imaging Sci. 2020;10:83.
- ↑ Kochar PS, Kumar Y, Sharma P, Kumar V, Gupta N, Goyal P. Isolated medial longitudinal fasciculus syndrome: Review of imaging, anatomy, pathophysiology and differential diagnosis. Neuroradiol J. 2018;31(1):95-99.
- ↑ Gil-Casas A, Piñero DP, Molina-Martin A. Binocular, Accommodative and Oculomotor Alterations In Multiple Sclerosis: A Review. Semin Ophthalmol. 2020;35(2):103-115.
- ↑ Jump up to: 38.0 38.1 Frohman, T. C., Frohman, E. M., O'Suilleabhain, P., Salter, A., Dewey, R. B., Jr, Hogan, N., Galetta, S., Lee, A. G., Straumann, D., Noseworthy, J., Zee, D., Corbett, J., Corboy, J., Rivera, V. M., & Kramer, P. D. (2003). Accuracy of clinical detection of INO in MS: corroboration with quantitative infrared oculography. Neurology, 61(6), 848–850. https://doi.org/10.1212/01.wnl.0000085863.54218.72
- ↑ Frohman, E. M., Zhang, H., Kramer, P. D., Fleckenstein, J., Hawker, K., Racke, M. K., & Frohman, T. C. (2001). MRI characteristics of the MLF in MS patients with chronic internuclear ophthalmoparesis. Neurology, 57(5), 762–768. https://doi.org/10.1212/wnl.57.5.762
- ↑ Ito K, Mizutani J, Murofushi T, Mizuno M. Bilateral pseudo-internuclear ophthalmoplegia in myasthenia gravis. ORL J Otorhinolaryngol Relat Spec. 1997;59(2):122-126. doi:10.1159/000276922
- ↑ McClard CK, Lyons LJ, Yalamanchili S. Bilateral pseudo-internuclear ophthalmoplegia in a patient with myasthenia gravis. Am J Ophthalmol Case Rep. 2018;12:76-78. Published 2018 Sep 19. doi:10.1016/j.ajoc.2018.09.008
- ↑ Diamond S, Schear HE, Leeds MF. Pseudo-internuclear oculomotor ophthalmoplegia secondary to Guillain-Barré polyneuronitis simulating myasthenia gravis in a air transport pilot. Aviat Space Environ Med. 1975;46(2):204-207.
- ↑ Chen BS, Newman NJ, Biousse V. Atypical presentations of idiopathic intracranial hypertension. Taiwan J Ophthalmol. 2020;11(1):25-38.
- ↑ Thakker R, Mohanty A. Reversible Progressive Multiple Cranial Nerve Paresis in the Isolated Fourth Ventricle following Placement of Fourth Ventricle Shunt: Case Report and Review of the Literature. Pediatr Neurosurg. 2019;54(6):405-410.
- ↑ Kanhai KMS, Nij Bijvank JA, Wagenaar YL, et al. Treatment of internuclear ophthalmoparesis in multiple sclerosis with fampridine: A randomized double-blind, placebo-controlled cross-over trial. CNS Neurosci Ther. 2019;25(6):697-703.
- ↑ Lin WV, Chévez-Barrios P, Sadaka A, Lee AG. Orbital metastasis mimicking internuclear ophthalmoplegia: A case report and review Can J Ophthalmol. 2017;52(4):e149-e151.
- ↑ Sklar B, Gervasio K, Karmazin K, Wu A. Orbital Metastasis From Urothelial Carcinoma: A Comprehensive Literature Review.Ophthalmic Plastic and Reconstructive Surgery. 2019; 35 (3): 213-217]
- ↑ Shibaki R, Murakami S, Oki K, Ohe Y. Nivolumab-induced autoimmune encephalitis in an anti-neuronal autoantibody-positive patient. Japanese Journal of Clinical Oncology. 2019;49(8):793-794
- ↑ Siegel CH, Finn RS, Ho MG. Multiple Cranial Neuropathies From Nivolumab in a Patient With Metastatic Hepatocellular Carcinoma. Mayo Clinic Proceedings. 2018;93(4):540-541
- ↑ Kao JC, Liao B, Markovic SN, et al. Neurological Complications Associated With Anti-Programmed Death 1 (PD-1) Antibodies. JAMA Neurol. 2017;74(10):1216-1222.
- ↑ Kanhai KMS, Nij Bijvank JA, Wagenaar YL, et al. Treatment of internuclear ophthalmoparesis in multiple sclerosis with fampridine: A randomized double-blind, placebo-controlled cross-over trial. CNS Neurosci Ther. 2019;25(6):697-703.
- ↑ Serra, A., Skelly, M. M., Jacobs, J. B., Walker, M. F., & Cohen, J. A. (2014). Improvement of internuclear ophthalmoparesis in multiple sclerosis with dalfampridine. Neurology, 83(2), 192–194. https://doi.org/10.1212/WNL.0000000000000567
- ↑ Jump up to: 53.0 53.1 Serra, Alessandro et al. “Improvement of Internuclear Ophthalmoparesis in Multiple Sclerosis with Dalfampridine.” Neurology 83.2 (2014): 192–194. PMC. Web. 9 Sept. 2018
- ↑ Jump up to: 54.0 54.1 Murthy, Ramesh, et al. "Botulinum toxin in the management of internuclear ophthalmoplegia." Journal of American Association for Pediatric Ophthalmology and Strabismus 11.5 (2007): 456-459.
- ↑ Jump up to: 55.0 55.1 Eggenberger E, Golnik K, Lee A, et al. Prognosis of ischemic internuclear ophthalmoplegia. Ophthalmology. 2002;109(9):1676-1678. doi:10.1016/s0161-6420(02)01118-1
- ↑ Jump up to: 56.0 56.1 Bolaños, I., Lozano, D., & Cantú, C. (2004). Internuclear ophthalmoplegia: causes and long-term follow-up in 65 patients. Acta neurologica Scandinavica, 110(3), 161–165. https://doi.org/10.1111/j.1600-0404.2004.00278.x