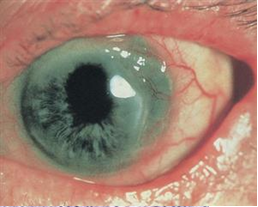
Mooren's Ulcer
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Mooren’s ulcer is characterized by painful peripheral corneal ulceration of unknown etiology. The disease generally begins with intense limbal inflammation and swelling in the episclera and conjunctiva [1]. Corneal changes begin within 2-3 mm from the limbus, first appearing as grey swellings that rapidly furrow, affecting the superficial one-third of the cornea and then proceeding circumferentially and centrally over 4-12 months. [1][2] The bed of the furrow becomes vascularized, with vessels advancing into the base of the undermined edges of the ulcers [1] (figure 1). These ulcers are often described as crescent-shaped and can leave behind an opaque and edematous central cornea. Alternatively, they can completely consume the corneal stroma, replacing it with a thin fibrovascular membrane. [3] Inflammation is not seen in the sclera adjacent to the peripheral ulcers, nor does it affect the underlying Descemet’s membrane. [4] Destruction of the cornea generally affects stromal tissue only, leaving behind an intact endothelium and epithelium. [5] The central edges of the ulcer can develop an overhanging edge with or without opacification, and neovascularization of the cornea can occur, extending from the limbus into the ulcer bed. [3] Neovascularization can occur up to the advancing edge of the ulcer but not beyond it. [5]
Etiology
Although the etiology of Mooren’s ulcer is unknown, there is evidence suggesting an autoimmune basis and possibly genetic and environmental factors contribute to the pathogenesis. Gottsch and colleagues have suggested that this disorder can result from a host response to calgranulin C, a normally hidden antigen expressed by keratinocytes in the corneal stroma. [6] This molecule has also been found in circulating polymorphonuclear leukocytes. [5] Receptors for this antigen have also been found on the surface of certain helminths, which has led to speculation regarding an association with helminthic infections. However, Mooren's ulcer has not been proven to be more prevalent in endemic areas of ascariasis. [4]
It has been found that certain HLA alleles may be associated with Mooren's ulcer. One study suggested that 83% of patients (n=12) with Mooren's ulcer were HLA-DR17 positive, and 83% were HLA-DQ2 positive, as compared to control populations where frequencies varied between 5% and 40% [5]. The same study found that only one out of 10 patients with another form of destructive sclerokeratitis expressed the HLA-DR17 allele, and three expressed HLA-DQ2. HLA-DQ5 has also been found in increased frequencies in patients with Mooren's ulcer [7]. A recent proteomic study suggested that anti-citrullinated protein antibody (ACPA; often referred to as anti-CCP) may be a signature for Mooren's ulcer and explain immunopathology.[8]
Risk Factors
Risk factors for Mooren's ulcer include corneal surgery, previous trauma, and infection. Although corneal surgery is a well-described risk factor, one study of 242 eyes affected by Mooren's ulcer in South India described an interesting caveat. In this study, Srinivasan and colleagues described 36 eyes that developed ulceration following extracapsular cataract extraction through a superior limbal incision; however, only 31% of these eyes developed ulceration at or contiguous to the site of the surgical wound. Nevertheless, this ratio of superior corneal involvement of Mooren's ulcer is still higher than expected when compared with 10% superior involvement found in eyes with no history of ocular surgery. A recent case report highlighted the potential for aggressive Mooren's ulcer during pregnancy, particularly in the presence of preexisting pterygium, suggesting that hormonal and immunological changes during pregnancy may exacerbate the condition.[9]
An association has also been demonstrated between Mooren’s ulceration and hookworm infection [10]. Certain helminths express receptors for calgranulin C, suggesting that there may be a helminthic antigen that cross-reacts with calgranulin C [5]. A previous infection has also been found to be a risk factor [11]. An association with pyoderma gangrenosum has been described, implying potential common pathogenesis between the two processes since both pyoderma gangrenosum and Mooren's ulcer show aseptic neutrophilic inflammation and both respond well to immunosuppression [12].
Epidemiology
Mooren’s ulceration is a rare disease. One study approximated the incidence to be 0.03% in China [2]. The disease is more common in the southern hemisphere, including Southern and Central Africa and India, indicating a genetic and/or geographic predisposition [5]. Although it is generally agreed upon that Mooren's ulcer is more common in men, figures differ from one geographic population to the next in terms of age distribution.
Studies from South India and China have suggested that patients tend to be affected between their sixth and eighth decades of life, with men being affected more than women by a ratio between 1.6:1 to 5:1. [3][10][11][13][14] However, this epidemiological picture may differ in African populations, as suggested by a study in Nigeria where the disease mostly affected men in their 20s-30s [15]. Another study from West Africa supported this epidemiologic finding, causing the authors to postulate that there were two distinct populations of patients with Mooren's ulcer [16]. The first population is older, more often develops unilateral disease, and responds to treatment, whereas the second population is younger, tends to have bilateral disease, which perforates more often, and does not respond to treatment. Further studies of patients from the Republic of Zaire, Togo, and Sierra Leone supported this hypothesis [3]. However, the categorization described by Wood and Kaufman has been refuted by others that found bilateral disease to be more common in older patients [17].
General Pathology
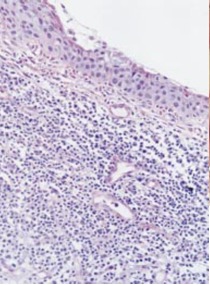
On histological examination (figure 2), samples of conjunctival and corneal specimens show a lymphocytic infiltration along with neutrophils, eosinophils, plasma cells, and mast cells [4],[18] . High levels of proteolytic enzymes have been found in affected conjunctival tissue [18]. When the limbal cornea is examined, it can show vascularization and infiltration of plasma cells and lymphocytes into the superficial stroma in addition to the destruction of the collagen matrix [4]. The midstroma may show disorganized collagen lamellae and hyperactive fibroblasts, while the deep stroma contains a macrophage infiltrate. The leading edge of the ulcer can show neutrophilic infiltration with evidence of degranulation. Adjacent conjunctiva may show a hyperplastic epithelium and subconjunctival lymphocytic and plasma cell infiltration [4]. It has also been shown that elevated NF-κB activity may be responsible for the pathology.[19]
Pathophysiology
It has been postulated that Mooren’s ulcer may result from an autoimmune etiology. Sensitization to calgranulin C, an antigen expressed by corneal stromal keratinocytes, may occur after trauma or infection of the cornea, causing the unveiling of this hidden corneal antigen [5]. The unveiling of the hidden antigen may also occur naturally in predisposed people with aging. It has been hypothesized that antigen-presenting cells at the limbus can present the unveiled self-antigens via certain HLA class II molecules to T-cells, priming them. Alternatively, antigen-presenting cells may present a helminthic antigen that cross-reacts with calgranulin C (since certain helminths express receptors that bind calgranulin C), resulting in a similar reaction [5]. Later, upon injury of the cornea, humoral and cellular responses result in Mooren's ulcer [4][5].
Martin and colleagues have suggested that infection, trauma, or a systemic disease can expose corneal antigens and stimulate an immune response in which complement is activated and neutrophils degranulate and release collagenases. Collagenases then destroy the corneal stroma, further perpetuating the process by exposing more altered corneal antigens. Eventually, the entire cornea becomes consumed [20]. Evidence for a humoral immune response comes from a study that found circulating IgG against corneal and conjunctival epithelium in patients with Mooren's ulcer [21]. Studies have also found antibodies and complements bound to the conjunctival epithelium in addition to elevated serum IgA levels [4].
Diagnosis
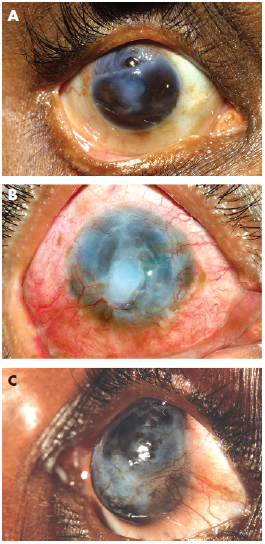
The diagnosis of Mooren's ulcer requires the absence of any ocular infection or systemic rheumatological diseases known to cause peripheral corneal ulceration [3]. Srinivasan and colleagues described three patterns of ulceration: partial peripheral, complete peripheral, and total corneal ulceration (Figure 3). In complete peripheral ulceration, the disease process has completely encompassed the corneal periphery, leaving behind a “central island of cornea” often opacified (Figure 3b). In total corneal ulceration, the corneal stroma has been completely replaced with a fibrovascular membrane (Figure 3c). Partial peripheral ulceration can be subdivided into nasal, temporal, superior, and inferior ulceration, of which temporal and nasal (the so-called intrapalpebral cornea) involvement is more common [3].
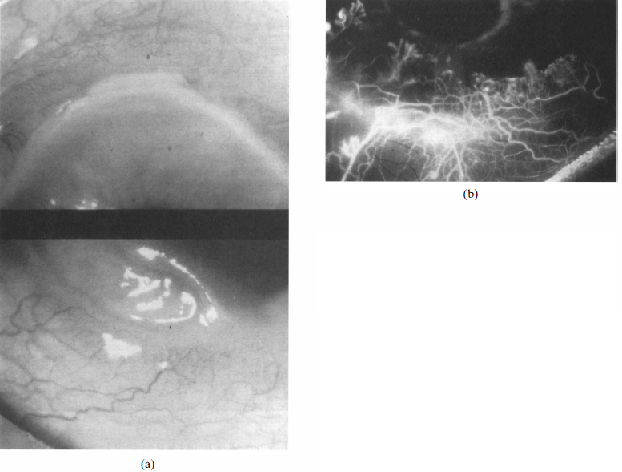
Watson and colleagues classified the disease into three types based on clinical features, anterior segment fluorescein angiographic findings, and treatment response.[1] The first type, unilateral Mooren’s ulceration (figure 4), is excessively painful and occurs in patients >60 years old. Affected eyes are seen to be red and congested, but inflammation does not extend beyond 3 mm from the limbus. Vascularization of the ulcer bed is seen along with leakage at the tips of these new vessels. Ulceration extends around the globe and often leaves a thick, opaque central cornea. The central corneal stroma eventually is removed completely and a thin layer of scar tissue remains overlying an intact endothelium and covered by an epithelium derived from the conjunctiva. When the stroma disappears, the pain subsides. If scar tissue retracts to the point where Descemet’s membrane is exposed, the same process will likely recur if a corneal transplant is performed [1]. Anterior segment fluorescein angiography shows venular occlusion of local episcleral and conjunctival blood vessels along with disruption of the limbal arcade and vascular leakage from deep vessels at the limbus and base of the ulcer. In addition, the vaso-obliteration of superficial vascular networks is characteristic of unilateral Mooren’s ulceration.
The second type, bilateral aggressive Mooren’s ulcer, occurs more in younger patients (between ages 14 and 40) and presents with pain that is less severe than in unilateral Mooren’s ulcer [1]. These patients may present with an ulcer in one eye and conjunctival congestion in the other, which eventually progresses to develop gray patches within the corneal stroma about 2 mm from the edge of the limbus. These gray patches then aggregate and lead to a typical Mooren's ulcer that progresses first circumferentially and then centrally. Fluorescein angiography (figure 5) shows vascular leakage and new vessel formation that reaches the base of the ulcer. Angiography can also show changes in the architecture of episcleral vessels and blockage in addition to the break-up of the limbal arcade. The superficial vascular plexus remains perfused, although it may be dilated.
The third type, bilateral indolent Mooren’s ulcer (figure 6), occurs in middle-aged (mid-50s) patients who exhibit corneal guttering in both eyes with little inflammation. Although both eyes tend to be involved at presentation, the disease is often more severe in one eye, and patients complain of discomfort rather than pain [1]. Most cases are gradually progressive, but some heal spontaneously. Others may reactivate after a long period of quiescence. Vascular architecture in this type is normal with the exception that new vessels may extend into the base of the ulcer [22].
Anterior segment optical coherence tomography (AS-OCT) can help in diagnosing Mooren's ulcer by providing high-resolution cross-sectional images of the cornea, which allow for the accurate evaluation of ulcer characteristics, including depth, extent, and disease progression. This imaging modality is non-invasive and offers detailed visualization of the corneal layers, which is crucial for assessing the severity and progression of the ulcer.
AS-OCT can identify specific patterns of corneal thinning and irregularities, which are characteristic of Mooren's ulcer. For instance, it can differentiate between arcuate and crab-claw patterns of corneal thinning, which have implications for the severity and location of the ulcer. [23]Additionally, AS-OCT can monitor the response to treatment by showing changes in corneal thickness and the resolution of inflammation over time. [24]
History and Physical examination
Patients often experience severe pain, photophobia, and tearing along with a red inflamed eye. About one-third of cases present bilaterally [22]. A slit-lamp examination may reveal a crescent-shaped peripheral corneal ulcer with an undermined central edge. A linear epithelial defect may develop at the central margin, followed by progressive stromal melting. The ulcer progresses circumferentially and centrally, resulting in a re-epithelialized, conjunctivalised thinned cornea [22]. Consequently, severe irregular astigmatism may result. Conjunctival and episcleral inflammation may occur; however, the sclera is spared. Decreased visual acuity can occur secondary to iritis, central corneal involvement, or irregular astigmatism [4].
Differential diagnosis
To rule out other causes of peripheral ulcerative keratitis, laboratory investigations include CBC with differential, platelet count, ESR, rheumatoid factor, anti-CCP, complement fixation, ANA, ANCA, circulating immune complexes, liver function tests, VDRL/FTA-ABS, BUN and creatinine, serum protein electrophoresis, and urinalysis [4]. In addition, cultures should be performed to rule out microbial keratitis.
Terrien’s marginal degeneration differs from Mooren’s ulceration in that it is a painless, non-inflammatory disease, causing peripheral corneal thinning without ulceration. Terrien’s marginal degeneration also usually begins in the superior cornea (rather than the interpalpebral region) and proceeds circumferentially but not centrally. A clear zone with superficial vascularization remains between the limbus and the infiltrate [4].
Pellucid marginal degeneration causes inferior corneal thinning and causes irregular against-the-rule astigmatism but lacks the inflammation and pain seen in Mooren's ulcer [4]. Senile furrow degeneration causes thinning between the limbus and an arcus in elderly patients; however, no inflammation or vascularization occurs [4].
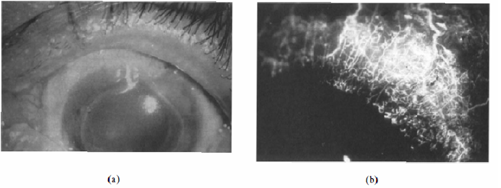
Rheumatoid arthritis can cause peripheral corneal ulcers that start as gray, swollen areas within 2 mm of the limbus [1]. Corneal thickness diminishes rapidly and may leave behind a fragile descemetocele. Angiography can show venous non-perfusion and disrupted limbal arcades with neovascularization reaching the base of the gutter. Scleritis may be associated, whereas Mooren's ulcer spares the sclera. Other findings in rheumatoid arthritis include keratoconjunctivitis sicca, episcleritis, and sclerosing keratitis [4]. Keratolysis is a disease of central corneal stromal disintegration that is often seen in patients with long-standing rheumatoid arthritis. First, the central cornea swells, and the epithelium begins to shed [1]. The disease spreads until the corneal stroma is fully resorbed and a fragile Descemet’s membrane is left. This disease is generally painless.
Other collagen vascular diseases in which peripheral ulcerative keratitis can occur include systemic lupus erythematosus, systemic sclerosis, and relapsing polychondritis [4]. Peripheral ulcerative keratitis has also been reported to occur in association with inflammatory bowel disease, giant cell arteritis, staphylococcal marginal keratitis, HSV, and acanthamoeba keratitis.
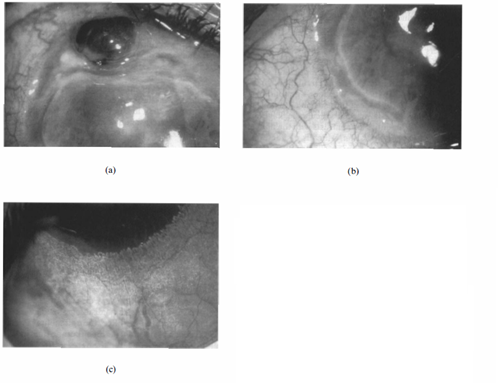
Ocular involvement in granulomatosis with polyangiitis (GPA; formerly known as Wegener's) can include proptosis, scleritis, and uveitis in addition to peripheral ulcerative keratitis. Polyarteritis nodosa can also cause choroidal or retinal vasculitis, scleritis, optic atrophy, papilledema, exudative retinal detachment, CRAO, and conjunctival lesions [4]. Granulomatosis with polyangiitis and polyarteritis nodosa can display severe leakage, and neovascularization is seen from the tips of the limbal arcades. In GPA, granulomas destroying limbal tissue can be seen in both the corneal and scleral side of the vascular changes [1].
Limbal involvement generally occurs in Mooren's ulcer, whereas other forms of peripheral ulcerative keratitis usually spare the limbus [4]. The distribution of vascular involvement can differ when comparing Mooren's ulcer to other causes of peripheral corneal ulceration. Mooren’s ulceration generally causes leakage from deep vascular networks that come from the long posterior ciliary circulation, with the unilateral form also blocking perfusion to the superficial network. In connective tissue disorders such as rheumatoid arthritis, angiography shows capillary and venous occlusion in the episcleral and conjunctival circulation [1]. In vascular disorders such as GPA and polyarteritis nodosa, marked vascular leakage and granulomatous inflammation may be seen involving the episcleral and venous vessels.
Management
Wood and Kaufman noted that the two types of Mooren's ulcer they described—one type being unilateral and developing in older patients, and the other type being bilateral in younger patients—respond differently to treatment. The first type tends to heal well after a conjunctival flap procedure or lamellar keratoplasty whereas the second type is not responsive to treatment.
Watson described three types of Mooren's ulcer, described above under “Diagnosis”. While the unilateral form has been said to be readily treatable, other studies have suggested otherwise [1]. The suggested treatment for this form involves a trial of aggressive systemic and local immunotherapy (described below), followed by surgical removal of the corneal stroma up to Descemet’s membrane. Corneal transplant often fails due to the recurrence of the disease or allograft rejection. Bilateral aggressive Mooren’s ulceration can also be treated with aggressive local and systemic immunosuppression. Bilateral indolent Mooren’s ulceration often resolves with dietary changes and treatment of any systemic infection that may be present. It can also be treated with local steroids and cyclosporine A [1].
The initial treatment of Mooren’s ulcer consists of topical corticosteroids. Topical use of 1% or 2% Cyclosporine and interferon alfa 2 a were also suggested for the treatment of Mooren's ulcer.[25][26] If corticosteroids do not control the inflammation, limbal conjunctival excision can be performed [22]. Excision of a 3-4 mm ring of limbal conjunctiva at least 2 clock hours adjacent to a Mooren's ulcer has been shown to be an effective treatment. Studies have shown that when less than half of the limbus is involved, the cure rate after conjunctival excision and corneal ulcer resection is performed is 51.3% [2]. When more than half of the limbus is involved, the cure rate after the procedure decreases to 36.8%. In a 1975 study where limbal conjunctival excision was performed, 8 out of 10 eyes healed, and one developed recurring ulcers which then healed upon re-treatment [27]. It was hypothesized that the limbal conjunctiva may contain antibodies that react with antigens in the corneal stroma in addition to enzymes that destroy the corneal stroma—therefore, excision may interrupt the disease process.
Surgical interventions can also include lamellar keratoplasty, keratoepithelioplasty, delimiting keratotomy, and conjunctival flap and patch grafts using periosteum or fascia lata [22]. Resection of the corneal lesion and adjacent conjunctiva combined with lamellar keratoplasty can achieve a final healing rate of 89.6%. If topical 1% cyclosporine A is added, a 95% final healing rate can be achieved [2].
Cryotherapy to the conjunctiva surrounding ulcers may temporarily halt the progression of the disease, but healing may not occur until the conjunctiva is excised [28]. Other potential local therapies include bandage contact lenses, tissue adhesive, subconjunctival heparin, artificial tears, and collagenase inhibitors [22]. Lecithinated superoxide dismutase has also been found to decrease epithelial defect size in patients with sterile corneal ulcers that do not respond to conventional therapy in some studies [29].
Systemic immunosuppression using cyclophosphamide, azathioprine, or methotrexate can be attempted if conjunctival resection yields no improvement [4][22]. Interferon alpha-2b has been used in patients who had hepatitis C and concomitant Mooren’s ulceration. In more aggressive cases, oral corticosteroids, cyclosporine A, and rituximab have also been used with some success [30][31].
A systematic review performed by Alhassan et al. found that there have not been any randomized controlled trials to compare the various treatment modalities for Mooren's ulcers. The authors recommend that studies measuring outcomes such as the percentage of area healed after treatment and speed of healing are necessary in order to ensure high-quality care for these patients.[22]
Complications
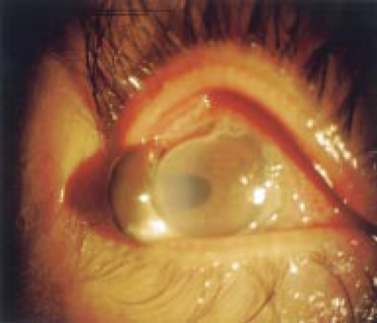
In addition to iritis being occasionally associated with Mooren’s ulceration, complications can include glaucoma, cataracts, and corneal perforation [4]. Anterior uveitis occurs 6.8% of the time and can involve fine dusty keratic precipitates and posterior synechiae locally [2]. Complicated cataracts can occur in 2.3% [2]. The rate of perforation has varied in different studies in various geographical locations. Perforation (figure 7) occurs most often in the limbal cornea, followed by the peripheral and then central cornea [2].
A study in South India described a perforation rate of 11% at the time of presentation [3]. This rate was higher for patients under 60 years old (27%) and was more likely to occur in patients with complete peripheral involvement of the corneal ulceration (73% of eyes with complete peripheral ulceration vs. 6% of those with partial peripheral ulceration). Other studies of Mooren's ulcer in South India described perforation rates varying from 0%[12] to 26% [11]. A study of 550 patients in China reported a perforation rate of 13% [2].
A Nigerian study suggested that, in addition to Mooren’s ulcer tending to affect more patients in their third and fourth decades of life, the perforation rate in these patients was more than one-third [15]. This study was further supported by another study consisting of 9 patients from West Africa [16].
Another complication that often occurs is postoperative recurrence. Chen and colleagues described a recurrence rate of 25.6% of the time, which can occur between 2 weeks and 15 years postoperatively, with 70.2% of recurrences occurring within 2-6 months. In recurrent cases, the first recurrence often occurs in the same location as the original ulcer and the interface of the donor graft and lamellar bed. Multiple recurrences, on the other hand, can occur in originally unaffected areas of the cornea, suggesting a difference between the immunological mechanisms of initial and multiple recurrences [2].
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Watson, P.G., Management of Mooren's ulceration. Eye (Lond), 1997. 11 ( Pt 3): p. 349-56.
- ↑ Jump up to: 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Chen, J., et al., Mooren's ulcer in China: a study of clinical characteristics and treatment. Br J Ophthalmol, 2000. 84(11): p. 1244-9.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Srinivasan, M., et al., Clinical characteristics of Mooren's ulcer in South India. Br J Ophthalmol, 2007. 91(5): p. 570-5.
- ↑ Jump up to: 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 Sangwan, V.S., P. Zafirakis, and C.S. Foster, Mooren's ulcer: current concepts in management. Indian J Ophthalmol, 1997. 45(1): p. 7-17.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Taylor, C.J., et al., HLA and Mooren's ulceration. Br J Ophthalmol, 2000. 84(1): p. 72-5.
- ↑ Gottsch, J.D., et al., Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol, 1999. 91(1): p. 34-40.
- ↑ Liang, C.K., et al., Association of HLA type and Mooren's Ulcer in Chinese in Taiwan. Br J Ophthalmol, 2003. 87(6): p. 797-8.
- ↑ Chi H, Hao W, Qi X, Zhang T, Dong Y, Gao H, Wei C, Shi W. A proteomic approach towards understanding the pathogenesis of Mooren's ulcer. Exp Eye Res. 2021 Feb 27;205:108509. doi: 10.1016/j.exer.2021.108509.
- ↑ Selvan H, Samantaray PP, Rana M. Bilateral Aggressive Mooren Ulcer in the Setting of Bilateral Pterygia and Pregnancy: A Unique Case. Cornea. 2024 Dec 1;43(12):1573-1577.
- ↑ Jump up to: 10.0 10.1 Zelefsky, J.R., et al., Hookworm infestation as a risk factor for Mooren's ulcer in South India. Ophthalmology, 2007. 114(3): p. 450-3.
- ↑ Jump up to: 11.0 11.1 11.2 Zegans, M.E., et al., Mooren ulcer in South India: serology and clinical risk factors. Am J Ophthalmol, 1999. 128(2): p. 205-10.
- ↑ Jump up to: 12.0 12.1 Nigi, Y., et al., Pyoderma gangrenosum with corneal perforation by Mooren's ulcer. J Dermatol, 2014. 41(5): p. 466-7.
- ↑ Gnandoss, A.S., An analysis of ten cases of Mooren's ulcer. Indian J Ophthalmol, 1974. 22(2): p. 22-4.
- ↑ Li, X., et al., Age distribution of various corneal diseases in China by histopathological examination of 3112 surgical specimens. Invest Ophthalmol Vis Sci, 2014. 55(5): p. 3022-8.
- ↑ Jump up to: 15.0 15.1 Kietzman, B., Mooren's ulcer in Nigeria. Am J Ophthalmol, 1968. 65(5): p. 679-85.
- ↑ Jump up to: 16.0 16.1 Wood, T.O. and H.E. Kaufman, Mooren's ulcer. Am J Ophthalmol, 1971. 71(1 Pt 2): p. 417-22.
- ↑ Lewallen, S. and P. Courtright, Problems with current concepts of the epidemiology of Mooren's corneal ulcer. Ann Ophthalmol, 1990. 22(2): p. 52-5.
- ↑ Jump up to: 18.0 18.1 Brown, S.I., Mooren's ulcer. Histopathology and proteolytic enzymes of adjacent conjunctiva. Br J Ophthalmol, 1975. 59(11): p. 670-4.
- ↑ Li L, Dong YL, Liu T, Luo D, Wei C, Shi WY. Increased succinate receptor GPR91 involved in the pathogenesis of Mooren's ulcer. Int J Ophthalmol. 2018 Nov 18;11(11):1733-1740. doi: 10.18240/ijo.2018.11.01.
- ↑ Martin, N.F., W.J. Stark, and A.E. Maumenee, Treatment of Mooren's and Mooren's-like ulcer by lamellar keratectomy: report of six eyes and literature review. Ophthalmic Surg, 1987. 18(8): p. 564-9.
- ↑ Schaap, O.L., T.E. Feltkamp, and A.C. Breebaart, Circulating antibodies to corneal tissue in a patient suffering from Mooren's ulcer (ulcus rodens corneae). Clin Exp Immunol, 1969. 5(4): p. 365-70.
- ↑ Jump up to: 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 Alhassan, M.B., M. Rabiu, and I.O. Agbabiaka, Interventions for Mooren's ulcer. Cochrane Database Syst Rev, 2014. 1: p. CD006131.
- ↑ Yoshihara M, Maeda N, Soma T, Fuchihata M, Hayashi A, Koh S, Oie Y, Nishida K. Corneal topographic analysis of patients with Mooren ulcer using 3-dimensional anterior segment optical coherence tomography. Cornea. 2015 Jan;34(1):54-9.
- ↑ Lucchino L, Mastrogiuseppe E, Giovannetti F, Bruscolini A, Marenco M, Lambiase A. Anterior Segment Optical Coherence Tomography for the Tailored Treatment of Mooren's Ulcer: A Case Report. J Clin Med. 2024 Sep 11;13(18):5384.
- ↑ Tandon R, Chawla B, Verma K, Sharma N, Titiyal JS. Outcome of treatment of mooren ulcer with topical cyclosporine a 2%. Cornea. 2008 Sep;27(8):859-61. doi: 10.1097/ICO.0b013e3181702d0c.
- ↑ Erdem U, Kerimoglu H, Gundogan FC, Dagli S. Treatment of Mooren's ulcer with topical administration of interferon alfa 2a. Ophthalmology. 2007 Mar;114(3):446-9. doi: 10.1016/j.ophtha.2006.09.024.
- ↑ Brown, S.I., Mooren's ulcer. Treatment by conjunctival excision. Br J Ophthalmol, 1975. 59(11): p. 675-82.
- ↑ Aviel, E., Combined cryoapplications and peritomy in Mooren's ulcer. Br J Ophthalmol, 1972. 56(1): p. 48-51.
- ↑ Shimmura, S., et al., Lecithin-bound superoxide dismutase in the treatment of noninfectious corneal ulcers. Am J Ophthalmol, 2003. 135(5): p. 613-9.
- ↑ Brown, S.I. and B.J. Mondino, Therapy of Mooren's ulcer. Am J Ophthalmol, 1984. 98(1): p. 1-6.
- ↑ Guindolet D, Reynaud C, Clavel G, Belangé G, Benmahmed M, Doan S, Hayem G, Cochereau I, Gabison EE. Management of severe and refractory Mooren's ulcers with rituximab. Br J Ophthalmol. 2017 Apr;101(4):418-422.