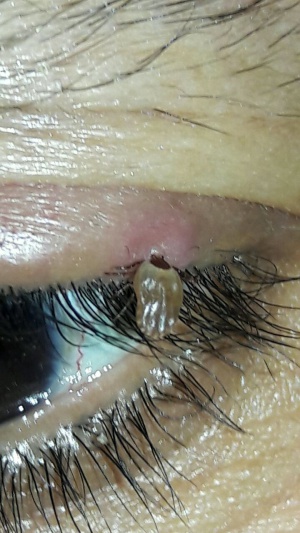
Lyme Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Lyme disease is a multisystem infection caused by the spirochete Borrelia burgdorferi which is typically transmitted through the Ixodes tick, the same carrier of the Babesia protozoa. The typical season outbreaks occur during summer and autumn. The animal reservoirs include rodents, deer, birds, cats and dogs.
The Ixodes ticks have three stages during their two year life span. During the first stage they are considered larvae and feed typically on the white-footed mice in the spring and summer, evolving later on into a nymph. During the next year the nymphs continue to feed on the white-footed mice and in late summer of their second year mature into adults. The adults attack larger hosts such as deer, cattle, or humans.
Disease Entity
Etiology
Borrelia burgdorferi is a spiral (spirochete) microorganism ranging from 20- 30 μm long and 0.2 to 0.3 μm wide. The microorganisms have a variable number of flagella (usually from 7 o 11) that make them highly capable for movement. This microorganism can be isolated from culture, but it takes a complex medium to do so; such as a Barbour-Stoenner-Kelly (BSK II) enriched with rifampin, fosfomycin and amphotericin B, to prevent contamination. It is usually easier to isolate the bacteria when the samples are taken from the erythematous lesions.
Risk Factors
Exposure to endemic areas
Pathophysiology
The infection occurs when there is human contact with the infected Ixodes tick and injects its salivary secretion into the human transmitting the spirochete. The microorganism adheres itself to the proteoglycans in the host cell through glycosaminoglycans that are present in the ''Borrelia''. After the injection, there is a lymphatic or hematologic dissemination of the bacteria to other skin regions, the musculoskeletal system and other internal organs.
Primary Prevention
Tick repellent spray may be helpful
Diagnosis
Clinical and laboratory tests.
History
There may be a history of exposure to endemic areas or wilderness exposure.
Physical Examination
Stage 1: Localized Infection
After an incubating period ranging from 3 to 32 days, a classic erythema migrans occurs at the site of the tick bite. It starts as a red macula or papule that expands slowly to form a large circular lesion. As the lesion expands and increases in size, it often develops a bright red outer border and central clearing, creating the classic target-like (bull’s eye) rash. The center of the lesion can become indurated, vesicular or necrotic. Even though the rash may start anywhere in the body, the usual places for the rash to begin are the thigh, groin, and armpits. It is important to mention that nearly 20% of patients do not exhibit the classic target-like lesion.
Stage 2: Disseminated Infection
In the USA it is common the B. burdorferi spread mainly hematogenously to many sites within days or weeks of the primary infection. In these cases, patients may develop secondary annular skin lesions far from the infection site accompanied by severe headache, mild stiffness of the neck fever, chills, migratory musculoskeletal pain, arthralgias without joint swelling, and profound malaise and fatigue.
If left untreated, approximately 15% of patients in this particular stage of the disease may develop neurologic involvement, such as meningeal irritation without pleocytosis, meningitis, encephalitic signs, cranial neuritis, motor or sensory radiculoneuropathy, peripheral neuropathy, mononeuritis multiplex, cerebellar ataxia or myelitis.
Likewise, if left untreated between 4 to 10% of patients may develop cardiac involvement such as auricular-ventricular block (of any degree), acute myopericarditis, left ventricular dysfunction, cardiomegaly or pericarditis.
Stage 3: Persistent Infection
If left untreated, several months after the onset of infection, 60% of patients develop frank arthritis. The typical pattern involves intermittent attacks of oligoarticular arthritis in large joints (especially the knees), lasting weeks to months in the selected joint.
If a sample is taken from the swollen joint, the usual findings are WBC counts between 500-110,000/μL, consisting mainly of polymorphonuclear leukocytes. Tests for rheumatoid arthritis or antinuclear antibodies are usually negative. If a synovial biopsy is taken the typical findings are fibrin deposits, villous hypertrophy, vascular proliferation, microangiopathic lesions, and a heavy infiltration of lymphocytes and plasma cells.
Ophthalmologic Findings
The ocular findings vary upon the stage of the disease.
Stage 1
The most common presentation is a follicular conjunctivitis, in approximately 11% of patients; and less commonly as an episcleritis.
Stage 2
The disease can manifest as anterior uveitis, intermediate uveitis, posterior uveitis or panuveitis. From these, the most common form of presentation is the intermediate uveitis. Vitritis may be severe and be accompanied by granulomatous anterior chamber reaction, papillitis, neuroretinitis, choroiditis, retinal vasculitis, and exudative retinal detachment.
Neuro-ophthalmic manifestations include multiple cranial nerves (II, III, IV, VI and most commonly VII), unilateral or bilaterally, presenting one and the other, or simultaneously. It is common that the patients develop a a bilateral facial paralysis. The optic nerve can be found with signs of optic neuritis, papilledema and papillitis.
Stage 3
The most common ocular manifestation in this stage is keratitis, and much less common episcleritis. These may present months to years after the primary infection. The usual presentation is a bilateral, patchy, focal, and stromal; or subepithelial infiltrate with no clear borders. The keratitis has been thought to be an immune response reaction rather than an infections process, due to the fact that there is good clinical response with the use of topical steroids alone.
Signs
Variable according to disease stage
Symptoms
Variable according to disease stage
Clinical Diagnosis
Clinical scenario and history
Differential diagnosis
Other tick-borne diseases with ocular manifestations include:
- Babesiosis[1]
- Babesiosis is most often caused by Babesia microti, primarily found in the Northeast and Upper Midwest region of the USA as well as Europe. It is transmitted by the deer tick (Ixodes scapularis) with the reservoir host being the white-footed mouse. Patients are typically asymptomatic but may present with malaise, fever, sweats, and chills 1-4 weeks after exposure. Thrombocytopenia and hemolytic anemia are common. Ocular manifestations include photophobia, conjunctival injection, retinal hemorrhage, papillitis, and nerve fiber layer infarcts.
- Colorado Tick Fever[1]
- Colorado tick fever is caused by the Colorado tick virus of the Reoviridae family and is primarily found in the Rocky Mountain region of the USA/Canada. It is transmitted by the wood tick (Dermacentor andersoni) with reservoir hosts being small rodents. Patients commonly present with a fever in a biphasic pattern with intervening periods of defervescence, pharyngeal erythema, and a maculopapular or petechial rash. Ocular manifestations include conjunctival injection.
- Ehrlichiosis[1]
- Ehrlichiosis is caused by a gram-negative coccus of the Rickettsiaceae family and has 2 main variants: Human granulocytic ehrlichiosis (HGE) and Human monocytic ehrlichiosis (HME). HGE is transmitted by the deer tick (Ixodes scapularis) with the reservoir host being the white-footed mouse and white-tailed deer. HME is spread by the dog tick (Dermacentor variabilis) and the Lone Star tick (Ambylomma Americanum) with the reservoir host being the white-tailed deer, primarily found in the South and Southeast USA. Both variants can present similarly with fever, headache, nausea, vomiting, arthralgia, and anorexia. Ocular manifestations are extremely rare with reports of HGE associated with optic neuritis and HME associated with trochlear nerve palsy.
- Powassan Encephalitis[1]
- Powassan encephalitis is caused by the Powassan virus of the Flaviviridae family and is primarily found in Canada, USA, Mexico and Russia. It is transmitted by the Ixodes family of ticks and reservoir host includes woodchucks and foxes. Patients may present with fever, malaise, sore throat, fatigue, nausea, vomiting, headache, and altered mental status 1-4 weeks after exposure. The ocular manifestations include optic disc swelling, retinal vessel tortuosity and ophthalmoplegia.
- Q Fever[1]
- Q fever is caused by Coxiella burnetii, an obligate intracellular gram-negative bacterium and is found worldwide. Although it can be transmitted by ticks, it is commonly transmitted via inhalation of spores from placental matter, excrement, milk, and urine from livestock. Reservoir hosts include cattle, sheep and goats. Patients are typically asymptomatic but may present with non-specific viral illness symptoms 2-3 weeks after exposure. Ocular manifestations include iridocyclitis, vitritis, choroiditis, retinal vessel sheathing, retinal vasculitis, optic disc edema, optic disc hemorrhage, optic disc pallor, neuroretinitis, and abducens nerve palsy.
- Rocky Mountain Spotted Fever[1]
- Rocky Mountain spotted fever is caused by Rickettsia rickettsii, an obligate intracellular gram-negative bacterium, and is found in the USA. It is spread via the dog tick (Dermacentor variabilis) or rocky mountain wood tick (Dermacentor andersoni).[2] Patients present with high fever, headache, malaise, and a centripetally spreading skin rash. Ocular manifestations include iridocyclitis, cotton wool spots, retinal venous tortuosity and dilation, retinal edema, retinal hemorrhage, white exudates in the posterior pole and periphery, vascular sheathing, focal branch retinal artery occlusion and neuroretinitis.
- Tick-Borne Relapsing Fever[1]
- Tick-borne relapsing fever is caused by the Borrellia species, a spirochete bacterium, and is found in the Western and Southwestern USA, east Africa, Central/South America, the Mediterranean and central Asia. In the USA, most cases are transmitted by the Ornithodoros hermsi or Ornithodoros turicata tick. Patients present with recurrent fevers, headaches, malaise, myalgia, and chills. Ocular manifestations include eyelid edema, conjunctivitis, marginal keratitis, iridocyclitis, choroiditis, vitritis, papillitis and cranial nerve palsies.
- Tularemia[1]
- Tularemia is caused by Francisella tularensis, a gram-negative bacterium, and is found in North America, Japan and Europe. The most common subtype is ulceroglandular disease which is spread via the Dermacentor variabilis or Amblyomma Americanum tick. The oculoglandular disease subtype is the rarest and is transmitted by eye rubbing with contaminated finger, contaminated aerosols or contaminated fluids. Patients present with painful lymphadenopathy. Ocular manifestations of oculoglandular disease include dacryocystitis, conjunctivitis, conjunctival ulcerations, corneal edema, corneal ulceration/perforation, acute angle closure glaucoma and optic nerve atrophy.
Diagnostic procedures
Besides the clinical characteristic findings (erythema chornicum migrans is diagnostic), diagnosis is confirmed with serologic data such as an ELISA test for IgM and IgG (which peak during the first month) followed by a Western blot test. The CDC recommends a two-step approach in which samples are tested initially with an ELISA, and those with equivocal or positive results are then tested by Western blotting with an RST1 strain. The CDC also establishes, for the American community, that an IgM Western blot is considered positive if two of the three bands are present: 23, 30 and 41 kDa; however caution must be taken since the combination of 23 and 41-kDa may still be a false positive result. An IgG blot is considered positive if 5 of the 10 bands are present (18, 23, 28, 30, 39, 41, 45, 58, 66 and 93 kDa). There are no criteria for European population.
Laboratory test
Serodiagnosis is insensitive in the first several weeks of infection
Care must be taken since Lyme disease can give a false positive test for syphilis (FTA-ABS)
PCR has been used to amplify the genomic and plasmid DNA from several tissues, including ocular fluids.
After antibiotic treatment, antibody titers slowly decline, but IgG and even IgM responses may persist for many years after treatment. So a positive IgM response cannot be used to interpret a recent infection or a reinfection without a clinical picture.
Management
General treatment
Early and Late Disease, Facial Nerve palsy:
- Doxycycline 100 mg PO bid x 10-21 days or
- Amoxixilin 500 mg PO tid x 14-21 days (25-50 mg/kg/day for children) or
- Cefuroxime 500 mg PO bid x 14-21 days (30 mg/kg/day for children)
Meningitis, Recurrent arthritis, CNS or peripheral nervous system disease:
- Ceftriaxone 2 g/d IV qd x 14-28 days
After the appropriate antibiotic therapy has begun, anterior segment inflammation can be treated with topical corticosteroids and mydriatics.
References
- Brooks, G; Butel, J; Morse, S. “Microbiología Médica de Javetz, Melnick y Adelberg” (2005). 18th edition. Manual Moderno
- Fauci, A; Kasper, D; Longo, D; Braunwald, E; Hauser, S; Jameson, J; Loscalzo; J. “Harrison’s Principles of Internal Medicine”. (2008). 17th edition. McGraw Hill.
- Krause, P; Bockenstedt, L. “Lyme Disease and the Heart”. (2013). American Heart Association. Circulation.
- Weinberg, R. “Uveitis Update Therapy”. (1999). Ophthalmology Clinics of North America. Volume 12, Number 1.
- Intraocular Inflammation and Uveitis. American Academy and Ophthalmology. Section 9. Basic and Clinical Science Course (2011-2012).
- Bennet, J; Dolin, R; Blaser, M “Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases(2010) 8th edition. Elsevier.
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Raja, H; Starr, M; Bakri, S. "Ocular manifestations of tick-borne diseases." Survey of Ophthalmology. 61(6):726 - 744.
- ↑ U.S. Centers for Disease Control and Prevention. "About Rocky Mountain Spotted Fever". Accessed Oct 31, 2024. https://www.cdc.gov/rocky-mountain-spotted-fever/about/index.html