Progressive Supranuclear Palsy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
- ICD10 - G23.1
Disease
Progressive supranuclear palsy (PSP) or Steele-Richardson-Olszewski syndrome is characterized by a progressive supranuclear ophthalmoplegia typically vertical but in particular downward limitation of eye movement. There is often associated loss of balance due to degeneration of neurons in the brainstem and basal ganglia.[1] PSP is recognized as a tauopathy, with a middle age to late age onset.[2] In PSP tau proteins aggregate in the brainstem and basal ganglia that are superior to the clusters of nuclei directly controlling eye movement, hence the term “supranuclear.” These aggregations can be used for pathological diagnosis of PSP in the form of neurofibrillary tangles, neuropil threads, 4-repeat tau protein, and tau-positive astrocytes and their processes in the basal ganglia and brainstem.[3] Definitive diagnosis of PSP depends on pathological findings but many cases can be diagnosed premortem.[4] PSP results in hallmark clinical signs including vertical gaze palsy (especially downgaze) and loss of balance.[4] In addition, patients with PSP may experience dysarthria, dysphagia, muscle rigidity, a Parkinsonian like resting tremor, postural instability leading to multiple falls, and psychomotor slowing characterized by forgetfulness.[5][6] This disease has an annual incidence of 5.3 new cases per 100,000 person years and a prevalence estimated to be 1.39 per 100,000 people in the United States.[7] However, PSP is likely to be under-diagnosed due to its similarities to other neurodegenerative disorders, chiefly Parkinson disease.[8] In regards to this chapter on PSP, the focus is on distinguishing it from other disorders with similar presentations by describing diagnostic, clinical, and pathological markers for the disease.
Etiology
PSP is a sporadic disease and the exact cause is not known. Potential hypotheses include:
- Drug/Toxin-induced: There have been three geographical clusters of illnesses similar to PSP in Guam, [9][10][11][12] Guadeloupe,[13] [14] and northern France.[15] The first two locations revealed a strong association with PSP and cycad neurotoxins in Guam,[16] and consumption of traditional medicines containing annonacin in Guadeloupe.[13] [14] However, the cluster in France is the most compelling case for environmental etiology, because the cases in Guam and Guadeloupe were not classic cases of PSP. The study in northern France found an increased prevalence of arsenic in the soil of chemical plants near the towns with increases in classic PSP diagnosis.[15]
- Genetic Factors: There are 10 different MAPT mutations that have been identified in PSP patients including the R5L mutation in exon 1. The remaining variants are in exon 10 and its spicing region (p.L284R, p.S285R, p. delN6, p.N296N, p.P301L, p.G303V, p.S305S, IVS10+3, and IVS10+6). These genetic abnormalities lead to neurofibrillary tangles and are currently under investigation as causative agents.[17]
- Although most cases of PSP are sporadic, there have been reports of familial aggregation. Among these groups with familial PSP, a stronger association with the MAPT H1 haplotype was noted when compared to controls and patients with sporadic PSP. MAPT encodes the Tau protein and genetic mutations in this gene are common in tauopathies. The familial cases of PSP were confirmed by autopsy adding to the validity of the study.[18] [19] However, the presence of the MAPT H1 haplotype does not definitively predict development of PSP and can only be regarded as a mild predisposition for it.[20]
- Degenerative: See pathology below.
Risk Factors
General Pathology
At autopsy, the brain of an affected individual with PSP may appear grossly normal or have mild atrophy in the posterior fossa structures, including the globus pallidus. An orange discoloration of the substantia nigra may be noted with definite hypopigmentation.[1] The locus coeruleus may also show pigment depletion macroscopically.[22][6] Under light microscopy there will be evidence of high concentrations of neuropil threads and/or neurofibrillary tangles in the brainstem and basal ganglia.[22][6] These defects are found more specifically in the pretectal area, pontomesencephalic tegementum, superior colliculus, substantia nigra (pars compacta), ventral tegmental area, hippocampus, parahippocampus, cuneiform nucleus, pedunculopontine nucleus, frontal cortex, insular region, periaqueductal gray, inferior olive, dentate, Edinger-Westphal nucleus, and interstitial nucleus of Cajal. [6][1] The subthalamic nucleus, striatum, pallidum, thalamus, and cortex may also demonstrate neurofibrillary tangles and neuropil threads to a lesser extent.[6][1]
Much like Alzheimer disease, PSP is characterized by neurofibrillary tangles causing nerve cell loss, gliosis, and sometimes ballooned argyrophilic neuronal degeneration. However, the neurofibrillary tangles of PSP are arranged in clusters of circling and interlacing bundles comprised of straight filaments with an average diameter of 12-15 nm, whereas in Alzheimer disease the filaments are paired and helical.[1] Also, amyloid deposits and plaques are absent in PSP, but a hallmark for Alzheimer disease.[6] The National Institute of Neurological Disorders and Stroke (NINDS) requires the presence of high density neurofibrillary tangles and neuropil threads in at least three of the following to meet definitive pathological diagnostic criteria for PSP: pallidum, substantia nigra, pons, or subthalamic nucleus. In addition there must be a low to high density in at least three of the following structures: oculomotor complex, dentate nucleus, striatum, or medulla.[4][6]
Pathophysiology
As stated previously, PSP is a known tauopathy. The gene that encodes tau protein is located on chromosome 17q21 and is comprised of 16 exons. There are six different isoforms of tau protein due to alternative splicing, and these proteins can be isolated into groups depending on how many microtubule-binding domains are repeated. The tau protein will either have three or four domain repeats, which is determined by whether or not the transcript of exon 10 is spliced out in the final tau protein.[1] The aggregation of four repeat tau protein in neurons and neuroglia in the brainstem and basal ganglia are typical findings in patients with PSP, with a ratio of 3:1 in favor of four repeat tau over three repeat.[17][23]
These four repeat tau aggregations contribute to formation of neurofibrillary tangles in the regions of the central nervous system mentioned previously. In a similar tauopathy resulting from an FTDP-17 mutation induced in mice, motor and behavioral deficits were noted due to the development of neurofibrillary tangles.[24] This discovery confirmed that tau dysfunction can lead to direct neurodegeneration, and is a plausible cause for the ocular motor deficits and dementia noted in PSP.[1] As stated previously, a polymorphism in the tau gene contributing to the presence of H1 haplotype may predispose individuals to developing these four repeat aggregations, and therefore neurofibrillary tangles. However, this is a modest genetic predisposition at best.[19][20] Höglinger et al. also discovered new significant (p< 5x10-8) genetic markers associated with PSP risk, which implicate the STX6, EIF2AK3 and MOBP genes. This suggests that proteins associated with vesicle membrane fusion at the golgi-endosomal interface, endoplasmic unfolded protein response, and a myelin structural component may contribute to the pathophysiology of this disease.[25]
The major pathways affected by the pathology of PSP are the dopaminergic nigrostriatal, the GABAergic, cholinoceptive striatal neurons, and the cholinergic brainstem and basal forebrain nuclei.[26][27] [28] Choline acetyltransferase activity is also reduced in the striatum, substantia inominata, and cerebral cortex. The degradation of cholinergic nuclei may contribute to some of the visual disturbances. However, this is mostly in regards to horizontal saccades, and not the more characteristic downgaze palsy of classic PSP.[29] Similarity in presentation of PSP and Parkinson disease may be attributable to the dopaminergic nigrostriatal pathway deficits. PSP patients commonly present with subcortical dementia in the later stages, and this has been associated with neuronal cell loss in the cortex and in the substantia nigra, globus pallidus, and subthalamic nucleus of the subcortex.[30]
Diagnosis
History
An accurate patient history is necessary for diagnosis of probable PSP. However a definitive diagnosis relies on histopathological findings, due to the fact that PSP is commonly misdiagnosed as other Parkinsonian disorders on the basis of history alone. The most common symptoms reported in patient history include postural instability with falls, dysarthria, bradykinesia, and visual disturbances.[31] [32]
Physical examination
A comprehensive physical examination for PSP should include motor, visual, and cognitive/behavioral tests.
Motor
- Masked facies with startled expression
- Bradykinesia
- Retrocollis
- Hypophonic, monotone voice
- Wide-based gait
- Axial rigidity greater than appendicular rigidity. [4] [31] [32]
- Postural instability: demonstrated by standing behind the patient and sharply pulling them backwards by their shoulders (“pull test”). If the patient falls backwards into your arms or takes several steps backwards to steady themselves this is a positive finding common in PSP.[1]
Ocular exam
- Evaluate the extra-ocular muscles in the nine cardinal directions of gaze. In PSP, impairments in the vertical plane but especially downgazeas well as convergence eye movements abnormalities are common.[33]
- Saccade testing is performed by instructing the patient to look quickly between two objects in a specified visual plane. Test for slow vertical saccades, square wave jerks, or complete ophthalmoplegia.[33] Usually the downgaze is affected first in PSP.
- Test both untargeted (asking the patient to look up and down) and targeted saccades (asking the patient to look between two targets in a vertical plane). In PSP, the untargeted saccades are usually affected first.[1]
- If improvement in supranuclear ophthalmoplegia is seen with the vestibular ocular reflex or the Bell phenomenon, the cause is likely supranuclear.[33]
- Eyelid retraction, eyelid opening or closing apraxia, blepharospasm, or lid lag may also be seen in PSP.[33]
Cognitive/behavioral
- Evaluation of the patient’s cognitive processing is important, as patients with PSP tend to have slower processing speed and difficulty in planning and putting tasks in sequential order. Impairments in memory and apathy may also be noted, as well as emotional lability.[5][34]
- When testing using Neuropsychiatric Inventory, a high apathy coupled with a low agitation and anxiety score suggest PSP diagnosis.[34]
- A behavioral motor disinhibition is also common in PSP patients. This can be demonstrated by the “signe d’applause,” in which you ask the patient to clap three times and they proceed with clapping their hands more than 3 times.[1]
Signs
Clinical signs include downgaze palsy, loss of balance, postural instability, muscle rigidity, dysphagia, dysarthria, emotional lability (pseudobulbar affect) and poor response to Levodopa therapy.[5] A patient with PSP may display a “rocket sign,” which entails trying to rapidly rise out of a chair and immediately falling backwards.[1] Usually symptoms of bulbar atrophy are noticed early in the progression of the disease and result in dysarthria. This causes a characteristic “growling” speech that can become completely unintelligible.[35]
Symptoms
Due to downward limitations of eye movement, patients commonly report that they have difficulty reading, may spill food while eating, and trip forwards or backwards.[5][36] “Vision problems” are a common complaint, and if a proper examination of extra-ocular muscles is not performed, a diagnosis of PSP may be overlooked. Photophobia can lead to patients wearing sunglasses constantly, even indoors. Patients may report “feeling dizzy” or “off balance,” and caregivers will often state that the patient appeared to tip over without even trying to brace themselves. Sleep disturbances and behavioral changes are common. Insomnia may be attributed to the marked rigidity in PSP patients, who frequently complain of difficulty finding a comfortable sleep position.[5]
Clinical diagnosis
Clinical diagnostic criteria have been defined by the National Institute of Neurological Disorders and the Society for Progressive Supranuclear Palsy: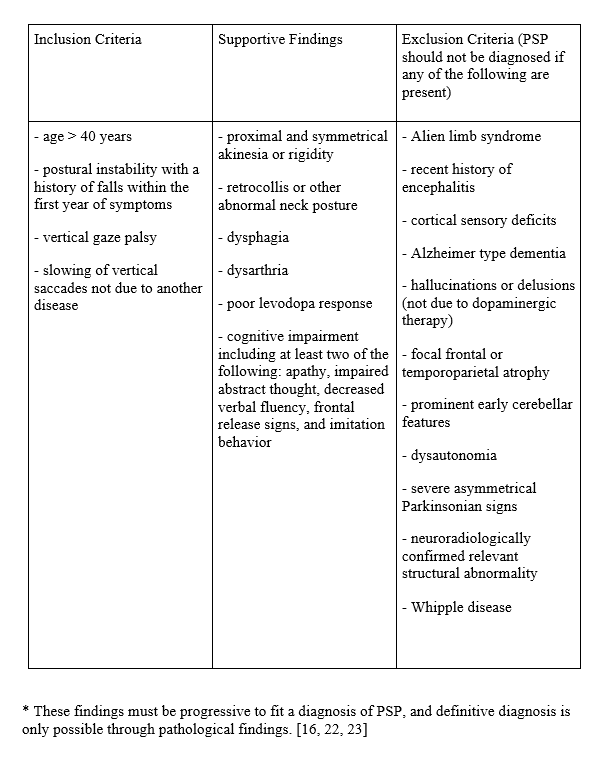
- These findings must be progressive to fit a diagnosis of PSP, and definitive diagnosis is only possible through pathological findings. [4] [31] [32]
Subtypes
Richardson syndrome (RS): This is the classic PSP type originally observed by Dr. Clifford Richardson in 1963. In a study done by D. R. Williams et al, 54% of all pathologically confirmed cases of PSP shared this phenotype. This subtype is characterized by falls and postural instability at early onset, and supranuclear gaze palsy and cognitive dysfunction in the later stages. The disease duration before death is significantly shorter for RS (5.9 years) and age at death is significantly younger (72.1 years) than in PSP-P. Also this subtype proved to be more prevalent in men than women. RS subtype also had a higher mean four repeat tau to three repeat tau ratio (2.84).[37]
PSP- Parkinsonism (PSP-P): The second subtype of PSP is commonly called atypical PSP and is distinguished by the presence of any tremor, an asymmetric onset of physical symptoms, and a moderate initial response to levodopa treatment. PSP-P is most often confused with Parkinson disease and has an even distribution between the sexes. In a study done by D.R. Williams et al. this subtype was found in 32% of the total confirmed PSP cases, and has a better prognosis. Compared to RS, patients with PSP-P had a longer disease duration (9.1 years) and later age at death (75.5 years). PSP-P subtype also had a lower mean four repeat tau to three repeat tau ratio (1.63) when compared to RS.[37]
PSP- pure akinesia with gait freezing (PSP-PAGF):The remaining 14% of cases of PSP could not be grouped into the RS or PSP-P categories based on the criteria for the Williams et al. study in 2005. [37] However, in 2007 Williams et al. identified a third PSP phenotype termed PSP- pure akinesia with gait freezing (PSP-PAGF). These patients do not present with the characteristic history of falls, vertical gaze palsy, rigidity, tremor, or cognitive dysfunction early in disease progression as in the previous two subtypes. [38] Patients with PSP-PAGF will more commonly present with start hesitation as an initial gait disturbance followed by gait, speech, or writing freezing. The speech and writing freezing leads to hypophonia and micrography, respectively. Gaze palsy took a mean of 9 years to manifest in these patients, and they did not improve with levodopa therapy. [39]
PSP- progressive nonfluent aphasia/apraxia of speech (PSP-PNFA/AOS): Josephs et al. found that a group of four patients suffering from a progressive motor speech disorder had a form of atypical PSP that was confirmed pathologically. These patients had PSP tau pathology in the neocortex which affected fluency. This caused anomia with an early preservation of semantic knowledge, episodic memory, and language comprehension. [40]
PSP-cerebellar ataxia (PSP-C): The cerebellar ataxia variant of PSP challenges the NINDS-SPSP criteria which names “prominent early cerebellar features” in their mandatory exclusion criteria. [4] The discovery of a variant characterized by predominant cerebellar ataxia is credited to a retrospective analysis of 22 pathologically confirmed cases of PSP in Japan. [41] Three of the patients in this study had cerebellar gait and limb ataxia early in disease progression, and one patient later developed gaze palsy and impaired cognition. Pathologic inspection revealed a higher prevalence of Purkinje cell tauopathy in PSP-C patients than in other PSP variants. Subsequent studies confirmed even more cases of PSP-C that were ruled out based on the exclusion criteria and therefore misdiagnosed. [39]
PSP phenomenologies are discovered continuously as pathological confirmation of PSP improves. In addition to the subtypes mentioned, other variants that have been described in the literature include PSP-corticobasal syndrome, PSP-behavioral variant frontotemporal dementia, PSP-primary lateral sclerosis, and PSP-pallido-nigro-luysian degeneration and axonal atrophy. One probable hypothesis for this spectrum of phenomenologies attributes the differences in clinical presentation to the differences in anatomical distribution of tau proteins. [39]
Diagnostic procedures
Due to the poor prognosis that accompanies diagnosis with PSP, an MRI of the brain is usually performed to rule out other causes for this set of symptoms, including cerebrovascular disease, hydrocephalus, and tumors. In addition, the MRI can reveal signal increase and atrophy of the midbrain and/or globus pallidus, poor differentiation of the substantia nigra, and atrophy of the putamen and/or red nucleus.[42] These features support the presence of PSP, and are found in the majority of patients with late stage disease. Volumetric MRI can demonstrate superior cerebellar peduncle atrophy and can distinguish PSP from Multiple System Atrophy (MSA), Parkinson disease (PD), and normal controls with a sensitivity of 74% and a specificity of 94%. However, this is not as reliable early in disease progression.[43][1]
Quattrone et al. recently used a Magnetic Resonance Parkinsonism Index (MRPI) for improved accuracy of PSP diagnosis. The MRPI is calculated by multiplying the midbrain/pons ratio by the middle cerebellar peduncle (MCP)/superior cerebellar peduncle (SCP) ratio. The MRPI value was significantly larger (P < .001) in patients with PSP when compared to PD, MSA, and control participants, without overlap between group values..[44]
Another study focusing on MR microscopy at high magnetic field strength found that non-heme iron protein was prevalent in the substantia nigra and globus pallidus of patients with PSP when compared to control brains. The presence of hemosiderin could be a potential biomarker and contrast enhancer for PSP when compared to normal controls. However, a comparison between hemosiderin levels in PSP and Parkinsonian brain specimens needs to be evaluated before this method can be used practically for diagnosis.[45]
Laboratory test
There are no lab tests used in diagnosis of PSP. These are only used for the purpose of ruling out diseases with similar presentations. Whipple disease, for example, has a similar presentation and can be diagnosed using polymerase chain reaction.
Differential diagnosis
PSP can mimic other tauopathies and disorders of the basal ganglia, which accounts for the frequency of misdiagnosis.
When considering a diagnosis of PSP, it is important to rule out Parkinson disease (PD) as these diseases share many chief characteristics including symmetrical bradykinesia, stooped gait, and bilaterally reduced arm swing.[1] PSP patients tend to have more postural instability and subsequent falls than those with PD. PD can cause postural instability, but this is usually later in progression, whereas it is the most common presenting symptom of PSP.[46][47] A PD patient may have an age-related slowing of vertical saccades, but this should not progress towards a restriction of vertical gaze like in PSP. Often PSP does not respond to levodopa therapy, whereas the rigidity accompanying PD should improve with therapy.[5] The hallmark “pill rolling” tremor of PD is usually absent in PSP, and rigidity has a tendency to be more axial than distal in PSP, unlike PD.[1]
Short term memory is often spared in PSP patients and when tested they outperform PD, Alzheimer, and Lewy Body dementia patients in memory tests. It is rare for patients with PSP to present with the cortical dementia that is common in Alzheimer disease. Instead they present with a subcortical dementia.[5][1]
Multiple system atrophy rarely manifests with a significant cognitive impairment, unlike PSP.[1] The cerebellar ataxia variant of PSP is commonly misdiagnosed as multiple system atrophy, and therefore the presence of cerebellar features should not necessarily exclude PSP from the differential diagnosis. [39] Patients with corticobasal degeneration, dementia with Lewy bodies, multiple system atrophy, and Parkinson-ALS-dementia complex of Guam all have the potential to present with a supranuclear gaze palsy and parkinsonism features, but these findings are rare outside of the context of PSP.[1] Other diseases to keep in the differential include Pick’s disease, and postencephalitic parkinsonism.[6] Cerebrovascular disease, hydrocephalus, and tumors can be ruled out using MRI.[42]
Management
General treatment
Management of PSP is directed at treatment of symptoms and physical therapy for fall reduction.
For postural instability, physical therapy can help prevent falls, improve mobility, and provide adequate walking support when necessary. Occupational therapy can help the patient rearrange his or her home to fit the limitations caused by PSP. Speech therapy can improve speech difficulties and provide communication devices.
If the patient is having difficulty swallowing he or she may also require percutaneous endoscopic gastrostomy feeding, which could prevent weight loss and aspiration pneumonia. Patients with the characteristic down gaze palsy can use mirror-prism glasses to help them with eating and reading or any other task requiring downward gaze. Specially tinted glasses can help reduce photophobia.[1] We advise against the use of progressive or multifocal lenses in PSP and prefer the use of single vision readers. In addition, eye drops can be used for dry eyes in order to prevent exposure keratitis, and botulinum toxin injections may reduce blepharospasm, dystonia, and retrocollis.[6][1] There are no effective medications in treating PSP directly, but options are available for symptomatic treatment. Dopaminergic replacement therapy is worth trying as it has been shown to mildly improve PSP symptoms. However, these improvements are often short lived. Antidepressants are often prescribed to treat patients with emotional apathy, depression, and pseudobulbar crying.[6] Amitriptyline can alleviate neck pain, insomnia, and excessive drooling, and gabapentin is useful in reducing dystonic pain.[1]
Prognosis
The prognosis for patients with PSP is poor, with the average time from onset of symptoms to death ranging from 5-9 years. Falls that occur early in the onset of disease may contribute to poor prognosis, as well as early difficulty with swallowing resulting in gastrostomy feeding.[1] The most common cause of death is pneumonia.[4] One study found that younger age at diagnosis correlated with an improved prognosis, however this has not been replicated in other research.[7]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 Warren N, Burn D. Progressive supranuclear palsy. Pract Neurol 2007; 7: 16–23.
- ↑ Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 1997;49:1284-1288.
- ↑ Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 2010;23:394-400.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994 Nov. 44(11):2015-9.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Boeve, Bradley F. Progressive Supranuclear Palsy. Parkinsonism & Related Disorders 2012;18S1:S192-S194.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 Rehman, H. U. "Progressive Supranuclear Palsy." Postgraduate Medical Journal 76.896 (2000): 333-36.
- ↑ Jump up to: 7.0 7.1 Golbe LI, Davis PH, Schoenberg BS, et al. Prevalence and natural history of progressive supranuclear palsy. Neurology 1988;38:1031–4.
- ↑ Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson- Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1996 Jun. 60(6):615-20.
- ↑ Arif M, Kazim SF, Grundke-Iqbal I, Garruto RM, Iqbal K. Tau pathology involves protein phosphatase 2A in parkinsonism-dementia of Guam. Proc Natl Acad Sci USA 2014;111:1144–1149.
- ↑ Lepore FE, Steele JC, Cox TA, et al.. Supranuclear disturbances of ocular motility in Lytico-Bodig. Neurology 1988;38:1849–1853
- ↑ Miklossy J, Steele JC, Yu S, et al.. Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam (ALS/PDC). Acta Neuropathol 2008;116:625–637.
- ↑ Winton MJ, Joyce S, Zhukareva V, et al.. Characterization of tau pathologies in gray and white matter of Guam parkinsonism-dementia complex. Acta Neuropathol 2006;111:401–412.
- ↑ Jump up to: 13.0 13.1 Caparros-Lefebvre D, Elbaz A. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a case-control study. Caribbean Parkinsonism Study Group. Lancet 1999;354:281–286.
- ↑ Jump up to: 14.0 14.1 Caparros-Lefebvre D, Sergeant N, Lees A, et al.. Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy. Brain 2002;125:801–811.
- ↑ Jump up to: 15.0 15.1 Caparros-Lefebvre,D., L. I. Golbe, V. Deramecourt, et al. “A Geographical Cluster of Progressive Supranuclear Palsy in Northern France.” Neurology 85.15 (2015): 1293-300.
- ↑ Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 2002; 58:956-959.
- ↑ Jump up to: 17.0 17.1 17.2 Im, Sun Young, Young Eun Kim, and Yun Joong Kim. “Genetics of Progressive Supranuclear Palsy.” Journal of Movement Disorders JMD 8.3 (2015): 122-29.
- ↑ Fujioka, S., A. A. Algom, M. E. Murray, A. Strongosky, A. et al. “Similarities between Familial and Sporadic Autopsy-proven Progressive Supranuclear Palsy.” Neurology 80.22 (2013): 2076-078.
- ↑ Jump up to: 19.0 19.1 Fujioka S, Van Gerpen JA, Uitti RJ, Dickson DW, Wszolek ZK. Familial progressive supranuclear palsy: a literature review. Neurodegener Dis 2014;13:180-182.
- ↑ Jump up to: 20.0 20.1 Pastor P, Tolosa E. Progressive supranuclear palsy: clinical and genetic aspects. Curr Opin Neurol 2002;15:429–37.
- ↑ Santacruz P, Uttl B, Litvan I, et al. Progressive supranuclear palsy: a survey of disease course. Neurology 1998;50:1637– 47.
- ↑ Jump up to: 22.0 22.1 Daniel SE, De Bruin VMS, Lees AJ. The clinical and patho- logical spectrum of Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy): a reappraisal. Brain 1995; 118:759–70.
- ↑ Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci 1998;21:428–33.
- ↑ Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant P301L tau protein. Nature Genetics 2000; 25:402-405.
- ↑ Höglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nature Genetics 2011; 43.7: 699-707.
- ↑ Agid Y, Javoy-Agid F, Ruberg M, et al. Progressive supranu- clear palsy: anatomoclinical and biochemical considera- tions. Adv Neurol 1987;45:191–206.
- ↑ Malessa S, Hirsch EC, Cervera P, et al. Catecholaminergic systems in the medulla oblongata in parkinsonian syndromes: a quantitative immunohistochemical study in Parkinson’s disease, progressive supranuclear palsy, and striatonigral degeneration. Neurology 1990;40:1739–43.
- ↑ Malessa S, Hirsch EC, Cervera P, et al. Progressive supranuclear palsy: loss of choline-acetyltransferase-like immunoreactive neurons in the pontine reticular formation. Neurology 1991;41:1593–7.
- ↑ Ruberg M, Javoy-Agid F, Hirsch E, et al. Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurology 1985;18:523–9.
- ↑ Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogenous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 1964;10:333– 359.
- ↑ Jump up to: 31.0 31.1 31.2 Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996 Jul. 47(1):1-9.
- ↑ Jump up to: 32.0 32.1 32.2 Litvan I, Agid Y, Jankovic J, et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology. 1996 Apr. 46(4):922-30.
- ↑ Jump up to: 33.0 33.1 33.2 33.3 Schmidt C, Herting B, Prieur S, Junghanns S, Schweitzer K, Globas C. Pupil diameter in darkness differentiates progressive supranuclear palsy (PSP) from other extrapyramidal syndromes. Mov Disord. 2007 Oct 31. 22(14):2123-6.
- ↑ Jump up to: 34.0 34.1 Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. 1996 Nov. 47(5):1184-9.
- ↑ Goetz CG, Leurgans S, Lang AE, et al. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003;60:917–22.
- ↑ Ling, Helen. "Clinical Approach to Progressive Supranuclear Palsy." Journal of Movement Disorders JMD 9.1 (2016): 3-13
- ↑ Jump up to: 37.0 37.1 37.2 Williams, D. R. "Characteristics of Two Distinct Clinical Phenotypes in Pathologically Proven Progressive Supranuclear Palsy: Richardson's Syndrome and PSP-parkinsonism." Brain 128.6 (2005): 1247-258.
- ↑ Williams DR et al. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord 2007;22:2235–41.
- ↑ Jump up to: 39.0 39.1 39.2 39.3 Lopez, G., K. Bayulkem, and M. Hallett. "Progressive Supranuclear Palsy (PSP): Richardson Syndrome and Other PSP Variants." Acta Neurologica Scandinavica Acta Neurol Scand (2016): DOI: 10.1111/ane.12546.
- ↑ Josephs KA et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and non- fluent aphasia. Neurocase 2005;11:283–96.
- ↑ Kanazawa M et al. Cerebellar involvement in progres- sive supranuclear palsy: a clinicopathological study. Mov Disord 2009;24:1312–8.
- ↑ Jump up to: 42.0 42.1 Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 2000;54:697–702.
- ↑ Paviour DC, Price SL, Stevens JM, et al. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 2005;64:675–9.
- ↑ Quattrone A, Nicoletti G, Messina D, et al. MR Imaging Index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 2008;246:214–221.
- ↑ Foroutan, Parastou, Melissa E. Murray, Shinsuke Fujioka, Katherine J. Schweitzer, Dennis W. Dickson, Zbigniew K. Wszolek, and Samuel C. Grant. "Progressive Supranuclear Palsy: High-Field-Strength MR Microscopy in the Human Substantia Nigra and Globus Pallidus." Radiology 266.1 (2013): 280-88.
- ↑ Birdi S, Rajput AH, Fenton M, et al. Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Move Disord 2002;17:1255–64.
- ↑ Nath U, Ben-Shlomo Y, Thomson RG, et al. Clinical features and natural history of progressive supranuclear palsy: A clinical cohort study. Neurology 2003;25:910–16.

