Giant Cell Arteritis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Giant cell arteritis (GCA) is the most common primary vasculitis in adults. Histopathologically, GCA is marked by generalized granulomatous inflammation of medium- to large-sized vessels that occurs in the elderly. The condition is also known as temporal arteritis. Based on United States census data from 2000, the prevalence of GCA is approximately 160,000. Since patients with GCA often present with vision loss, ophthalmologists are on the front lines of diagnosing the disorder. In addition to vision loss, patients commonly note associated symptoms such as headache, jaw claudication, diplopia, myalgias, and constitutional symptoms. The most commonly feared sequela of GCA is permanent visual loss secondary to arteritic anterior ischemic optic neuropathy (AAION). Since the vision loss from AAION can progress rapidly, and can involve the fellow eye within a matter of days, GCA is considered an ophthalmologic emergency. When GCA is suspected, treatment with corticosteroids is indicated on an urgent basis, as further vision loss and fellow eye involvement are usually preventable. There is a significant amount of research and discussion regarding the underlying etiology, pathogenesis, and appropriate management of patients with GCA. This review serves to highlight the essential concepts in our current understanding of the management of patients with GCA.
History
References to GCA can be found dating all the way back to 10th century Baghdad. The earliest observation was recorded by an ophthalmologist named Ali Ibn Isa al-Kahhal. He had discussed a potential relationship between inflamed arteries and visual signs and symptoms. He noted that “these diseased conditions may terminate in loss of sight.”[1] In his observations, he suggested excising the temporal arteries in order to treat these patients and save their vision. The next time GCA is noted was in the English literature in 1890 by Jonathan Hutchinson[2]. Hutchinson was treating an 80-year-old patient who had been experiencing “painful red streaks” on his head that were so uncomfortable he was unable to wear his hat. Hutchinson described these findings as “thrombotic arteritis of the aged.” At the time, there was no known link between atherosclerosis/arteriolosclerosis and this arteritis.
Then in 1932, Bayard Horton reported two patients presenting with symptoms that would eventually be highly characteristic of giant cell arteritis[3]. Their presentations included systemic symptoms such as fever, weakness, headache, pain, and difficulty chewing, as well as ophthalmologic symptoms of transient diplopia and vision loss. Horton and his team were the first to perform a temporal artery biopsy and describe the now well-known histopathological findings of granulomatous vasculitis. At the time, the disease was known as “Horton’s disease”. The pathology of the disease was further explained by Gilmoure in 1941 when he described the presence of giant cells as part of the disease. The term “temporal arteritis” began to gain popularity in the 1930s and was further linked to other inflammatory and rheumatological diseases such as polymyalgia rheumatica (PMR) by Jennings in 1938[4]. Current literature estimates that approximately one-third of patients with GCA have PMR at presentation.
Epidemiology and Risk Factors
Giant cell arteritis is the most common primary systemic vasculitis in adults. The incidence is negligible in patients less than 50 years old and rises relative to the patient’s age, with a median age of 75 years. Specifically, the average estimates range between 2.3 per 100,000 cases per year in the sixth decade of life to 44.7 per 100,000 cases per year in patients in their ninth decade.[5][6] The average age of presentation is 72.5 years for women, and 70.3 years old for men.[7] Furthermore, women are affected between 2 and 6 times more often than men.[8] This increased incidence in women might be explained by the higher proportion of women in the elderly population. When examining the role of race and epidemiology in GCA, white patients of northern European descent have the highest rates (about 30⁄100,000 in Norway) while African, Asian and Arab populations had the lowest rates (1.47⁄100,000 in Japan).[9] Other risk factors may include smoking, low body mass index, early menopause, and relative adrenal hypofunction.[10][11]
Etiology
The underlying etiology of GCA is complex and has been widely researched, yet is still not well understood. This includes genetic and possibly infectious factors, which go on to trigger an immune response.
A genetic predisposition for GCA has been suspected, due to increased reports of GCA among first degree relatives and rare familial forms of GCA.[12] There have also been reports of monozygotic twin concordance, further increasing this suspicion.[13] [14] Certain genes within the human leukocyte antigen (HLA) class I and class II regions, specifically, HLA DRB1*04, DRW6, and DR3 have been associated with increased susceptibility to GCA as well[15]. These genes encode amino acids in the antigen-binding pocket of the HLA molecule, which suggests that the disease may be antigen-driven and further highlights the role of adaptive immunity in the pathogenesis.[16]
Other linked genes are those related to cytokine and chemokine expression, which can alter the clinical presentation of GCA in different patients. For example, polymorphisms at the tumor necrosis factor-a (TNF-a) locus and the interleukin (IL)-10 promoter region have independently correlated to a greater risk of developing GCA. Similarly so, genes encoding vascular endothelial growth factor (VEGF), interferon-c and platelet glycoprotein receptor (PGR) are linked to an increased risk of ischemic complications in GCA.[17]
In addition to genetic factors, an infectious or environmental factor has been suspected due to successive studies of a stable population in Minnesota, where a cyclic fluctuation in the incidence of GCA was noted. [18] A range of infectious stimuli have been implicated, including Chlamydia pneumoniae, varicella virus, and parvovirus B19. [19][20][21] However, there are conflicting reports that argue against these infectious stimuli in select patients. [22][23] Additionally, some have suggested that an endogenous element in the arterial wall stimulates the initial inflammatory event.[24] Furthermore, the age-specific expression of GCA may be explained by age-related calcifications in the lamina, elastin and extracellular matrix proteins.[25]
After the initial trigger, regardless of etiology, a dual immune response begins.[26] One involves a systemic inflammatory reaction and the other is a maladaptive, antigen-specific immune response. The systemic inflammatory reaction results from over-activation of the innate acute phase response: a non-antigen-driven, non-adaptive defense mechanism to overall stress and injury. This response is mediated by IL-6, produced by circulating macrophages, neutrophils and monocytes[27]. IL-6 levels have correlated with the intensity of the immune response and other acute phase reactants such as C-reactive protein, haptoglobin, fibrinogen, and complement. The combination of these reactants under the systemic inflammatory reaction leads to the general signs of inflammation seen in GCA such as fevers, chills, sweats, myalgias, anorexia, and weight loss. In comparison, the antigen-specific immune response damages the arterial walls and results in the focal ischemic complications seen in GCA. The combination of these two processes results in the systemic inflammatory syndrome, and arteritis, respectively.
GCA preferentially affects three-layered vessels. These layers include an outer adventitia, a muscular medial layer, and an elastic lamina separating the inner intimal layer. The primary site of injury is in the adventitial layer[28]. This is due to the fact that the adventitial layer is the only vascularized layer – composed of the vasa vasorum. Macrophages and T cells then utilize the vasa vasorum to enter the arterial wall. The luminal side is not usually affected because cell adhesion and entry is prohibited by the high velocity of blood flow through these arteries.
Activated T-cells in the vasa vasorum of medium and large vessels up-regulate and activate macrophages, which then form a granulomatous immune reaction. Activated T-cells lead to the maturation of dendritic cells in the adventitia of the arteries, which produce chemokines that trigger the recruitment of CD4+ T cells. These CD4+ T cells then become activated, multiply and polarize to Th1 and Th17 cells, which in turn produce IFN-γ and IL-17 respectively. In response to IFN-γ release, endothelial cells and smooth muscle cells produce chemokines which lead to the recruitment of Th1 cells, CD8+ T cells and monocytes. The monocytes differentiate into macrophages, which form giant cells, the histological trademark of GCA. This results in the destruction and remodeling of the arterial wall and progressive occlusion of the lumen responsible for the ischemic symptoms of GCA.[29]
Metalloproteinases and reactive oxygen intermediates expressed by macrophages ultimately cause destruction within the blood vessel wall.[30] After this initial immune response, the vessel undergoes a healing response to injury, which includes intimal thickening, myofibroblast proliferation, and extracellular matrix deposition – all of which contribute to vascular stenosis and occlusion. This vascular stenosis and occlusion ultimately cause a variable set of signs and symptoms, depending on the territory supplied by the affected vessel. For example, short posterior ciliary artery occlusion results in ischemic optic neuropathy. Of note, there has been no evidence demonstrating the role of B cells in the development of GCA. This is an important distinguishing point between large vessel vasculitides and ANCA-associated vasculitides.[31]
General Pathology
GCA is characterized by a nodular granulomatous inflammation of medium- and large-sized arteries.[32] Pathological examination of affected vessels usually reveals a fragmented internal elastic lamina, with a cellular infiltrate extending transmurally.[33] It is important to remember that skip lesions are common, so typically a two centimeter biopsy of the temporal artery is recommended. Giant cells are not necessary on the pathologic specimen to confirm the diagnosis; however, when they are present, they are most often situated near the fragmented internal elastic membrane.[34] The most common vessels affected are the superficial temporal artery, the ophthalmic artery, the posterior ciliary arteries, and the vertebral arteries.[35] Less commonly, the aorta, coronary arteries, and carotid circulation are affected; intracranial arteries are typically spared.[34] The intracerebral arteries are typically spared from the vasculitic attack of GCA, presumably because of the paucity of elastic tissue in their walls[28]
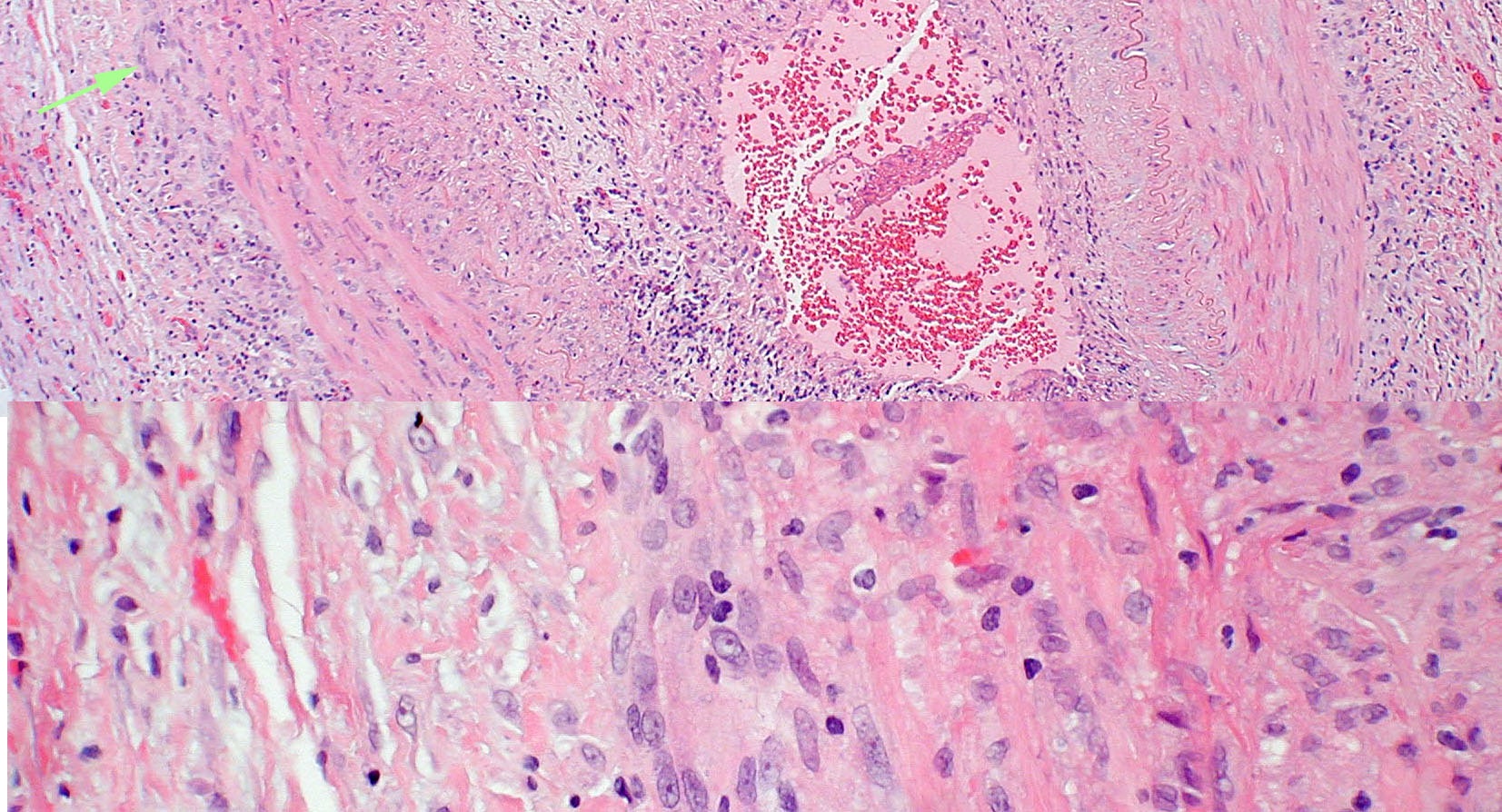
Figure 1. Top panel: Pathologic specimen from a patient with biopsy-proven giant cell arteritis. Multi-nucleated giant cells (arrow) are present in the vessel wall. Bottom panel: Higher power view. Photograph courtesy of Ben Glasgow, M.D.
Pathophysiology
Ocular manifestations are common in GCA and can arise at any point of the disease including during treatment. The most common ocular manifestation of GCA is visual loss, most commonly secondary to Arteritic Anterior Ischemic Optic Neuropathy (AAION).[36] In these cases, the short posterior ciliary arteries are occluded by intimal hyperplasia, secondary to systemic inflammation.[36][37][38][39][40] Once occluded, the short posterior ciliary arteries cannot provide blood flow to the prelaminar and laminar portions of the optic nerve head, this results in an ischemic injury to the anterior portion of the optic nerve (AAION). Pallid optic nerve edema is a common finding.
A similar phenomenon, classically not associated with GCA is non-arteritic ischemic optic neuropathy (NAION), which is due to hypoperfusion of the terminal para-optic branches of the short posterior ciliary artery leading to optic disc ischemia. NAION presents with either segmental or diffuse optic nerve head edema with hemorrhage, which can evolve to pallor. However, there have been cases reported of biopsy confirmed AAION secondary to GCA presenting with segmental disk edema without pallor, mimicking NAION. These cases were presumed to be due to GCA involving only a subset of the short posterior ciliary arteries supplying the optic nerve head. Therefore, it is important to have a high index of suspicion for GCA when examining patients with atypical presentation of NAION.
Although AAION is the most common manifestation, GCA can affect the entire visual pathway from the retina to the occipital lobe. Retinal ischemia can result from co-existent (or isolated) central retinal artery involvement. This may be evident on funduscopic examination or fluorescein angiography. [41]
Less common causes of ischemic visual loss include posterior ischemic optic neuropathy, cilioretinal artery occlusion, choroidal infarction and rarely ischemia to the optic chiasm or post-chiasmal visual pathway. Furthermore, patients with cortical visual loss may experience visual hallucinations arising from the focal areas of ischemia and usually resolve after a few weeks. Ischemia to other ocular structures, such as the extraocular muscles, can result in dysfunction of the affected structure (see “Physical Examination” section).
In addition to the ocular findings described above, patients often complain of headache and scalp tenderness. These symptoms can be attributed to involvement of the temporal artery, and resultant localized ischemia. Jaw claudication, another common symptom of GCA, is due to vascular involvement in the territory of the masticatory muscles. Systemic symptoms, such as fevers and malaise, are likely secondary to the release of inflammatory cytokines, such as interleukin-6, during the acute inflammatory phase.
Diagnosis
The diagnosis of GCA is complicated by the fact that there is no single lab value, imaging result or biopsy that will always be positive in all patients. Therefore, no test can be used to rule it in or out. Additionally, patients can present with a broad range of symptoms, including both systemic and ocular complaints; none of which are pathognomonic for GCA. The transient nature of many of the characteristic signs and symptoms further complicates diagnosis. There are several features in the history, physical examination, and in ancillary tests that can aid the clinician in making the diagnosis. It is crucial that clinicians maintain a high index of suspicion in any elderly patient with a new onset headache or visual changes. However, clinicians must consider each patient on a case-by-case basis, using the constellation of symptoms, examination features, and ancillary test to prompt diagnosis.
History
Ocular Manifestations
The most common ocular manifestation in GCA is acute unilateral vision loss, which has been reported in 7-60% of patients with GCA. Typically, the vision loss is secondary to optic nerve ischemia (AAION); it is less commonly due to a central retinal artery occlusion. Other, less common causes of vision loss include posterior ischemic optic neuropathy (PION), resulting from interrupted blood flow to the retrobulbar portion of the optic nerve, and cerebral ischemia, which can lead to homonymous hemianopsia. Even with effective medical therapy, reports of permanent partial or complete vision loss range between 8.2% to 20%.[42] Once permanent visual loss has ensued, it is irreversible. Furthermore, some estimates in untreated patients have shown that vision loss in the unaffected eye can ensue within one week of the primary insult[43]. Fortunately, the rapid initiation of steroid therapy prior to loss of vision significantly reduces chances of subsequent ocular complications. There are currently no effective methods of stratifying risk factors for developing permanent visual loss in GCA. Although numerous risk factors were analyzed, prior transient vision loss is currently the only strong risk factor associated with developing permanent vision loss[44].
In approximately 30% of patients with permanent vision loss, there are antecedent episodes of amaurosis fugax.[36] Patients typically complain of partial field defects or a curtain covering a portion of vision in one eye. These episodes of transient visual loss typically begin about 8.5 days prior to permanent loss, and are secondary to transient retinal, choroidal, and/or optic nerve ischemia.[45] Other than vision loss, patients may complain of diplopia, eye pain, or symptoms from cranial neuropathies (i.e. ptosis, anisocoria, diplopia).
Systemic Manifestations
Systemic symptoms are present in most patients, may be acute or gradual, and often precede the ocular manifestations.[46][47] The most common systemic complaint of GCA patients is a new onset headache (temporal or occipital), which is present in 90% of cases.[48][49] Headaches are often associated with localized or diffuse scalp tenderness[32]; patients may complain of discomfort when combing their hair or placing their head on a pillow. Patients often note a wide range of constitutional symptoms, such as fatigue, fevers, and weight loss. In addition, they may complain of jaw or tongue claudication, and pain in other areas, including the face, ears, and mouth. Tongue numbness and vertigo have also been reported.[50] Myalgias, especially of the proximal muscles, are also associated with GCA. Less often, neurological symptoms, such as peripheral neuropathies[51][52], strokes[53], scalp necrosis[54] and dementia[55][56] have been attributed to GCA.
Symptoms of polymyalgia rheumatica (PMR) are present in up to 50% of patients.[48][57] PMR is a more common inflammatory syndrome that seems to share a similar underlying etiology with GCA, as well as a similar demographic of commonly affected patients. Symptoms of PMR include bilateral aching neck, shoulder pain or stiffness and pelvic pain or stiffness. Additionally, many patients with PMR note constitutional symptoms, such as fever, weight loss, and anorexia.
Physical Examination
The physical examination of a patient in whom GCA is suspected should include careful evaluation of the following elements:
- Detailed examination of the temporal artery to detect prominence (Figure 2), nodularity, or tenderness to palpation of the artery and the surrounding skin. In addition, an evaluation of the strength of the temporal artery pulse is crucial.
- Detailed ophthalmologic examination evaluating visual acuity, pupils (looking for a relative afferent defect), intraocular pressures, anterior segment examination, motility examination (looking for ocular misalignment and/or evidence of cranial neuropathies), and a dilated fundus examination (evaluating for signs of optic nerve or retinal ischemia)
- Visual fields testing
- Auscultation of the carotid artery for bruits
- Auscultation of the heart for an aortic regurgitation murmur to evaluate for aortic aneurysm
Figure 2. Preoperative appearance of a patient with a prominent left superficial temporal artery. The vessel was tender and pulseless. Histopathology confirmed a result positive for temporal arteritis in this case. Image courtesy of Marcus M. Marcet, MD FACS.
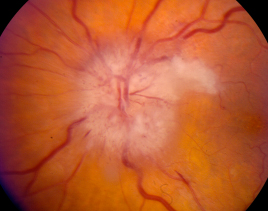
AAION is characterized by acute monocular vision loss accompanied by optic disc edema. The optic disc edema in AAION is usually diffusely “chalk white”, and may be accompanied by disc hemorrhage, retinal whitening or cotton wool spots.[58] Adjacent retinal whitening and cotton wool spots are crucial findings that indicate concurrent retinal ischemia, and should raise a clinician’s suspicion for GCA. Visual acuity is often 20/200 or worse, with up to 21% demonstrating no light perception.[59] An obvious afferent pupillary defect is often present. Visual field loss in patients with AAION is typically in a central, arcuate, or altitudinal pattern.
A much less common cause of GCA-associated optic neuropathy is posterior ischemic optic neuropathy. In cases of posterior ischemic optic neuropathy, patients will present with similar symptoms to those with AAION, and will have similarly decreased visual acuity, abnormal visual fields, and a relative afferent pupillary defect. However, in posterior ischemic optic neuropathy, the optic disc will appear perfectly normal; therefore, a high index of suspicion for posterior ischemic optic neuropathy is warranted in elderly patients presenting with acute visual loss and a normal appearing optic nerve.
In addition to the optic nerve circulation, the central retinal artery can also be affected by GCA: approximately 10% of patients with ocular involvement experience a central retinal artery occlusion.[36][60] Central retinal artery occlusions are manifest by retinal whitening with an associated “cherry red spot” in the macula. If the retinal whitening is more diffuse, and there is absence of a “cherry red spot”, then ophthalmic artery occlusion should be suspected. GCA should be considered in any patient with a central retinal artery occlusion over the age of 50.
Localized ischemia to the extra-ocular muscles and/or cranial nerves can result in diplopia and ocular misalignment in 2-15% of patients.[61][62] Additionally, Horner’s syndrome[50], anterior segment ischemia[63], and hypotony (likely secondary to decreased aqueous humor production)[64] have all been reported in patients with GCA.
Figure 3. Typical findings of a patient with arteritic ischemic optic neuropathy. Note the pallid disc edema, associated hemorrhages, and adjacent cotton wool spot. Photograph courtesy of Dr. Nicholas Volpe.
Signs
Although ocular involvement is common in GCA, the incidence of ocular findings in all GCA patients is not well-defined. In various studies, ocular findings range from 14-70%[7][36][50][61][65][66][67](this wide range is largely due to differing study populations). The visual loss can initially be transient, and can become bilateral and permanent, with typical involvement of the second eye often within 14 days in untreated cases.[36][68] In addition, GCA can present with any ocular symptoms (vision loss or diplopia) without additional systemic findings; this type of presentation is considered “occult”, and can occur in up to 38% of cases.[59]
Symptoms
Reliability of symptoms
The sensitivity and specificity of many ocular and non-ocular findings have been intensively studied in order to delineate clear indications for further testing. Meta-analyses of several studies have determined that both jaw claudication and diplopia are the most diagnostically powerful indicators of GCA.[69][70] Furthermore, temporal artery abnormalities such as temporal artery pain and an abnormal temporal artery on palpation are good indicators of disease.[69]
The 1990 American College of Rheumatology classification criteria for GCA include the presence of at least 3 of the following 5 findings[71]:
- Greater than 50 years old
- New onset of headache
- Temporal artery abnormality on examination
- Elevated erythrocyte sedimentation rate (ESR) using the Westergreen method (>50 mm/h)
- Abnormal arterial biopsy demonstrating a necrotizing vasculitis with predominance of mononuclear cells and granulomatous inflammation.
These criteria have been shown to have a sensitivity of 93.5% and a specificity of 91.2% in GCA in classifying patients with vasculitis.[71] However, the importance of the clinical history and setting cannot be understated when diagnosing GCA. Furthermore, these criteria were determined by rheumatologists, and studies that defined them were likely subject to under-representation of patients with solely ocular manifestations.[69] Several criticisms have been raised regarding these criterion, including the lack of specificity of headache[72][73], the lack of inclusion of several very significant markers such as CRP, jaw claudication, and neck pain, and the possibility of GCA with a normal ESR or temporal artery biopsy.[73] A 2012 retrospective chart review of all patients undergoing biopsy at a single institution showed that 9 out of 35 patients with positive biopsies would not have been diagnosed with GCA using the 1990 guidelines. Furthermore, 11 out of 39 patients with negative biopsies met the criteria and would have been diagnosed with GCA[74].
The 2022 American College of Rheumatology/EULAR classification criteria for GCA[75] include the presence of:
- Age ≥50 years at diagnosis is an absolute requirement.
- Score of ≥6 points, considering:
- positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5)
- erythrocyte sedimentation rate ≥50 mm/hour or C reactive protein ≥10 mg/L (+3)
- sudden visual loss (+3)
- morning stiffness in shoulders or neck, jaw or tongue claudication, new temporal headache, scalp tenderness, temporal artery abnormality on vascular examination, bilateral axillary involvement on imaging and fluorodeoxyglucose-positron emission tomography activity throughout the aorta (+2 each).
The chewing gum test for diagnosing GCA is no better than a clinical history of jaw claudication. However, if there is an onset of pain after 2 minutes of chewing, specificity increases, which may assist clinicians in distinguishing GCA-related jaw claudication from other causes of jaw pain.[76]
Giant cell arteritis probability score
The Southend giant cell arteritis (GCA) probability score (GCAPS)[77] is a pre-test tool that helps assess the likelihood of a patient having GCA. The GCAPS is based on a patient's: symptoms, signs, laboratory findings, demographics, and competing differentials. The GCAPS score is used to categorize patients into risk groups: Low risk: GCAPS score of less than 9; Medium risk: GCAPS score of 9–12; High risk: GCAPS score of more than 12. A GCAPS score of less than 10 may be enough to rule out GCA without further review. Patients with a GCAPS score of 13 or higher are considered high risk and may benefit from empirical glucocorticoids. The GCAPS has been shown to be an effective diagnostic tool and risk assessment tool. It has a high negative predictive value (NPV) and an excellent area under the receiver operating characteristic curve (ROC AUC). A score of 9 or 10 or more steroid medications are considered.
Clinical diagnosis
The diagnosis of GCA should be considered in any patient over the age of 50 with new headaches, acute visual changes, symptoms of polymyalgia rheumatica, unexplained constitutional symptoms, or jaw claudication. Since individual patients with GCA can present with a wide range of symptoms and examination findings, and many of the symptoms may be transient, patients must be questioned directly about symptoms of GCA. In any patient in whom GCA is suspected based on history, examination findings, or an elevated ESR, C-reactive protein or thrombocytosis, a temporal artery biopsy and initiation of corticosteroid treatment should be considered. In addition, several ancillary tests may help to confirm the diagnosis.
Although mathematical prediction models for GCA do not substitute for clinical experience, they can objectively guide shared doctor-patient decision-making. A ten factor diagnostic pretest prediction model for GCA with geographic external validation and compliance with transparent reporting guidelines is available.[78] There are accompanying posttest probability tables after the result of either ultrasound, MRI or a negative temporal artery biopsy.[79]
Diagnostic procedures
Temporal artery biopsy
Unfortunately, there is no single test that is positive in all cases of GCA. Temporal artery biopsy is the gold standard (Figure 4); however, a negative biopsy does not confirm a negative diagnosis. The biopsy should be obtained as soon as possible after the patient’s presentation, but should not delay the initiation of treatment with corticosteroids. Most authors feel that the sensitivity of the biopsy is not significantly affected for the first 2 weeks after starting therapy.[80] However, there has been one study that demonstrated a decrease in sensitivity from 82% to 60% after 1 week of steroid therapy.[81]
False negatives are relatively common (5-13%) because of “skip lesions”, or areas without disease within the vessels. In order to avoid false negatives, it has been recommended that the biopsy sample have a length of 1 cm to up to 2.5 cm of artery. In general, longer biopsy specimens have led to increased chances of positive results[82]. But more contemporary studies have challenged this belief, showing that shorter biopsy lengths may be adequate[83]. However, it is also important to note that shrinkage of the specimen will occur with fixation and should be taken into account. Approximately 10 percent shrinkage can be expected on average.[84][85]
Initial negative biopsy results may warrant follow-up with a contralateral biopsy depending on the clinical context. Physicians should evaluate the overall clinical picture including any additional imaging studies available before making this decision. Studies have demonstrated that repeat biopsy may be positive in 1-15% of patients.[72][86][87][88][89][90]
All sections should be stained with hematoxylin and eosin. Furthermore, staining for elastin using an elastic Van Gieson stain has been proposed in order to identify the internal elastic lamina. However, a 2010 retrospective case series studying 105 biopsies showed no increase in diagnostic sensitivity using this method. Van Gieson staining may be helpful in cases of supplementary investigation, such as repeat biopsies.[91]
Most commonly, a panarteritis consisting of lymphocytes and macrophages with or without granuloma formation is seen during inspection. Additionally, intimal thickening and fragmentation of the internal elastic lamina should be sought. The procedure for obtaining a temporal artery biopsy is relatively safe, with rare reports of complications including hematoma, infection[72], nerve damage[92], stroke[93] and scalp necrosis.[94]
Figure 4. Initial intraoperative appearance of a temporal artery biopsy. The ruler along the left side is located posteriorly in this case. The incision is made posterior to the hairline (hair shaved) to avoid damage to the temporalis branch of the facial nerve. The right superficial temporal artery is clearly seen prior to complete dissection and biopsy. Except for grossly visible age-related calcification, the intraoperative appearance of the vessel was otherwise normal. The histopathology confirmed a negative result. Image courtesy of Marcus M. Marcet, MD FACS.
Ancillary imaging tests
Patients with GCA have abnormalities on several ancillary tests. Fluorescein angiography has been found to show delayed choroidal and central retinal artery filling, with possible choroidal non-perfusion, especially in the peri-papillary area. [37][36][73][95] Angiographic findings are usually seen within the first days of symptom onset.
Color Doppler ultrasound (CDUS) has been gaining popularity as an alternative to temporal artery biopsy. CDUS provides many advantages such as being non-invasive, safe, and providing real time imaging and analysis in addition to visualizing multiple arteries including the facial, occipital and vertebral arteries. [96][97] Ultrasonography may demonstrate arterial edema, which is represented by hypoechoic halos immediately adjacent to the arterial wall;[98][99][100] this finding has been shown to have a sensitivity of 69% and specificity of 82% in a recent meta-analysis.[101] In a single study, the specificity reached 100% when the halos were found bilaterally.[102] Furthermore, CDUS is largely operator-dependent and lack of experience and standardization of image acquisition can hinder its potential assistance in diagnosis.
Magnetic resonance imaging (MRI) has recently been utilized in the diagnosis of GCA, but may demonstrate blood vessel wall thickening and/or enhancement (in the same area as the “halo-sign” on ultrasound).[103] Comparing MRI results with clinically diagnosed GCA via meta-analysis demonstrated a pooled sensitivity and specificity of 73% and 83% respectively[104]. Similarly so, when compared to a positive temporal artery biopsy, the sensitivity and specificity were 9% and 81%, respectively. Limitations to this method include cost, radiation exposure, and diminishing sensitivity after initiation of glucocorticoid therapy.
Ultrasound biomicroscopy (UBM) has also been used preliminarily to detect the halo sign.[105] Finally, computerized tomography (CT), MRI and PET scanning of the abdomen and chest may be useful to evaluate the aorta and other great vessels if large-vessel vasculitis is suspected.
Patients with a negative initial evaluation via biopsy or ultrasound who continue to raise high clinical suspicion may warrant additional testing. Reasons for negative evaluation may include incorrect biopsy location, non-cranial artery involving GCA, or no GCA. Physicians may opt to evaluate patients for potential large-vessel GCA using imaging studies such as CDUS, CT, and MRI of the vascular system.
Comparison of different diagnostic imaging modalities
| Modality | Typical Findings | Advantages | Disadvantages |
|---|---|---|---|
| CDUS |
|
|
|
| CT/CT-A |
|
|
|
| MRI/MR-A |
|
|
|
| PET-CT |
|
|
|
Adapted from Berger et al.[106]
There is a lack of substantial data directly comparing the different imaging modalities. Furthermore, a benchmark for comparison is lacking since histological confirmation is not always available. In one cohort of 24 patients with suspected GCA, PET/CT was shown to be superior to CTA (without contrast-enhanced venous phase) with a specificity of 100% vs 84.7% and comparable sensitivities of 66.7% vs 73.3% respectively. A different study in a temporal artery biopsy-positive cohort, with 15 GCA patients and 9 non-GCA patients, compared PET vs. CT-A. PET was slightly superior to CTA due to false positive findings with CTA, which effectively lowered the positive predictive value in that group. Other studies also found comparable outcomes of PET/CT and CTA, with a slightly better performance of PET/CT, as it was more sensitive in detecting inflammation in a given segment. In summary, PET/CT slightly outperforms CTA for initial GCA diagnosis, but the two techniques show good concordance.
Laboratory tests
The most commonly utilized laboratory test in the diagnosis of GCA is the ESR, measured by Westergren method.[107] The ESR can occasionally be normal (ie. <30-40 mm/hr) in up to 30% of patients with biopsy proven GCA. [61][70][72][73][108][109][110] A meta-analysis of many studies showed that an ESR >100 mm/hr had a positive likelihood ratio (LR) of 2.466 and a negative LR of 0.775. However, the ESR can be normalized by corticosteroids or immunosuppression, and there is often difficulty in decision making when the ESR ranges between 50 and 100. In addition, the ESR may be elevated in other disease states such as anemia, inflammatory disease, malignancy, or infection.[32]Therefore, the ESR must be interpreted within the clinical context of a given scenario.
The C-reactive protein (CRP) has been utilized as an adjunct test to the ESR. The CRP has certain advantages over the ESR, including a lack of variation with age, sex or hematological factors. The sensitivity and specificity of the CRP for the diagnosis of GCA has been reported as 100% and 97%, respectively.[72] Some authors have asserted that the CRP is more sensitive in the diagnosis of GCA.[72] A retrospective chart review of 119 patients with biopsy-proven GCA demonstrated a 99% sensitivity when ESR and CRP were utilized together.[111]
Platelets are also an acute phase reactant. Numerous studies have shown the utility of platelets as a predictor of GCA.[78][69][73][107][112][113][114] [115]Anti-cardiolipin antibodies[116], elevated IFN gamma[117], and elevated interleukin-6.[118] have not been proven to have definite correlation with GCA in large studies, and are thus subjects for future research.
Differential diagnosis
The most common disease in the differential diagnosis of GCA with decreased vision is non-arteritic ischemic optic neuropathy (NAION). There are several differences in the patient population, clinical signs and symptoms that are important to elicit. NAION is associated with small-crowded optic nerves, hypertension, diabetes, and hyperlipidemia[32], whereas AAION patients do not necessarily have the same vascular risk factors. In addition, patients with GCA are typically older than those with NAION (i.e. 8th-9th decade vs. 6th-7th decade). In NAION, patients will usually not note any associated systemic symptoms such as headache, jaw claudication, scalp tenderness, weight loss, anorexia, fever, or myalgias/arthralgias. In general, the vision loss from GCA is more severe than NAION, with one third of the AAION patients demonstrating a visual acuity worse than 20/200[119], and most NAION patients retaining a visual acuity near 20/80. In addition, AAION patients may complain of preceding amaurosis fugax, as well as other ocular symptoms such as diplopia, fellow eye involvement, or ophthalmoplegia.
The physical examination also differs between these two diseases. Unlike NAION, AAION patients demonstrate a diffuse “chalky” white edema[37][59], eventual cupping of the disc, and possible coexistent retinal ischemia. NAION often demonstrates segmental optic nerve edema with eventual sectoral or total flattening and pallor. In addition, laboratory evaluations such as the ESR and CRP are usually normal in patients with NAION.
Aside from NAION, one must consider other systemic causes of vasculitis which may present with similar systemic and possibly ophthalmic manifestations. These conditions include rheumatoid arthritis, systemic lupus erythematosus, polyarteritis nodosa, polymyositis, and Takayasu arteritis.[32] In addition, one should consider malignancy, systemic infections, dental disease, trigeminal neuralgia, and amyloidosis in the appropriate clinical setting.
Table 1. Important features that may help to differentiate arteritic ischemic optic neuropathy (AAION) from non-arteritic ischemic optic neuropathy (NAION). 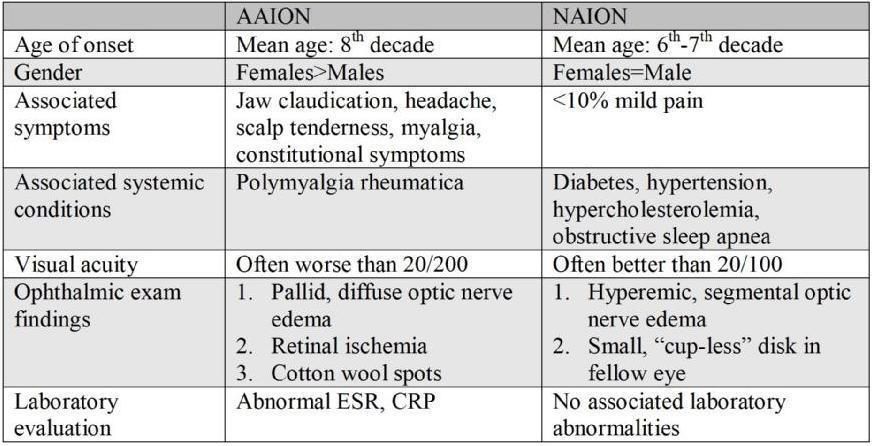
Management
Medical therapy
The standard of care for the initial treatment of GCA is corticosteroids. Corticosteroids should be started immediately after patients are suspected of having GCA, and should not be held until a biopsy can be undertaken. There is no standardized regimen for the optimal dose of corticosteroids for either induction or maintenance therapy. It has been shown, however, that higher doses of corticosteroids should be utilized in higher risk patients, ie. those who have had severe vision loss, or those with the potential for severe vision loss or cerebrovascular disease.[73][94] Suggested dosages of prednisone for initiation of treatment range from 40 mg to 100 mg per day, with a common starting point of 1 mg/kg/day.[116] If the diagnosis of GCA is confirmed with a biopsy, prednisone dosage should be increased to 80-100 mg/day, or intravenous corticosteroids (ie. 1 gram of methylprednisone for 3 days followed by 1-2 mg/kg/day of oral prednisone) should be utilized.[48][73]
Some have suggested that IV steroids be utilized in patients with amaurosis fugax, marked loss of vision in one eye, or signs of fellow eye involvement.[48][73] The suggested method is administering “pulses” of 500-1000mg methylprednisolone intravenously each day due to the importance of protecting the patient’s vision. However, there is no consensus regarding the use of IV steroids, since there has not been any significant benefit demonstrated in several studies[120][121][122] and the elderly population is at high risk of serious adverse reactions to high dose IV steroid pulses. However, IV methylprednisone has been shown to demonstrate higher serum concentrations more rapidly than oral dosing, and is probably useful in certain clinical scenarios.[123]
In select situations, steroid-sparing agents may be utilized. Situations in which the need for these agents may arise include those patients who do not respond to corticosteroids or for those who require less exposure to steroids because of side effects. Methotrexate has been shown to suppress disease activity when used with lower dosages of corticosteroids over a shorter course.[124][125] Other drugs under investigation include anti-tumor necrosis factor alpha antibody[126], azathioprine[127], cyclophosphamide[128], and anti-platelet agents, such as aspirin.[129]
New treatment strategies
In GCA IL-6 is up regulated within the inflamed arteries and peripheral circulation. It has been proposed that the pharmacologic inhibition of the IL-6 can ameliorate vascular inflammation in this setting. GiACTA is a Phase III, global, randomized, double-blind, placebo-controlled trial investigating the efficacy and safety of Actemra/RoActemra® (tocilizumab) a humanized anti-interleukin-6 as a novel treatment for GCA. In published reports, tocilizumab therapy leads to rapid and maintained improvement, both clinically and serologically, in patients with refractory/relapsing GCA and/or with unacceptable side effects related to corticosteroids. However, the risk of infection should be kept in mind when using this drug in patients with GCA. [130] [131] [132]
Patients who were eligible to enroll in the study presented with either new-onset or relapsing GCA, and were diagnosed using either a positive temporal artery biopsy or proof of large vessel disease using imaging (angiography, MRA, CTA or PET-CT) with associated elevations in acute-phase reactants. Tocilizumab was found to be effective either when used in conjunction with corticosteroid therapy or after steroid taper. It was not found to be effective in flares.[133]
As tocilizumab is associated with infection, gastric perforation, and abnormal lab values including thrombocytopenia, neutropenia, transaminitis, and elevated lipids, patients should have routine lab screenings. These include neutrophil count, platelet count and liver function tests starting four to eight weeks after initiating therapy and every three months afterwards. Similarly, lipids should be initially monitored between four and eight weeks of therapy and every few months afterwards. Other biologics that are currently being tested include TNF-a blockers, IL-12/IL-23 blockers, IL-1b blockers, and T-cell mediated CD28 blockers.
Medical follow up
Once corticosteroid treatment is initiated, it is important to follow the patient closely to determine the response to therapy. Some have recommended following the ESR and CRP from every 3 days to weekly, depending on the severity and route of administration of the corticosteroids. Although symptomatic improvement may be reached within days of therapy initiation, the ESR can take several weeks to decrease. Once an effective dose of corticosteroid is reached (based upon improvement in symptoms), the treatment at this dose should be continued for a minimum of 4-6 weeks.[134] However, treatment courses typically last for 1 to 2 years, given the importance of a very slow steroid taper to avoid a relapse of the GCA.[135]
There is no consensus on how frequently patients should be seen after starting treatment. A 2019 article in the British Medical Journal recommended follow-ups in weeks 0, 1, 3, and 6, and then monthly follow up afterwards for the first year.[133]Physicians may choose to design their own follow-up schedules and should individualize management focusing on patient disease progression, symptoms, and complications. Each visit should screen for progressing GCA and PMR including cranial symptoms and ischemic symptoms. Adverse effects to steroids should also be monitored closely. Proper patient education and access to support should not be overlooked as GCA is a chronic condition.
Once the decision is made to taper the corticosteroids, it is usually decreased at a rate of 5-10 mg per month until a dose of 10-15 mg per day is reached, and then the taper is decreased by 1-5 mg per month.[136] The patient should be followed closely for any relapse of symptoms, or increase in ESR or CRP. Relapses typically occur within the first 1.5 years, with a median time of approximately 7 months; however, active disease has been reported up to 9 years after initiating therapy.[137]
Complications
Patients with known GCA can rarely develop sequelae of large vessel vasculitis, including aortic aneurysms or dissection, after steroid treatment has been tapered. Studies using positron emission tomography (PET) and autopsy suggest that more than 50% of patients with GCA may have subclinical aortitis.[138][139] Fortunately, the rate of catastrophic events from aortic aneurysms in this population is probably less than 10%.[140][141] In addition, GCA can affect the coronary arteries, and patients with GCA have an increased relative risk of ischemic cardiovascular events, such as angina or myocardial infarction.[142]
There are several side effects and complications related to the use of long-term corticosteroids. Most commonly, patients are at increased risk for osteoporosis and gastrointestinal disturbances. For this reason, it is recommended that patients on long-term corticosteroids should be given calcium, vitamin D supplementation, and an H2 antagonist or proton pump inhibitor. Furthermore, one should consider the use of bisphosponates in selected patients. Other complications for which patients must be monitored include diabetes mellitus, hypertension, immunosuppression, glaucoma, and cataracts.
Population-based studies have estimated approximately 43% of patients suffer one or more relapses of GCA either during or after initial treatment.[143][144][145][146][147] However, differing studies have reported values ranging from 34 percent to 74 percent. The reason for variation is multifactorial. Signs and symptoms of relapse can vary from patient to patient and inflammatory markers may be normal during the flare. Physicians should ask about new-onset ischemic complications, symptoms similar to original presentation, or classic manifestations of GCA or PMR. Due to the lack of consensus in defining a relapse, accurate diagnosis is not possible and therefore physicians must have a high index of suspicion. In the event of diagnosed relapse, patients should be re-started on glucocorticoid therapy. In general, most cases of GCA relapse have been shown to occur at prednisone doses below 20mg/day especially during the first year of treatment.
Prognosis
The visual prognosis is highly dependent on the rapidity with which steroids are started, and the status of the patient’s vision upon presentation. If the patient has already had an ischemic event resulting in AAION or a CRAO, it is rare that vision will improve appreciably.[60][148] However, up to one-third of treated cases may demonstrate some small degree of improvement in acuity after treatment has been initiated.[59][60][121][149][150]
In general, vision often stabilizes once steroids are started; however, if it deteriorates on steroid therapy, it tends to be within 5 days[60][122] and is rare after 1 month.[151] It has been suggested that 9-17% of patients may have a deterioration of their vision while on corticosteroids.[151][152] Additionally, one small study suggested that approximately 9% of patients can progress to fellow eye involvement after therapy is initiated;[60] in contrast, 20-62% of untreated patients may progress to this stage.[151] If there is visual deterioration after treatment is initiated, the prognosis is grim, with 80% of patients progressing to light perception or no light perception in one small study.[60] Features that are associated with a worse visual prognosis include visual symptoms before the steroids are initiated, older age, fever, weight loss, antecedent transient visual loss, diplopia, and jaw claudication.[59][68][73][151] In addition, studies have demonstrated a worse ocular prognosis in patients with a high concentration of hemoglobin and a lower ESR.[46]
Patients with GCA may also be at increased risk for cardiovascular disease within the first year of diagnosis[153], including myocardial infarction from coronary artery vasculitis[154], aortic disease[155], and stroke.[58] These patients also may experience complications from long-term steroid use.[156] Finally, it has been suggested that GCA patients are at increased risk for malignancy, which has been observed in 7% of patients.[157] Fortunately, despite all of these potential complications, the life expectancy of this population is not significantly different than that of the general population.[66][158][159]
Additional Resources
- Turbert D, Janigian RH. Giant Cell Arteritis. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/giant-cell-arteritis-list. Accessed March 13, 2019.
- Patient Information Brochure for Giant Cell Artheritis. . North American Neuro-Ophthalmology Society (NANOS). Patient information Brochures. https://www.nanosweb.org/i4a/pages/index.cfm?pageid=4161. Accessed March 3, 2025
References
- ↑ Kawasaki A, Purvin V. Giant cell arteritis: an updated review. Acta Ophthalmol. 2009;87(1):13-32.
- ↑ Shelley BP. Temporal arteritis; Bayard Taylor Horton's headache era. Arch Med Health Sci 2013;1:188-90
- ↑ Horton BT, Magath TB, Brown GE. An undescribed form of arteritis of the temporal vessels. Mayo Clin Proc 1932;7:700-1
- ↑ Jennings GH. Arteritis of the temporal arteries. Lancet 1938;1:424-8
- ↑ Bengtsson B, Malmvall, BE. The epidemiology of giant cell arteritis including temporal arteritis and polymyalgia rheumatica, incidences of different clinical presentations and eye complications. Arthritis Rheum 1981;24:899-904.
- ↑ Machado E, Michet, CJ, Ballard, DJ, et al. Trends in incidence and clinical presentation of temporal arteritis in Olmsted County, Minnesota, 1950-1985. Arthritis Rheum 1988;31:745-749.
- ↑ 7.0 7.1 Baldursson O, Steinsson, K, Bjornsson, J, et al. Giant cell arteritis in Iceland. An epidemiologic and histopathologic analysis. Arthritis Rheum 1994;37:1007-1012.
- ↑ Salvarani C, Crowson CS, O'fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51(2):264-8.
- ↑ Hunder GG. Epidemiology of giant-cell arteritis. Cleve Clin J Med. 2002;69 Suppl 2:SII79-82.
- ↑ Larsson K, Mellstron, D, Nordborg, E, Oden, A, Nordborg, E. Early menopause, low body mass index, and smoking are independent risk factors for developing giant cell arteritis. Ann Rheum Dis 2006;65:529-532.
- ↑ Narvaez J, Bernad, B, Diaz Torne, C, et al. Low serum levels of DHEAS in untreated polymyalgia rheumatica/giant cell arteritis. J Rheumatol 2006;33:1293-1298.
- ↑ Fietta P, Manganelli P, Zanetti A, Neri TM. Familial giant cell arteritis and polymyalgia rheumatica: aggregation in 2 families. J Rheumatol. 2002;29(7):1551-5.
- ↑ Kemp A. Monozygotic twins with temporal arteritis and ophthalmic arteritis. Acta Ophthalmol 1977;55:183-190.
- ↑ Wernick C, Duvey, M, Bonafede, P. Familial gianct cell arteritis: report of an HLA-typed sibling pair and review of the literature. Clin Exp Rheumatol 1994;12:63-66.
- ↑ Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. The HLA-DRB1 locus as a genetic component in giant cell arteritis. Mapping of a disease-linked sequence motif to the antigen binding site of the HLA-DR molecule. J Clin Invest. 1992;90(6):2355-61.
- ↑ Carmona FD, Mackie SL, Martín JE, et al. A large-scale genetic analysis reveals a strong contribution of the HLA class II region to giant cell arteritis susceptibility. Am J Hum Genet 2015; 96:565.
- ↑ Rueda B, Roibas B, Martin J, Gonzalez-gay MA. Influence of interleukin 10 promoter polymorphisms in susceptibility to giant cell arteritis in Northwestern Spain. J Rheumatol. 2007;34(7):1535-9.
- ↑ Salvarani C, Gabriel SE, O'Fallon WM, Hunder GG. The incidence of giant cell arteritis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med 1995;123:192-4.
- ↑ Gabriel S, Espy, M, Erdman, DD, Bjornsson, J, Smith, TF, Hunder, GG. The role of parvovirus B19 in the pathogenesis of giant cell arteritis. Arthritis Rheum 1999;42:1255-1258.
- ↑ Nordborg C, Nordborg, E, Petursdottir, V, et al. Search for Varicella zoster virus in giant cell arteritis. Ann Neurol 1998;44:413-414.
- ↑ Rimeti G, Blasi, F, Cosentini, R, et al. Temporal arteritis associated with Chlamydia pneumoniae DNA detected in an arterial specimen. J Rheumatol 2001;28:1738-1739.
- ↑ Rodriguez-Pla A, Bosch-Gil, JA, Echevarria-Mayo, JE, et al. No detection of parvovirus B19 or herpesvirus DNA in giant cell arteritis. J Clin Virol 2004;31:11-15.
- ↑ Regan M, Wood, BJ, Hsieh, YH, et al. Temporal arteritis and Chlamydia pneumoniae: failure to detect the organism by polymerase chain reaction in ninety cases and ninety controls. Arthritis Rheum 2002;46:1056-60.
- ↑ Ma-Krupa W, Kwan M, Goronzy JJ, Weyand CM. Toll-like receptors in giant cell arteritis. Clin Immunol 2005;15:38-46.
- ↑ Toll-like receptors in giant cell arteritis Ma-Krupa W, Kwan M, Goronzy JJ, Weyand CM Clin Immunol, 2005
- ↑ Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139(6):505-15.
- ↑ Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139(6):505-15.
- ↑ 28.0 28.1 Weyand CM, Ma-krupa W, Goronzy JJ. Immunopathways in giant cell arteritis and polymyalgia rheumatica. Autoimmun Rev. 2004;3(1):46-53.
- ↑ Samson M, Corbera-Bellalta M, Audia S, Planas-Rigol E, Martin L, Cid MC,
Bonnotte B. Recent advances in our understanding of giant cell arteritis
pathogenesis. Autoimmun Rev. 2017 Aug;16(8):833-844. - ↑ Weyand C, Goronzy, JJ. Pathogenic principles in giant cell arteritis. Int J Cardiol 2000;75:S9-15.
- ↑ Uppal S, Hadi M, Chhaya S. Updates in the Diagnosis and Management of Giant Cell Arteritis. Curr Neurol Neurosci Rep. 2019;19(9):68.
- ↑ 32.0 32.1 32.2 32.3 32.4 Rahman W, Rahman, FZ. Giant cell (temporal) arteritis: an overview and update. Surv Ophthalmol 2005;50:415-428.
- ↑ Lie J. Histopathologic specificity of systemic vasculitis. Rheum Dis Clin North Am 1995;21:883-909
- ↑ 34.0 34.1 Weyand C, Bartley, GB. Giant cell arteritis: new concepts in pathogenesis and implications for management. Am J Ophthalmol 1997;123:392-395.
- ↑ Wilkinson I, Russell, RW. Arteries of the head and neck in giant cell arteritis. Arch Neurol 1972;27:378-391.
- ↑ 36.0 36.1 36.2 36.3 36.4 36.5 36.6 Hayreh S, Podhajsky, PA, Zimmerman, B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol 1998;125:509-520.
- ↑ 37.0 37.1 37.2 Hayreh S. Anterior ischaemic optic neuropathy II. Fundus on ophthalmoscopy and fluorescein angiography. Br J Ophthalmol 1974;58:964-980.
- ↑ Hayreh S, et al. Acute ischemic disorders of the optic nerve: pathogenesis, clinical manifestations, and management. Ophthalmol Clin North Am 1996;9:407-442.
- ↑ Henkind P, Charles, NC, Pearson, J. Histopathology of ischaemic optic neuropathy. Am J Ophthalmol 1970;69:78-90.
- ↑ Weyand C, Goronzy, JJ. Medium and large-vessel vasculitis. N Engl J Med 2003;349:160-169.
- ↑ Miller NR. Visual manifestations of temporal arteritis. Rheum Dis Clin North Am. 2001;27(4):781-97, vi.
- ↑ Chen JJ, Leavitt JA, Fang C, Crowson CS, Matteson EL, Warrington KJ. Evaluating the Incidence of Arteritic Ischemic Optic Neuropathy and Other Causes of Vision Loss from Giant Cell Arteritis. Ophthalmology. 2016;123(9):1999-2003.
- ↑ Danesh-meyer H, Savino PJ, Gamble GG. Poor prognosis of visual outcome after visual loss from giant cell arteritis. Ophthalmology. 2005;112(6):1098-103.
- ↑ Liozon E, Dalmay F, Lalloue F, et al. Risk Factors for Permanent Visual Loss in Biopsy-proven Giant Cell Arteritis: A Study of 339 Patients. J Rheumatol. 2016;43(7):1393-9.
- ↑ Kawasaki A, Purvin V. Giant cell arteritis: an updated review. Acta Ophthalmol 2009;87:13-32.
- ↑ 46.0 46.1 Cid M, Font, C, Oristrell, J, et al. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complicatios in giant cell (temporal) arteritis. Arthritis Rheum 1998;41:26-32.
- ↑ Ghanchi F, Weir, C, Dudgeon, J. Facial swelling in giant cell (temporal) arteritis. Eye 1996;10:747-749.
- ↑ 48.0 48.1 48.2 48.3 Salvarani C, Cantini, F, Boiardi, L, et al. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002;347:261-271.
- ↑ Tovilla-Canales J. Ocular manifestations of giant cell arteritis. Curr Opin Ophthalmol 1998;9:73-79.
- ↑ 50.0 50.1 50.2 Caselli R, Hunder, GG, Whisnant, JP. Neurologic disease in biopsy-proven giant cell (temporal) arteritis. Neurology 1988;38:352-359.
- ↑ Chess J, Albert, DM, Bhan, AK, et al. Serologic and immunopathologic findings in temporal arteritis. Am J Ophthalmol 1983;96:283-289.
- ↑ Reich K, Giansiracusa, DF, Strongwater, Sl. Neurologic manifestations of giant cell arteritis. Am J med 1990;89:67-72.
- ↑ Sandercock P, Warlow, CP, Jones, LN, et al. Predisposing factors for cerebral infarction: the Oxfordshire community stroke project. BMJ 1989;298:75-80.
- ↑ Manetas S, Moutzouris, DA, Falagas, ME. Scalp necrosis: a rare complication of temporal arteritis. Clin Rheumatol 2006;26:1169.
- ↑ Caselli R. Giant cell (temporal) arteritis: a treatable cause of multi-infarct dementia. Neurology 1990;40:753-755.
- ↑ Paulley J, Hughes, JP. Giant cell arteritis or arthritis of the aged. BMJ 1960;2:1562-1567.
- ↑ Healey L. The relation of giant cell arteritis to polymyalgia rheumatica. Baillieres Clin Rheumatol 199;5:371-378.
- ↑ 58.0 58.1 Carroll S, Gaskin, BJ, Danesh-Meyer, HV. Giant cell arteritis. Clin Experiment Ophthalmol 2006;34:159-173.
- ↑ 59.0 59.1 59.2 59.3 59.4 Liu G, Glaser, JS, Schatz, NJ, Smith, JL. Visual morbidity in giant cell arteritis. Clinical characteristics and prognosis of vision. Ophthalmology 1994;101:1779-1785.
- ↑ 60.0 60.1 60.2 60.3 60.4 60.5 Danesh-Meyer H, Savino, PJ, Gamble, GD. Poor prognosis of visual outcome after visual loss from giant cell arteritis. Ophthalmology 2005;112:1098-1103.
- ↑ 61.0 61.1 61.2 Huston K, Hunder, GG, Lie, JT, et al. Temporal artertitis: a 25-year epidemiologic, clinical, and patholgic study. Ann Intern Med 1978;88:162-167.
- ↑ Meadows S. Temporal or giant cell arteritis. Proc R Soc Med 1966;85:251-258.
- ↑ McKillop E, Tejawani, D, Weir, C, Jay, J. Anterior segment ischaemia with giant cell arteritis. Can J Ophthalmol 2006;41:201-203.
- ↑ Calcagni A, Claes, CA, Maheshwari, M, Jacks, AS. Hypotony as a presentation of giant cell arteritis. Eye 2007;21:123-4.
- ↑ Graham E, Holland, A, Avery, A, et al. Prognosis in giant-cell arteritis. Br Med J 1981;282:269-271.
- ↑ 66.0 66.1 Jonasson F, Cullen, JF, Elton, RA. Temporal arteritis: a 14-year epidemiological clinical and prognostic study. Scott Med J 1979;24:111-117.
- ↑ Whitfield A, Bateman, M, Trevor Cooke, W. Temporal arteritis. Br J Ophthalmol 1963;47:555-566.
- ↑ 68.0 68.1 Cornblath W, Eggenberger, ER. Progressive visual loss from giant cell arteriits despite high-dose intravenous methylprednisonolone Ophthalmology 1997;104:854-858.
- ↑ 69.0 69.1 69.2 69.3 Niederkohr R, Levin, LA. Management of the patient with suspected temporal arteritis. A decision-analytic approach. Ophthalmology 2005;112:744-756.
- ↑ 70.0 70.1 Smetana G, Shmerling, RH. Does this patient have temporal arteritis. JAMA 2002;287:92-101.
- ↑ 71.0 71.1 Hunder G, Bloch, DA, Michel, BA. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122-1128.
- ↑ 72.0 72.1 72.2 72.3 72.4 72.5 Hayreh S, Podhajsky, P, Raman, R, Zimmerman, B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol 1997;123:285-296.
- ↑ 73.0 73.1 73.2 73.3 73.4 73.5 73.6 73.7 73.8 Hayreh S, Zimmerman, B. Management of giant cell arteritis. Our 27-year clinical study: new light on old controversies. Ophthalmologica 2003;217:239-259.
- ↑ Murchison AP, et al. Validity of the American College of Rheumatology Criteria for the Diagnosis of Giant Cell Arteritis. Am J Ophthalmol 2012;154:722–729.
- ↑ Ponte C, Grayson PC, Robson JC, Suppiah R, Gribbons KB, Judge A, Craven A, Khalid S, Hutchings A, Watts RA, Merkel PA, Luqmani RA; DCVAS Study Group. 2022 American College of Rheumatology/EULAR classification criteria for giant cell arteritis. Ann Rheum Dis. 2022 Dec;81(12):1647-1653. doi: 10.1136/ard-2022-223480. Epub 2022 Nov 9. Erratum in: Ann Rheum Dis. 2023 Feb;82(2):e52. PMID: 36351706.
- ↑ Xiong J, Sammel A, Laurent R, Fraser CL. Diagnostic utility of the chewing gum test in giant cell arteritis. Clin Exp Ophthalmol. 2021 Jan;49(1):83-84. doi: 10.1111/ceo.13879. Epub 2020 Nov 12. PMID: 33141471.
- ↑ Laskou F, Coath F, Mackie SL, Banerjee S, Aung T, Dasgupta B. A probability score to aid the diagnosis of suspected giant cell arteritis. Clin Exp Rheumatol. 2019 Mar-Apr;37 Suppl 117(2):104-108. Epub 2019 Feb 15. PMID: 30767870. Melville AR, Donaldson K, Dale J, Ciechomska A. Validation of the Southend giant cell arteritis probability score in a Scottish single-centre fast-track pathway. Rheumatol Adv Pract. 2021 Dec 15;6(1):rkab102. doi: 10.1093/rap/rkab102. PMID: 35059557; PMCID: PMC8765789.
- ↑ 78.0 78.1 Ing EB, Miller NR, Nguyen A, et al. Neural network and logistic regression diagnostic prediction models for giant cell arteritis: development and validation. Clin Ophthalmol. 2019;13:421‐430.
- ↑ Liu J, Chuo J, Torun N, Ing E. The post-test probability of giant cell arteritis after ultrasound, MRI, or negative biopsy. The 2020 Canadian Ophthalmological Society Annual Meeting and Exhibition. May 1, 2020 https://doi.org/10.26226/morressier.5e9852a0df8760da4ba873fc
- ↑ Achkar A, Lie, JT, Hunder, GG, et al. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis. Ann Intern Med 1994;120:987-992.
- ↑ Allison M, Gallagher, PJ. Temporal artery biopsy and corticosteroid treatment. Ann Rheum Dis 1984;1984:416-417.
- ↑ Gonzalez-gay MA, Garcia-porrua C, Llorca J, Gonzalez-louzao C, Rodriguez-ledo P. Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum. 2001;30(4):249-56.
- ↑ Ypsilantis E, Courtney ED, Chopra N, et al. Importance of specimen length during temporal artery biopsy. Br J Surg. 2011;98(11):1556-60.
- ↑ Sudlow C. Diagnosing and managing polymyalgia rheumatica and temporal arteritis. Sensitivity of temporal artery biopsy varies with biopsy length and sectioning strategy. BMJ. 1997;315(7107):549.
- ↑ Gordon LK, Levin LA. Visual loss in giant cell arteritis. JAMA. 1998;280(4):385-6.
- ↑ Danesh-Meyer H, Savino, PJ, Eagle, RC, Kubis, KC, Sergott, RC. Low diagnostic yield with second biopsies in suspected giant cell arteritis. J Neuroophthalmol 2000;20:213-215.
- ↑ Hall S, Hunder, GG. Is temporal artery biopsy prudent? Mayo Clin Proc 1984;59:793-796.
- ↑ Klein R, Campbell, RJ, Hunder, GG. Skip lesions in temporal arteritis. Mayo Clin Proc 1976;51:504-510.
- ↑ Ponge T, Barrier, JH, Grolleau, JY, Ponge, A, Vlasak, AM, Cottin, S. The efficacy of selective unilateral temporal artery biopsy versus bilateral biopsies for diagnosis of giant cell arteritis. J Rheumatol 1988;15:997-1000.
- ↑ Hall J, Volpe, NJ, Galetta, SL, et al. The role of unilateral temporal artery biopsy. Ophthalmology 2003;110:543-548.
- ↑ Foss F, Brown LAn elastic Van Gieson stain is unnecessary for the histological diagnosis of giant cell temporal arteritisJournal of Clinical Pathology2010;63:1077-1079.
- ↑ Bhatti M, Goldstein, MH. Facial nerve injury following superficial temporal artery biopsy. Dermatol Surg 2001;27:15-17.
- ↑ Stuart R. Temporal artery biopsy in suspected temporal arteritis: a five-year survey. N Z Med J 1989;102:431-433.
- ↑ 94.0 94.1 Mizen T, et al. Giant cell arteritis: diagnostic and therapeutic considerations. Ophtahl Clin North Am 1991;4:547-556.
- ↑ Siatkowski R, Gass, DM, Glaser, JS, Smith, JL, Schartz, NJ, Schiffman, J. Fluorescein angiography in the diagnosis of giant cell arteritis. Am J Ophthalmol 1993;115:57-63.
- ↑ Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord 2010; 11:44.
- ↑ Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005; 142:359.
- ↑ Ghanchi F, Williamson, TH, Lim, CS, et al. Colour doppler imaging in giant cell (temporal) arteritis: serial examination and comparison with non-arteritic anterior ischaemic optic neuropathy. Eye 1996;10:459-464.
- ↑ Hunder G, Weyand, CM. Sonography in giant cell arteritis. N Engl J Med 1997;337:1385-1386.
- ↑ Schmidt W, Kraft, HE, Vorpahl, K, Volker, L, Gromnica-Ihle, EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336-1342.
- ↑ Karassa F, Matsaga, MI, Schmidt, WA, Ioanndis, JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005;142:359-369.
- ↑ Karahaliou M, Vaiopoulos, G, Papaspyrou, S, Kanakis, MA, Revenas, K, Sfikakis, PP. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther 2006;8:R116.
- ↑ Bley TW, O, Uhl,M, Thiel, J, Schmidt, D, Langer, M. High-resolution MRI in giant cell arteries: imaging of the wall of the superficial temporal artery. Am J Roentgenol 2005;184:283-287.
- ↑ Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018; 4:e000612.
- ↑ Vianna R, Mansour, M, Ozdal, PC, et al. The role of ultrasound biomicroscopy in predicting the result of temporal artery biopsy in temporal arteritis patients: a preliminary study. Eur J Ophthalmol 2005;15:655-659.
- ↑ Berger Christoph T., Sommer Gregor, Aschwanden Markus, Staub Daniel, Rottenburger Christof, Daikeler Thomas. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Medical Weekly.2018;148:w14661. https://smw.ch/article/doi/smw.2018.14661. Accessed [8/25/19].
- ↑ 107.0 107.1 Hazleman B. Laboratory investigations useful in the evaluation of polymyalgia rheumatica (PMR) and giant cell arteritis. Clin Exp Rheumatol 2001;18:S29-31.
- ↑ Ellis M, Ralston, S. ESR in the diagnosis and management of polymyalgia rheumatica/giant cell arteritis syndrome. Ann Rheum Dis 1983;42:168-170.
- ↑ Kyle V, Causton, TE, Hazleman, BL. Erythrocyte sedimentation rate and C reactive protein in the assessment of polymyalgia rheumatica/giant cell arteritis on presentation and during follow up. Ann Rheum Dis 1989;48:667-671.
- ↑ Salvarni C, Hunder, GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001;45:140-145.
- ↑ Parikh M, Miller, NR, Lee, AG, et al. Prevalence of a normal C-reactive protein with an elevated erythrocyte sedimentation rate in biopsy-proven giant cell arteritis. Ophthalmology 2006;113:1842-1845.
- ↑ Gonzalez-Alegre P, Ruiz-Lopez, AD, Abara-Costalago, M, et al. Increment of the platelet count in temporal arteritis: response to therapy and ischemic complications. Eur Neurol 2001;45:43-45.
- ↑ Foroozan R, Danesh-Meyer H, Savino PJ, Gamble G, Mekari-Sabbagh ON, Sergott RC. Thrombocytosis in patients with biopsy-proven giant cell arteritis. Ophthalmology. 2002;109(7):1267‐1271. doi:10.1016/s0161-6420(02)01076-x
- ↑ De Lott LB, Burke JF; Michigan Neuro-Ophthalmology Research Consortium. Use of laboratory markers in deciding whether to perform temporal artery biopsy. JAMA Ophthalmol. 2015;133(5):605‐606. doi:10.1001/jamaophthalmol.2014.5861
- ↑ Lincoff NS, Erlich PD, Brass LS. Thrombocytosis in temporal arteritis rising platelet counts: a red flag for giant cell arteritis. J Neuroophthalmol. 2000;20(2):67‐72.
- ↑ 116.0 116.1 Bhatti M, Tabandeh, H. Giant cell arteritis: diagnosis and management. Curr Opin Ophthalmol 2001;12:393-399.
- ↑ Weyand C, Tetzlaff, N, Bjornsson, J, et al. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum 1997;40:19-26.
- ↑ Hernandez-Rodriguez J, Segarra, M, Vilardell, C, et al. Elevated production of interleukin-6 is associated with a lower incidence of disease-related ischemic events in patients with giant-cell arteritis: angiogenic activity of interleukin-6 as a potential protective mechanism. Circulation 2003;107:2428-2434.
- ↑ Danesh-Meyer H, Savino, PJ, Eagle, RC, Sergott, RC. The prevalence of cupping in end-stage arteritic and nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2001;108:593-598.
- ↑ Chevalet P, Barrier, JH, Pottier, P, et al. A randomized, multicenter, controlled trial using intravenous pulses of methylprenisolone in the initial treament of simple forms of giant cell arteritis: a one year followup study of 164 patients. J Rheumatol 2000;27:1484-1491.
- ↑ 121.0 121.1 Hayreh S, Zimmerman, B, Kardon, RH. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of the literature. Acta Ophthalmol Scand 2002;80:355-367.
- ↑ 122.0 122.1 Hayreh S, Zimmerman, B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003;110:1204-1215.
- ↑ Hayball P, Cosh, DG, Ahern, MJ, Schultz, DW, Roberts-Thomson, PJ. High-dose oral methylprednisolone in patients with rheumatoid arthritis: pharmokinetics and clinical response. Eur J Clin Pharmacol 1992;42:85-88.
- ↑ Caporali R, Cimmino, MA, Ferraccioli, GF, et al. Prednisone plus methotrexate for polymyalgia rheumatica. Ann Intern Med 2004;141:495-501.
- ↑ Jover J, Hernandez-Garcia, C, Morado, IC, et al. Combined treatment of giant-cell arteritis with methotrexate and prednisolone, a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001;134:106-114.
- ↑ Airo P, Antoniolo, CM, Vianelli, M, Toniati, P. Anti-tumour necrosis factor treament with infliximab in a case of giant cell arteritis resistant to steroid and immunosuppressive drugs. Rheumatology 2002;41:347-349.
- ↑ De Silva M, Hazleman, BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double blind study. Ann Rheum Dis 1986;45:136-138.
- ↑ De Vita S, Tavoni, A, Jeracitano, G, et al. Treatment of giant cell arteritis with cyclophosphamide pulses. J Intern Med 1992;232:373-375.
- ↑ Nesher G, Berkun, Y, Mates, M, Baras, M, Rubinow, A, Sonnenblick, M. Low-dose aspirin and prevention of cranial ischemic complications of giant cell artertis. Arthritis Rheum 2004;50:1332-1337.
- ↑ Christidis D, Jain S, Gupta BD. Successful use of tocilizumab in polymyalgic onset biopsy positive GCA with large vessel involvement. BMJ Case Rep 2011. doi:10.1136/bcr.04.2011.4135.
- ↑ Beyer C, Axmann R, Sahinbegovic E, et al. Antiinterleukin 6 receptor therapy as rescue treatment for giant cell arteritis. Ann Rheum Dis 2011; 70:1874– 1875.
- ↑ Loricera J, Blanco R, Hernández JL, et. al. Tocilizumab in giant cell arteritis: Multicenter open-label study of 22 patients. Semin Arthritis Rheum. 2015 Jun;44(6):717-23. doi: 10.1016/j.semarthrit.2014.12.005. PubMed PMID:25697557.
- ↑ 133.0 133.1 Lazarewicz K, Watson P. Giant cell arteritis. BMJ. 2019 May 30;365:l1964. doi:10.1136/bmj.l1964. PubMed PMID: 31147398.
- ↑ Provon A, Gabriel, SE, Orces, C, O'Fallon, WM, Hunder, GG. Glucocorticoid therapy in giant cell arteritis:duration and treament outcomes. Arthritis Rheum 2003;49:703-708.
- ↑ Fauchald P, Rygvold, O, Oystese, B. Temporal arteritis and polymyalgia
- ↑ Goodman BJ. Temporal arteritis. Am J Med 1979;67:839-852.
- ↑ Blumberg S, Giansiracusa, DF, Docken, WP, et al. Recurrence of temporal arteriis. Clinical recurrence nine years after initial illness. JAMA 1980;244:1713.
- ↑ Ostberg G. Temporal arteritis in a large necropsy series. Ann Rheum Dis 1971;30:224-235.
- ↑ Blockmans D, de Ceunnick L, Vanderscheuren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluoro-deoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006;55:131-7.
- ↑ Levine SM, Hellman DB. Giant cell arteritis. Curr Opin Rheumatol 2002;14:3-10.
- ↑ Gonzalez-Gay MA, Garcia-Porrua C, Pineiro A, Pego-Reigosa A, Llorca J, Hunder GG. Aortic aneurysm and dissection in biopsy-proven giant cell arteritis patients from Northwest Spain. A population-based study. Medicine 2004;83:335-41.
- ↑ Le Page L, Duhaut P, Seydoux D. Incidence of cardiovascular events in giant cell arteritis: preliminary results of a prospective double cohort study. Rev Med Intern 2005;27:98-105.
- ↑ Kermani TA, Warrington KJ, Cuthbertson D, et al. Disease Relapses among Patients with Giant Cell Arteritis: A Prospective, Longitudinal Cohort Study. J Rheumatol 2015; 42:1213.
- ↑ Martinez-Lado L, Calviño-Díaz C, Piñeiro A, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore) 2011; 90:186.
- ↑ Alba MA, García-Martínez A, Prieto-González S, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore) 2014; 93:194.
- ↑ Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55:347.
- ↑ Restuccia G, Boiardi L, Cavazza A, et al. Flares in Biopsy-Proven Giant Cell Arteritis in Northern Italy: Characteristics and Predictors in a Long-Term Follow-Up Study. Medicine (Baltimore) 2016; 95:e3524.
- ↑ Hayreh S. Ophthalmic features of giant cell arteritis. Baillieres Clin Rheumatol 1991;5:431-459.
- ↑ Chan C, Pain, M, O'Day, J. Steroid management in giant cell arteritis. Br J Ophthalmol 2001;85:1061-1064.
- ↑ Foroozan R, Deramo, VA, Buono, LM, et al. Recovery of visual function in patients with biopsy-proven giant cell arteritis. Ophthalmology 2003;110:539-542.
- ↑ 151.0 151.1 151.2 151.3 Aiello P, Trautmann, JC, McPhee, TJ, et al. Visual prognosis in giant cell arteritis. Ophthalmology 1993;100:550-555.
- ↑ Calamia K, Hunder, GG, et al. Clnical manifestations of giant-cell (temporal) arteritis. Clin Rheum Dis 1980;6:389-403.
- ↑ Uddhammar A, Eriksson, Al, Nystrom, L, et al. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol 2002;29:737-742.
- ↑ Morris C, Scheib, JS. Fatal myocardial infarction resulting from coronary arteritis in a patient with polymyalgia rheumatica and biopsy-proven temporal artertis. A case report and review of the literature. Arch Intern Med 1994;154:1158-1160.
- ↑ Evans J, O'Fallon, WM, Hunder, GG. Increased incidence of aortic aneurysm and dissection in giant cell (Temporal)artertiis - a population based study. Ann Intern Med 1995;122:502-507.
- ↑ Nesher G, Sonnenblick, M, Friedlander, Y. Analysis of steroid related complications and mortality in temporal arteritis: a 15-year survey of 43 patients. J Rheumatol 1994;21:1283-1286.
- ↑ Liozon E, Loustaud, V, Fauchais, AL, et al. Concurrent temporal (giant cell) arteritis and malignancy: report of 20 patients with review of the literature. J Rheumatol 2006;33:1606-1614.
- ↑ Matteson E, Gold, KN, Bloch, DA, et al. Long-term survival of patients with giant cell arteritis in the American College of Rheumatology giant cell arteritis classification criteria cohort. Am J Med 1996;100:193-196.
- ↑ Nordborg E, Bengtsson, BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ 1989;299:549=550.